Electrochemical Degradation of Methylene Blue Using a Ni-Co-Oxide Anode
Abstract
:1. Introduction
2. Results and Discussion
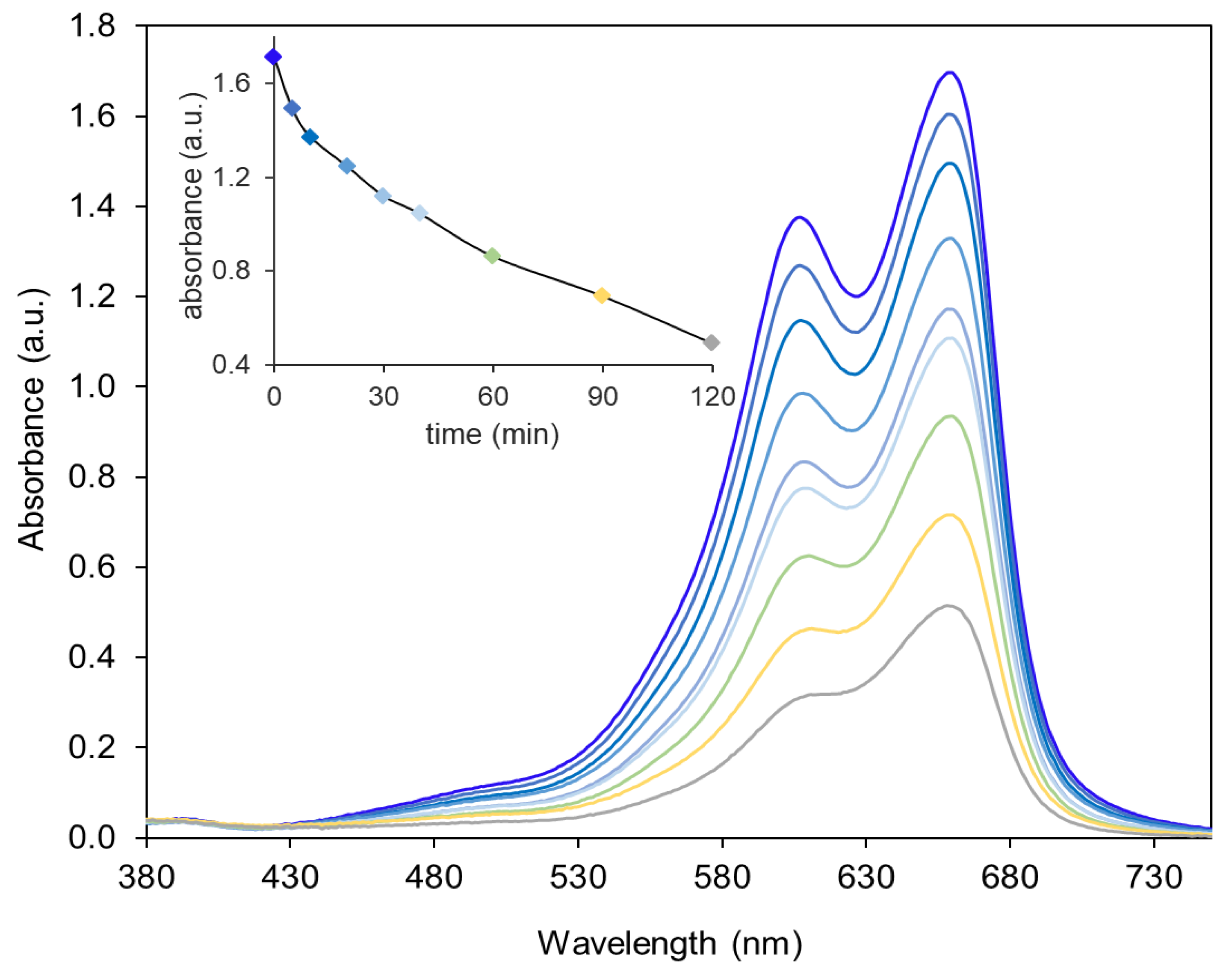
2.1. Electrochemical Degradation of MB in the Absence of Chlorides
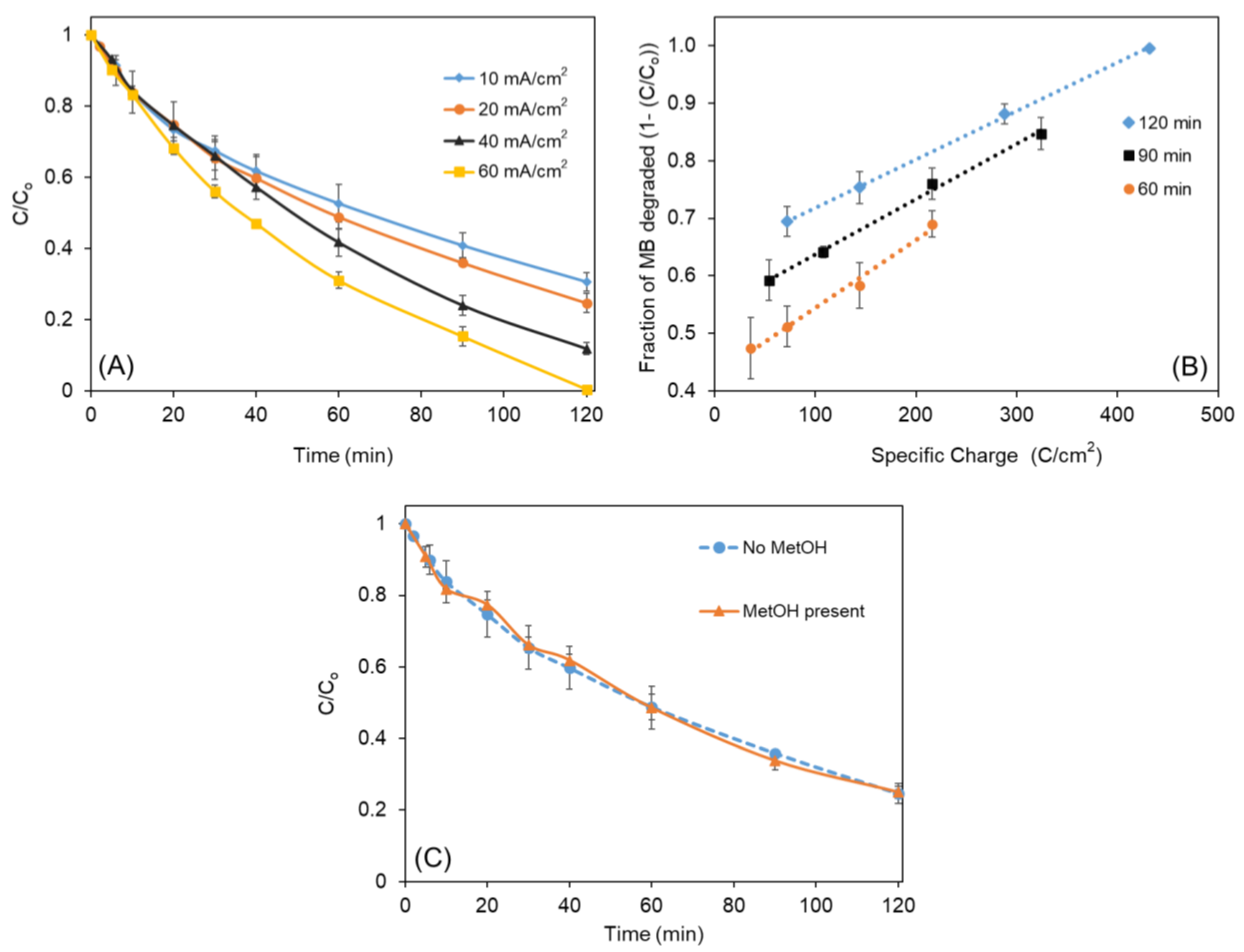
2.2. Influence of Chloride-Ion Concentration on the Kinetics of MB Degradation
2.3. Influence of Current Density on the Kinetics of MB Degradation in the Presence of Chlorides
2.4. Chemical Oxygen Demand, Total Organic Carbon and Color Removal Efficiency
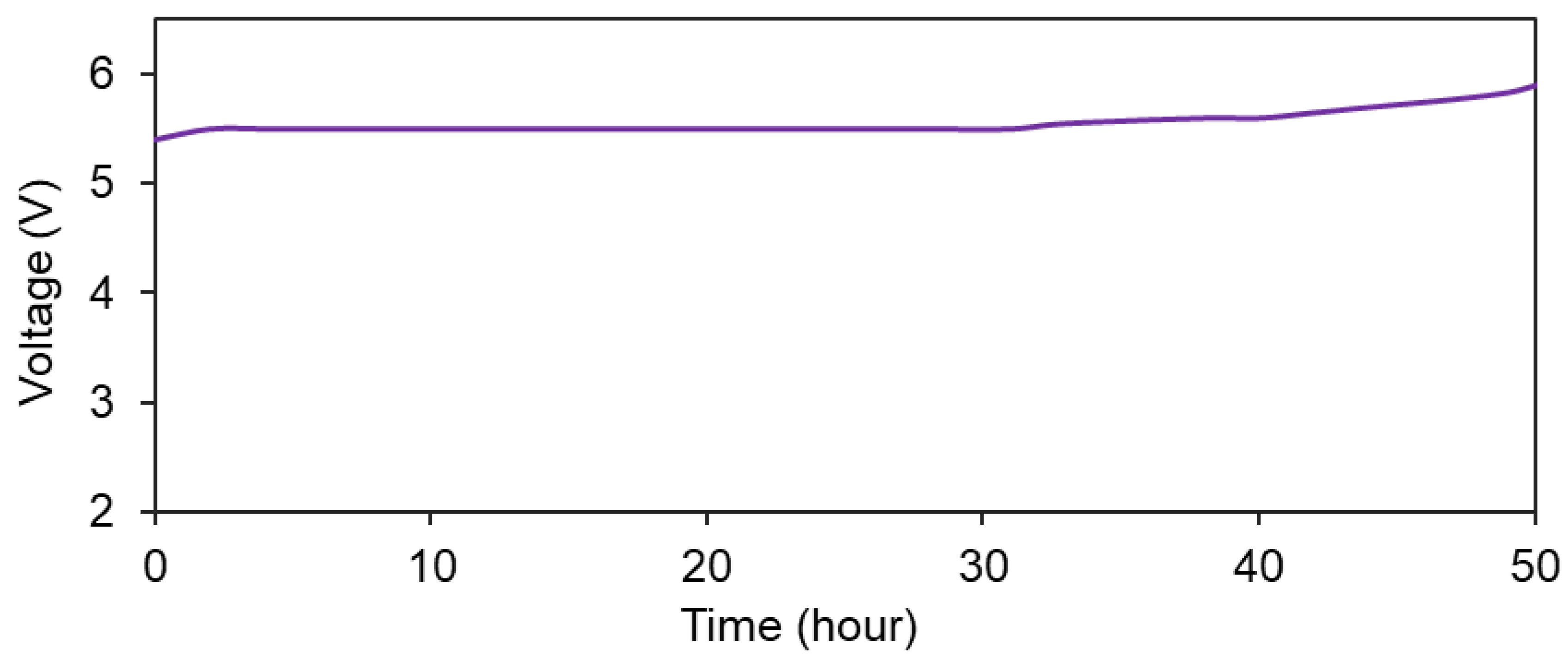
2.5. Stability of the Anode Material
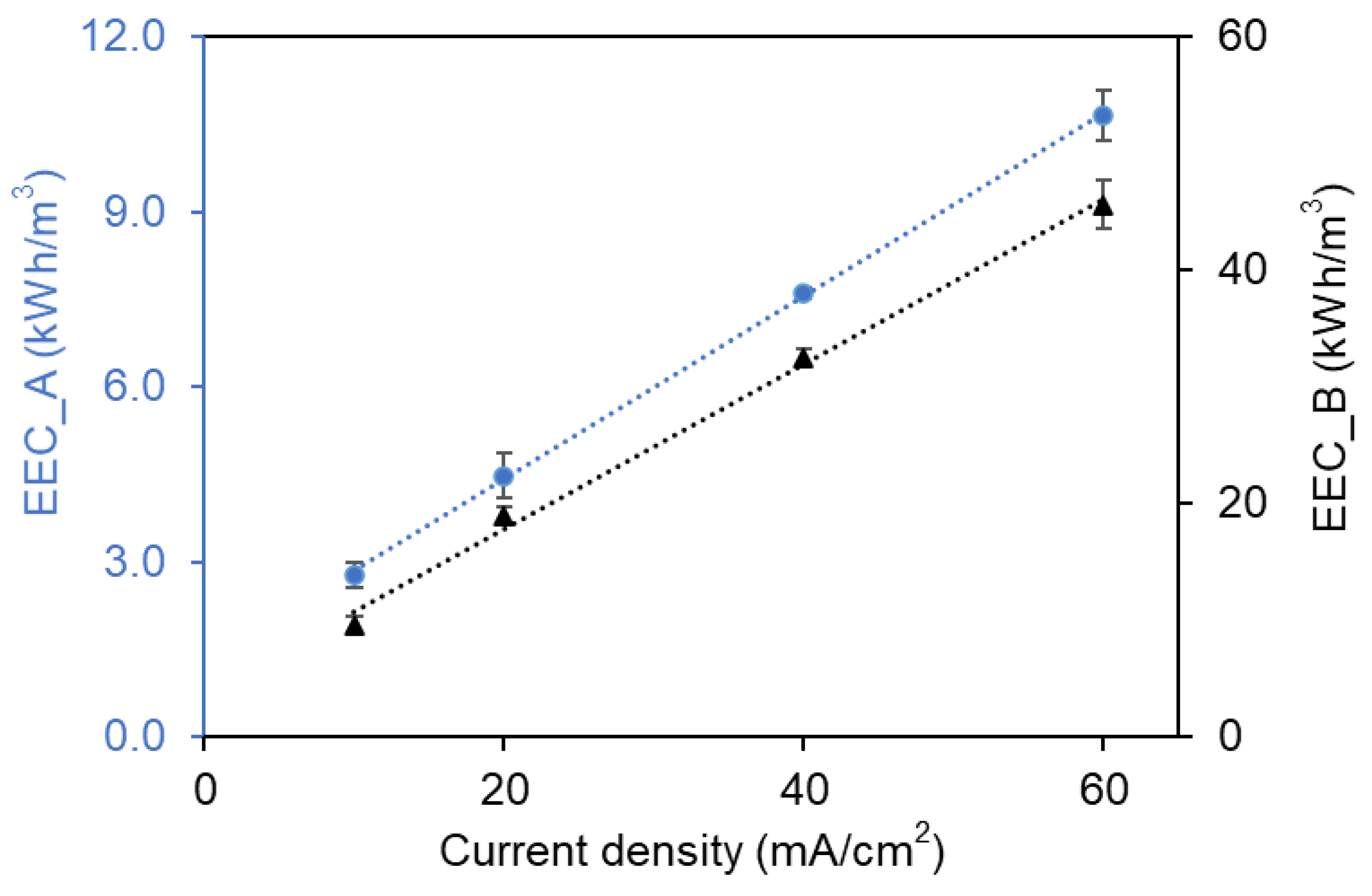
2.6. Energy Consumption Determination
3. Materials and Methods
3.1. Anode Preparation
3.2. Electrochemical Degradation of Methylene Blue
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mathur, N.; Bhatnagar, P.; Bakre, P. Assessing mutagenicity of textile dyes from Pali(Rajasthan) using Ames bioassay. Appl. Ecol. Environ. Res. 2006, 4, 111–118. [Google Scholar] [CrossRef]
- Indu, M.; Gupta, A.; Sahoo, C. Electrochemical oxidation of methylene blue using lead acid battery anode. APCBEE Procedia 2014, 9, 70–74. [Google Scholar] [CrossRef]
- Panizza, M.; Barbucci, A.; Ricotti, R.; Cerisola, G. Electrochemical degradation of methylene blue. Sep. Purif. Technol. 2007, 54, 382–387. [Google Scholar] [CrossRef]
- Kraft, A. Electrochemical water disinfection: A short review. Platin. Metals Rev. 2008, 52, 177–185. [Google Scholar] [CrossRef]
- Anglada, A.; Urtiaga, A.; Ortiz, I. Contributions of electrochemical oxidation to waste-water treatment: Fundamentals and review of applications. J. Chem. Technol. Biotechnol. 2009, 84, 1747–1755. [Google Scholar] [CrossRef]
- Ghasemian, S.; Omanovic, S. Fabrication and characterization of photoelectrochemically-active Sb-doped Snx-W(100-x)%-oxide anodes: Towards the removal of organic pollutants from wastewater. Appl. Surf. Sci. 2017, 416, 318–328. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced oxidation processes (AOPs) in wastewater treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Dutta, K.; Mukhopadhyay, S.; Bhattacharjee, S.; Chaudhuri, B. Chemical oxidation of methylene blue using a Fenton-like reaction. J. Hazard. Mater. 2001, 84, 57–71. [Google Scholar] [CrossRef]
- Gottschalk, C.; Libra, J.A.; Saupe, A. Ozonation of Water and Waste Water: A Practical Guide to Understanding Ozone and Its Applications, 2nd ed.; John Wiley & Sons GmbH: Weinheim, Germany, 2010; pp. 46–55. [Google Scholar]
- Ghasemian, S.; Nasuhoglu, D.; Omanovic, S.; Yargeau, V. Photoelectrocatalytic degradation of pharmaceutical carbamazepine using Sb-doped Sn80%-W20%-oxide electrodes. Sep. Purif. Technol. 2017, 188, 52–59. [Google Scholar] [CrossRef]
- Lei, J.; Duan, P.; Liu, W.; Sun, Z.; Hu, X. Degradation of aqueous cefotaxime in electro-oxidation—electro-Fenton—persulfate system with Ti/CNT/SnO2–Sb–Er anode and Ni@ NCNT cathode. Chemosphere 2020, 250, 126163. [Google Scholar] [CrossRef]
- Zhao, W.; Xing, J.; Chen, D.; Jin, D.; Shen, J. Electrochemical degradation of Musk ketone in aqueous solutions using a novel porous Ti/SnO2-Sb2O3/PbO2 electrodes. J. Electroanal. Chem. 2016, 775, 179–188. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, L.; Liu, J.; Logan, B.E. Electrochemical technologies for wastewater treatment and resource reclamation. Environ. Sci. Water Res. Technol. 2016, 2, 800–831. [Google Scholar] [CrossRef]
- Ghasemian, S.; Asadishad, B.; Omanovic, S.; Tufenkji, N. Electrochemical disinfection of bacteria-laden water using antimony-doped tin-tungsten-oxide electrodes. Water Res. 2017, 126, 299–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Lo, S.L.; Srivastava, V.C.; Hiwarkar, A.D. Comparative study of electrochemical oxidation for dye degradation: Parametric optimization and mechanism identification. J. Environ. Chem. Eng. 2016, 4, 2911–2921. [Google Scholar] [CrossRef]
- Gattrell, M.; Kirk, D. A study of the oxidation of phenol at platinum and preoxidized platinum surfaces. J. Electrochem. Soc. 1993, 140, 1534. [Google Scholar] [CrossRef]
- Chatzisymeon, E.; Dimou, A.; Mantzavinos, D.; Katsaounis, A. Electrochemical oxidation of model compounds and olive mill wastewater over DSA electrodes: 1. The case of Ti/IrO2 anode. J. Hazard. Mater. 2009, 167, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Comninellis, C. Electrocatalysis in the electrochemical conversion/combustion of organic pollutants for waste water treatment. Electrochim. Acta 1994, 39, 1857–1862. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Application of diamond electrodes to electrochemical processes. Electrochim. Acta 2005, 51, 191–199. [Google Scholar] [CrossRef]
- Lacasa, E.; Tsolaki, E.; Sbokou, Z.; Rodrigo, M.A.; Mantzavinos, D.; Diamadopoulos, E. Electrochemical disinfection of simulated ballast water on conductive diamond electrodes. Chem. Eng. J. 2013, 223, 516–523. [Google Scholar] [CrossRef]
- Katsoni, A.; Mantzavinos, D.; Diamadopoulos, E. Sequential treatment of diluted olive pomace leachate by digestion in a pilot scale UASB reactor and BDD electrochemical oxidation. Water Res. 2014, 57, 76–86. [Google Scholar] [CrossRef]
- Solano, A.M.S.; de Araújo, C.K.C.; de Melo, J.V.; Peralta-Hernandez, J.M.; da Silva, D.R.; Martínez-Huitle, C.A. Decontamination of real textile industrial effluent by strong oxidant species electrogenerated on diamond electrode: Viability and disadvantages of this electrochemical technology. Appl. Catal. B Environ. 2013, 130, 112–120. [Google Scholar] [CrossRef]
- Zhou, M.; Dai, Q.; Lei, L.; Ma, C.A.; Wang, D. Long life modified lead dioxide anode for organic wastewater treatment: Electrochemical characteristics and degradation mechanism. Environ. Sci. Technol. 2005, 39, 363–370. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, T.; Su, Q.; Tang, Y.; Xu, X.; Akram, M.; Jiang, B. Highly efficient and mild electrochemical degradation of bentazon by nano-diamond doped PbO2 anode with reduced Ti nanotube as the interlayer. J. Colloid Interface Sci. 2020, 575, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Polcaro, A.; Palmas, S.; Renoldi, F.; Mascia, M. On the performance of Ti/SnO2 and Ti/PbO2 anodesin electrochemical degradation of 2-chlorophenolfor wastewater treatment. J. Appl. Electrochem. 1999, 29, 147–151. [Google Scholar] [CrossRef]
- Wu, T.; Zhao, G.; Lei, Y.; Li, P. Distinctive tin dioxide anode fabricated by pulse electrodeposition: High oxygen evolution potential and efficient electrochemical degradation of fluorobenzene. J. Phys. Chem. C 2011, 115, 3888–3898. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, X.; Miao, J.; Zhang, R.; Zhang, J.; Zhou, M.; Lu, W. Enhanced performance of an Al-doped SnO2 anode for the electrocatalytic oxidation of organic pollutants in water. Mater. Today Commun. 2020, 24, 101164. [Google Scholar] [CrossRef]
- Nwanebu, E.O.; Omanovic, S. The influence of NixCo1-x-oxide composition on its electrocatalytic activity in the oxygen evolution reaction. Mater. Chem. Phys. 2019, 228, 80–88. [Google Scholar] [CrossRef]
- Morgounova, E.; Shao, Q.; Hackel, B.J.; Thomas, D.D.; Ashkenazi, S. Photoacoustic lifetime contrast between methylene blue monomers and self-quenched dimers as a model for dual-labeled activatable probes. J. Biomed. Opt. 2013, 18, 056004. [Google Scholar] [CrossRef] [Green Version]
- Usacheva, M.N.; Teichert, M.C.; Biel, M.A. The role of the methylene blue and toluidine blue monomers and dimers in the photoinactivation of bacteria. J. Photochem. Photobiol. B Biol. 2003, 71, 87–98. [Google Scholar] [CrossRef]
- Spencer, W.; Sutter, J.R. Kinetic study of the monomer-dimer equilibrium of methylene blue in aqueous solution. J. Phys. Chem. 1979, 83, 1573–1576. [Google Scholar] [CrossRef]
- Chung, S.-K.; Toshihiko, O. Hydroxyl radical scavengers from white mustard (Sinapis alba). Food Sci. Biotechnol. 1998, 7, 209–213. [Google Scholar]
- Mishra, O.P.; Popov, A.V.; Pietrofesa, R.A.; Christofidou-Solomidou, M. Gamma-irradiation produces active chlorine species (ACS) in physiological solutions: Secoisolariciresinol diglucoside (SDG) scavenges ACS-A novel mechanism of DNA radioprotection. Biochim. Biophys. Acta 2016, 1860, 1884–1897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu Ghalwa, N.M.; Zaggout, F.R. Electrodegradation of methylene blue dye in water and wastewater using lead oxide/titanium modified electrode. J. Environ. Sci. Health A 2006, 41, 2271–2282. [Google Scholar] [CrossRef]
- Samarghandi, M.R.; Dargahi, A.; Shabanloo, A.; Nasab, H.Z.; Vaziri, Y.; Ansari, A. Electrochemical degradation of methylene blue dye using a graphite doped PbO2 anode: Optimization of operational parameters, degradation pathway and improving the biodegradability of textile wastewater. Arab. J. Chem. 2020, 13, 6847–6864. [Google Scholar] [CrossRef]
- Comninellis, C.; Pulgarin, C. Electrochemical oxidation of phenol for wastewater treatment using SnO2 anodes. J. Appl. Electrochem. 1993, 23, 108–112. [Google Scholar] [CrossRef]
- Zeng, L.; Wei, X.; Miao, J.; Zhang, R.; Zhang, J.; Zhou, M.; Lu, W. Preparation and investigation of a Ni–B-assisted SnO2–Sb anode for electrooxidation of phenol. J. Solid State Electrochem. 2021, 25, 1541–1553. [Google Scholar] [CrossRef]
- Nwanebu, E.O.; Yao, Y.; Omanovic, S. The Influence of Ir Content in (Ni0.4Co0.6)1-xIrx-oxide Anodes on their Electrocatalytic Activity in Oxygen Evolution by Acidic and Alkaline Water Electrolysis. J. Electroanal. Chem. 2020, 865, 114122. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nwanebu, E.O.; Liu, X.; Pajootan, E.; Yargeau, V.; Omanovic, S. Electrochemical Degradation of Methylene Blue Using a Ni-Co-Oxide Anode. Catalysts 2021, 11, 793. https://doi.org/10.3390/catal11070793
Nwanebu EO, Liu X, Pajootan E, Yargeau V, Omanovic S. Electrochemical Degradation of Methylene Blue Using a Ni-Co-Oxide Anode. Catalysts. 2021; 11(7):793. https://doi.org/10.3390/catal11070793
Chicago/Turabian StyleNwanebu, Emmanuel Onyekachi, Xiaocheng Liu, Elmira Pajootan, Viviane Yargeau, and Sasha Omanovic. 2021. "Electrochemical Degradation of Methylene Blue Using a Ni-Co-Oxide Anode" Catalysts 11, no. 7: 793. https://doi.org/10.3390/catal11070793






