Enhanced Performance of the OMS-2-Supported CuOx Catalysts for Carbon Monoxide, Ethyl Acetate, and Toluene Oxidation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties of OMS-2 and xCu/OMS-2
2.1.1. Crystal Phase Composition
2.1.2. Structure
2.1.3. Morphology
2.1.4. Thermal Stability
2.1.5. Component Analysis
2.1.6. Oxygen Desorption Behavior
2.1.7. Reducibility
2.2. Catalytic Performance
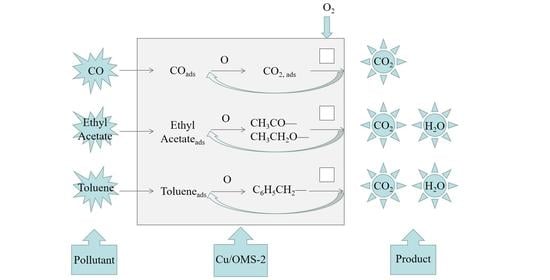
2.3. In Situ DRIFTS Spectra and Catalytic Oxidation Mechanism
2.3.1. CO Oxidation over 15Cu/OMS-2
2.3.2. Ethyl Acetate Oxidation over 15Cu/OMS-2
2.3.3. Toluene Oxidation over 15Cu/OMS-2
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ghosh, R.; Gardner, J.W.; Guha, P.K. Air pollution monitoring using near room temperature resistive gas sensors: A review. IEEE Trans. Electron. Dev. 2019, 66, 3254–3264. [Google Scholar] [CrossRef]
- Mao, M.; Lv, H.; Li, Y.; Yang, Y.; Zeng, M.; Li, N.; Zhao, X. Metal support interaction in Pt nanoparticles partially confined in the mesopores of microsized mesoporous CeO2 for highly efficient purification of volatile organic compounds. ACS Catal. 2015, 6, 418–427. [Google Scholar] [CrossRef]
- Sanz, O.; Delgado, J.J.; Navarro, P.; Arzamendi, G.; Gandía, L.M.; Montes, M. VOCs combustion catalysed by platinum supported on manganese octahedral molecular sieves. Appl. Catal. B Environ. 2011, 110, 231–237. [Google Scholar] [CrossRef]
- Deng, H.; Kang, S.; Ma, J.; Zhang, C.; He, H. Silver incorporated into cryptomelane-type Manganese oxide boosts the catalytic oxidation of benzene. Appl. Catal. B Environ. 2018, 239, 214–222. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, Z.; Shangguan, W. Low-temperature catalysis for VOCs removal in technology and application: A state-of-the-art review. Catal. Today 2016, 264, 270–278. [Google Scholar] [CrossRef]
- He, J.; Chen, S.; Tang, W.; Dang, Y.; Kerns, P.; Miao, R.; Dutta, B.; Gao, P.; Suib, S.L. Microwave-assisted integration of transition metal oxide nanocoatings on manganese oxide nanoarray monoliths for low temperature CO oxidation. Appl. Catal. B Environ. 2019, 255, 117766. [Google Scholar] [CrossRef]
- Tokura, Y.; Nagaosa, N. Orbital physics in transition-metal oxides. Science 2000, 288, 462–468. [Google Scholar] [CrossRef] [Green Version]
- Sithambaram, S.; Nyutu, E.K.; Suib, S.L. OMS-2 catalyzed oxidation of tetralin: A comparative study of microwave and conventional heating under open vessel conditions. Appl. Catal. A Gen. 2008, 348, 214–220. [Google Scholar] [CrossRef]
- Chen, X.; Shen, Y.; Suib, S.L.; O’Young, C.L. Characterization of manganese oxide octahedral molecular sieve (M−OMS-2) materials with different metal cation dopants. Chem. Mater. 2002, 14, 940–948. [Google Scholar] [CrossRef]
- Pisal, D.S.; Yadav, G.D. Single-step hydrogenolysis of furfural to 1,2-pentanediol using a bifunctional Rh/OMS-2 catalyst. ACS Omega 2019, 4, 1201–1214. [Google Scholar] [CrossRef]
- Liu, J.; Ke, L.; Liu, J.; Sun, L.; Yuan, X.; Li, Y.; Xia, D. Enhanced catalytic ozonation towards oxalic acid degradation over novel copper doped manganese oxide octahedral molecular sieves nanorods. J. Hazard. Mater. 2019, 371, 42–52. [Google Scholar] [CrossRef]
- Li, Y.; Fan, Z.; Shi, J.; Liu, Z.; Zhou, J.; Shangguan, W. Modified manganese oxide octahedral molecular sieves M’-OMS-2 (M’ = Co, Ce, Cu) as catalysts in post plasma-catalysis for acetaldehyde degradation. Catal. Today 2015, 256, 178–185. [Google Scholar] [CrossRef]
- Nur, H.; Hayati, F.; Hamdan, H. On the location of different titanium sites in Ti-OMS-2 and their catalytic role in oxidation of styrene. Catal. Commun. 2007, 8, 2007–2011. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Song, Y.; Fu, Z.; Ye, Q.; Cheng, S.; Kang, T.; Dai, H. Effect of preparation method on the surface characteristics and activity of the Pd/OMS-2 catalysts for the oxidation of carbon monoxide, toluene, and ethyl acetate. Appl. Surf. Sci. 2017, 396, 599–608. [Google Scholar] [CrossRef]
- Özacar, M.; Poyraz, A.S.; Genuino, H.C.; Kuo, C.; Meng, Y.; Suib, S.L. Influence of silver on the catalytic properties of the cryptomelane and Ag-hollandite types manganese oxides OMS-2 in the low-temperature CO oxidation. Appl. Catal. A Gen. 2013, 462–463, 64–74. [Google Scholar] [CrossRef]
- Sun, M.; Yu, L.; Ye, F.; Diao, G.; Yu, Q.; Hao, Z.; Zheng, Y.; Yuan, L. Transition metal doped cryptomelane-type manganese oxide for low-temperature catalytic combustion of dimethyl ether. Chem. Eng. J. 2013, 220, 320–327. [Google Scholar] [CrossRef]
- Fu, J.; Dong, N.; Ye, Q.; Cheng, S.; Kang, T.; Dai, H. Enhanced performance of the OMS-2 catalyst by Ag loading for the ox-idation of benzene, toluene, and formaldehyde. New J. Chem. 2018, 42, 18117–18127. [Google Scholar] [CrossRef]
- Hou, J.; Li, Y.; Mao, M.; Ren, L.; Zhao, X. Tremendous effect of the morphology of birnessite-type manganese oxide nanostructures on catalytic activity. ACS Appl. Mater. Interfaces 2014, 6, 14981–14987. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Chen, S.; Wang, P.; Quan, X. Catalytic oxidation of toluene over manganese oxide octahedral molecular sieves (OMS-2) synthesized by different methods. Chem. Eng. J. 2011, 178, 191–196. [Google Scholar] [CrossRef]
- Li, X.; Ma, J.; Jia, X.; Xia, F.; Huang, Y.; Xu, Y.; Xu, J. Al-doping promoted aerobic amidation of 5-hydroxymethylfurfural to 2,5-furandicarboxamide over cryptomelane. ACS Sustain. Chem. Eng. 2018, 6, 8048–8054. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, J.; Chen, B.; Jing, Z.; Zhao, P. Copper supported on H+-modified manganese oxide octahedral molecular sieves (Cu/H-OMS-2) as a heterogeneous biomimetic catalyst for the synthesis of imidazo[1,2-a]-N-heterocycles. Catal. Sci. Technol. 2016, 6, 890–896. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, L.; Guan, S.; Zhang, X.; Wang, B.; Cao, X.; Yu, Z.; He, Y.; Evans, D.G.; Feng, J.; et al. Ultrathin and vacancy-rich CoAl-layered double hydroxide/graphite oxide catalysts: Promotional effect of cobalt vacancies and oxygen vacancies in alcohol oxidation. ACS Catal. 2018, 8, 3104–3115. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, J.; Lyu, L.; Hu, C. Enhanced catalytic degradation of ciprofloxacin over Ce-doped OMS-2 microspheres. Appl. Catal. B Environ. 2016, 181, 561–569. [Google Scholar] [CrossRef]
- Tian, H.; He, J.; Liu, L.; Wang, D.; Hao, Z.; Ma, C. Highly active manganese oxide catalysts for low-temperature oxidation of formaldehyde. Microporous Mesoporous Mater. 2012, 151, 397–402. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, S.; Li, H.; Yang, J.; Yang, Z.; Zhao, J.; Peng, H.; Shih, K. Elemental mercury oxidation over manganese oxide octahedral molecular sieve catalyst at low flue gas temperature. Chem. Eng. J. 2019, 356, 142–150. [Google Scholar] [CrossRef]
- Aguilera, D.A.; Perez, A.; Molina, R.; Moreno, S. Cu-Mn and Co-Mn catalysts synthesized from hydrotalcites and their use in the oxidation of VOCs. Appl. Catal. B Environ. 2011, 104, 144–150. [Google Scholar] [CrossRef]
- Song, Y.; Liu, L.; Fu, Z.; Ye, Q.; Cheng, S.; Kang, T.; Dai, H. Excellent performance of Cu-Mn/Ti-sepiolite catalysts for low-temperature CO oxidation. Front. Environ. Sci. Eng. 2017, 11, 77–86. [Google Scholar] [CrossRef]
- Huang, Q.; Yan, X.; Li, B.; Xu, X.; Chen, Y.; Zhu, S.; Shen, S. Activity and stability of Pd/MMnOx (M = Co, Ni, Fe and Cu) supported on cordierite as CO oxidation catalysts. J. Ind. Eng. Chem. 2013, 19, 438–443. [Google Scholar] [CrossRef]
- Dou, B.; Zhao, R.; Yan, N.; Zhao, C.; Hao, Q.; Hui, K.S.; Hui, K.N. A facilitated synthesis of hierarchically porous Cu-Ce-Zr catalyst using bacterial cellulose for VOCs oxidation. Mater. Chem. Phys. 2019, 237, 121852. [Google Scholar] [CrossRef]
- Alvarez-Merino, M.A.; Ribeiro, M.F.; Silva, J.M.; Carrasco-Marín, F.; Maldonado-Hódar, F.J. Activated carbon and tungsten oxide supported on activated carbon catalysts for toluene catalytic combustion. Environ. Sci. Technol. 2004, 38, 4664–4670. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tang, Y.; Ma, Y.; Zhang, Z.; Tao, F.F.; Qu, Y. In situ formation of isolated bimetallic PtCe Sites of single-dispersed Pt on CeO2 for low-temperature CO oxidation. ACS Appl. Mater. Interfaces 2018, 10, 38134–38140. [Google Scholar] [CrossRef]
- Wang, Y.; Widmann, D.; Lehnert, F.; Gu, D.; Schuth, F.; Behm, R.J. Avoiding self-poisoning: A key feature for the high activity of Au/Mg(OH)2 catalysts in continuous low-temperature CO oxidation. Angew. Chem. Int. Ed. 2017, 56, 9597–9602. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Han, Q.; Shi, W.; Zhang, C.; Li, E.; Zhu, T. Catalytic oxidation of ethyl acetate over Ru-Cu bimetallic catalysts: Further insights into reaction mechanism via in situ FTIR and DFT studies. J. Catal. 2019, 369, 482–492. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, X.; Zhu, T.; Zhu, T. Catalytic oxidation of ethyl acetate over silver catalysts supported on CeO2 with different morphologies. Mater. Chem. Phys. 2019, 229, 32–38. [Google Scholar] [CrossRef]
- Genuino, H.C.; Dharmarathna, S.; Njagi, E.C.; Mei, M.C.; Suib, S.L. Gas-phase total oxidation of benzene, toluene, ethylbenzene, and xylenes using shape-selective manganese oxide and copper manganese oxide catalysts. J. Phys. Chem. C 2012, 116, 12066–12078. [Google Scholar] [CrossRef]
- Gandhe, A.R.; Rebello, J.S.; Figueiredo, J.L.; Fernandes, J.B. Manganese oxide OMS-2 as an effective catalyst for total oxidation of ethyl acetate. Appl. Catal. B Environ. 2007, 72, 129–135. [Google Scholar] [CrossRef]
- Sun, H.; Liu, Z.; Chen, S.; Quan, X. The role of lattice oxygen on the activity and selectivity of the OMS-2 catalyst for the total oxidation of toluene. Chem. Eng. J. 2015, 270, 58–65. [Google Scholar] [CrossRef]
- Bentrup, U.; Martin, A.; Lücke, B. Infrared characterization of the surface intermediates in the oxidation of toluene on vanadyl pyrophosphate catalysts. Top. Catal. 2000, 11, 139–145. [Google Scholar] [CrossRef]











| Samples | Thermal Analysis (Weight Loss wt%) | |||
|---|---|---|---|---|
| 50–250 (°C) | 250–550 (°C) | 550–700 (°C) | 700–850 (°C) | |
| OMS-2 | 1.54 | 1.29 | 3.69 | 3.07 |
| 1Cu/OMS-2 | 2.08 | 0.86 | 3.55 | 2.69 |
| 5Cu/OMS-2 | 3.15 | 0.91 | 3.78 | 3.28 |
| 15Cu/OMS-2 | 3.62 | 2.21 | 3.12 | 2.65 |
| 20Cu/OMS-2 | 4.54 | 0.79 | 2.37 | 2.02 |
| Sample | CuO Content (wt%) a | Surface Atomic Ratio b | |
|---|---|---|---|
| Mn3+/Mn4+ | Oads/Olatt | ||
| OMS-2 | 0.00 | 1.63 | 0.379 |
| 1Cu/OMS-2 | 0.23 | 1.97 | 0.447 |
| 5Cu/OMS-2 | 1.05 | 2.05 | 0.456 |
| 15Cu/OMS-2 | 3.37 | 3.05 | 0.530 |
| 20Cu/OMS-2 | 4.58 | 2.79 | 0.526 |
| Sample | Temperature (°C) | H2 Consumption (mmol/gcat) | ||||||
|---|---|---|---|---|---|---|---|---|
| α | β | γ | α | β | γ | (α + β)/γ | Total | |
| OMS-2 | 320 | 370 | 420 | 1.46 | 4.85 | 3.14 | 2.01 | 9.45 |
| 1Cu/OMS-2 | 220 | 300 | 350 | 1.32 | 3.54 | 2.87 | 1.69 | 7.73 |
| 5Cu/OMS-2 | 205 | 310 | 345 | 1.10 | 3.20 | 2.95 | 1.46 | 7.25 |
| 15Cu/OMS-2 | 190 | 285 | 330 | 1.56 | 3.51 | 2.52 | 2.01 | 7.59 |
| 20Cu/OMS-2 | 200 | 300 | 340 | 1.63 | 3.20 | 2.55 | 1.89 | 7.38 |
| Catalyst | rcat (molCO/(gcat s)) | rcat (molethyl acetate/(gcat s)) | rcat (moltoluene/(gcat s)) | Reference |
|---|---|---|---|---|
| OMS-2 | 1.36 × 10−7 | 1.96 × 10−6 | 1.96 × 10−6 | This study |
| 1Cu/OMS-2 | 2.73 × 10−7 | 2.51 × 10−6 | 2.62 × 10−6 | This study |
| 5Cu/OMS-2 | 1.09 × 10−6 | 2.84 × 10−6 | 2.94 × 10−6 | This study |
| 15Cu/OMS-2 | 4.09 × 10−6 | 3.49 × 10−6 | 3.71 × 10−6 | This study |
| 20Cu/OMS-2 | 1.77 × 10−6 | 3.27 × 10−6 | 3.27 × 10−6 | This study |
| PdCoMn/Cord | 3.41 × 10−7 (at 100 °C) | - | - | [28] |
| Cu–Ce–Zr | - | 2.30 × 10−7 | - | [29] |
| OM-CuMn0.25 | - | - | 4.09 × 10−7 | [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Z.; Chen, M.; Ye, Q.; Dong, N.; Dai, H. Enhanced Performance of the OMS-2-Supported CuOx Catalysts for Carbon Monoxide, Ethyl Acetate, and Toluene Oxidation. Catalysts 2021, 11, 713. https://doi.org/10.3390/catal11060713
Fu Z, Chen M, Ye Q, Dong N, Dai H. Enhanced Performance of the OMS-2-Supported CuOx Catalysts for Carbon Monoxide, Ethyl Acetate, and Toluene Oxidation. Catalysts. 2021; 11(6):713. https://doi.org/10.3390/catal11060713
Chicago/Turabian StyleFu, Zhidan, Mengyue Chen, Qing Ye, Ning Dong, and Hongxing Dai. 2021. "Enhanced Performance of the OMS-2-Supported CuOx Catalysts for Carbon Monoxide, Ethyl Acetate, and Toluene Oxidation" Catalysts 11, no. 6: 713. https://doi.org/10.3390/catal11060713