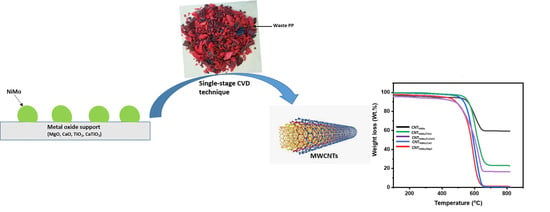
Effect of Different Catalyst Supports on the Quality, Yield and Morphology of Carbon Nanotubes Produced from Waste Polypropylene Plastics
Abstract
:1. Introduction
2. Results and Discussion
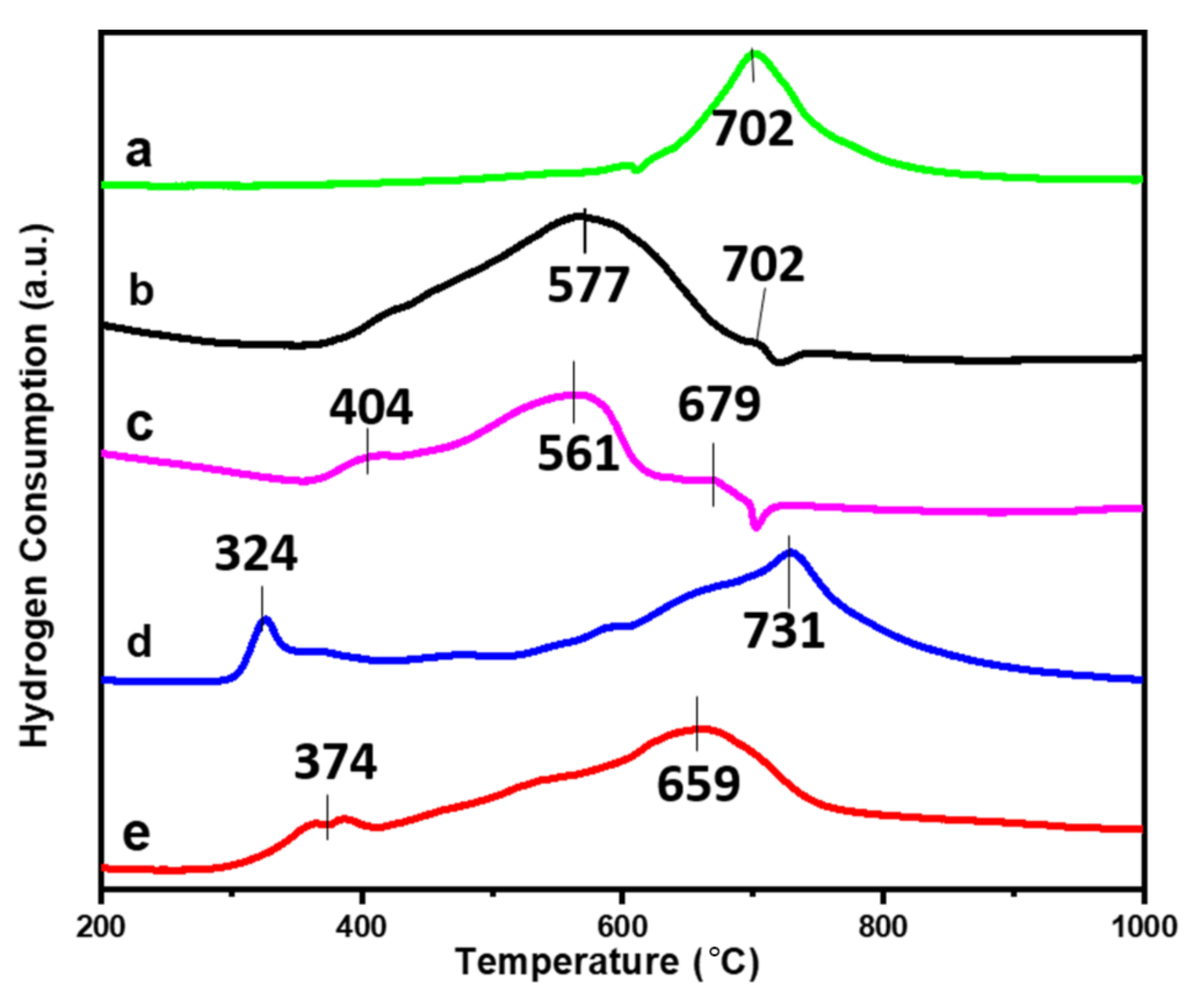
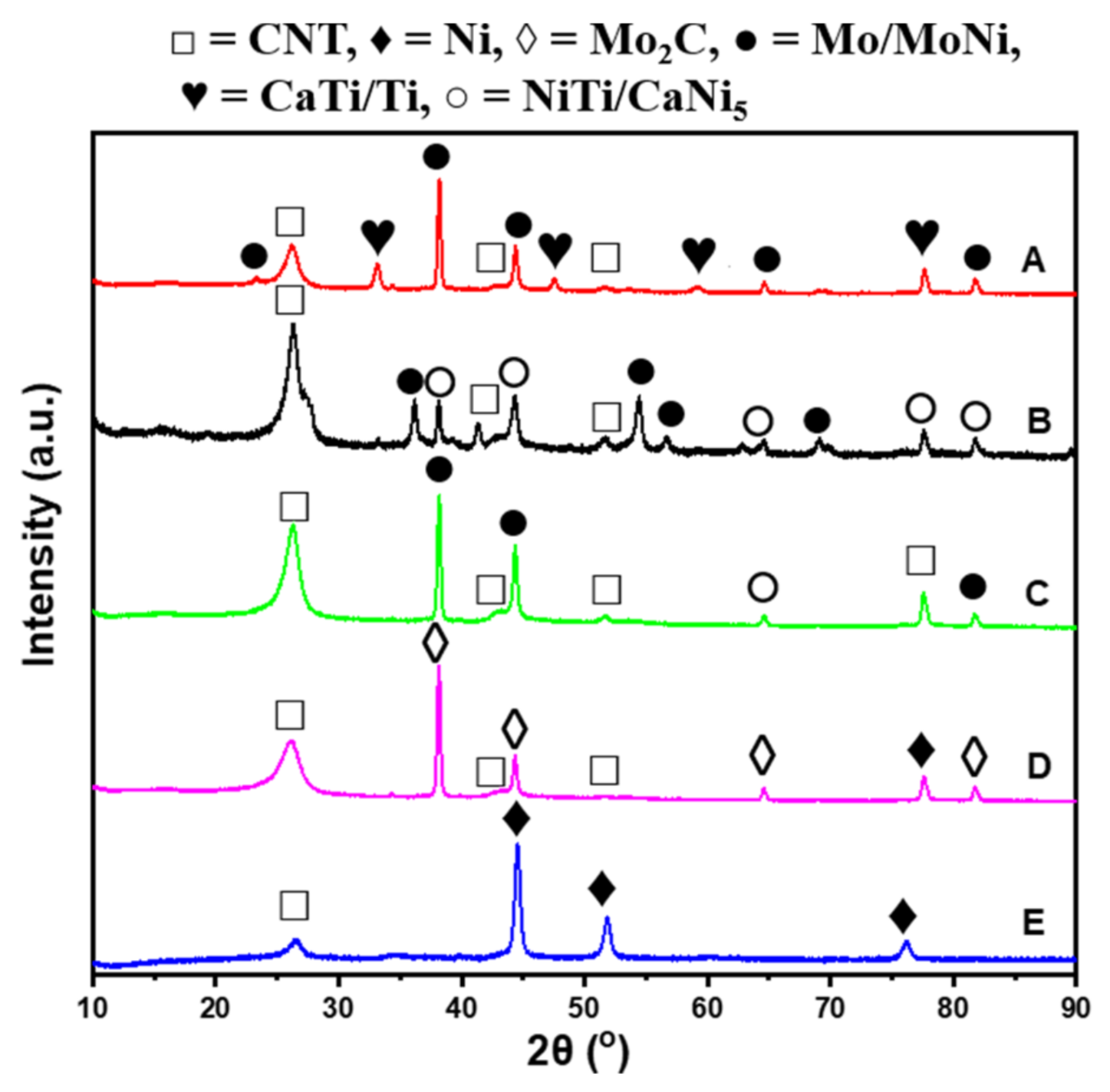
2.1. Characterization of Catalysts
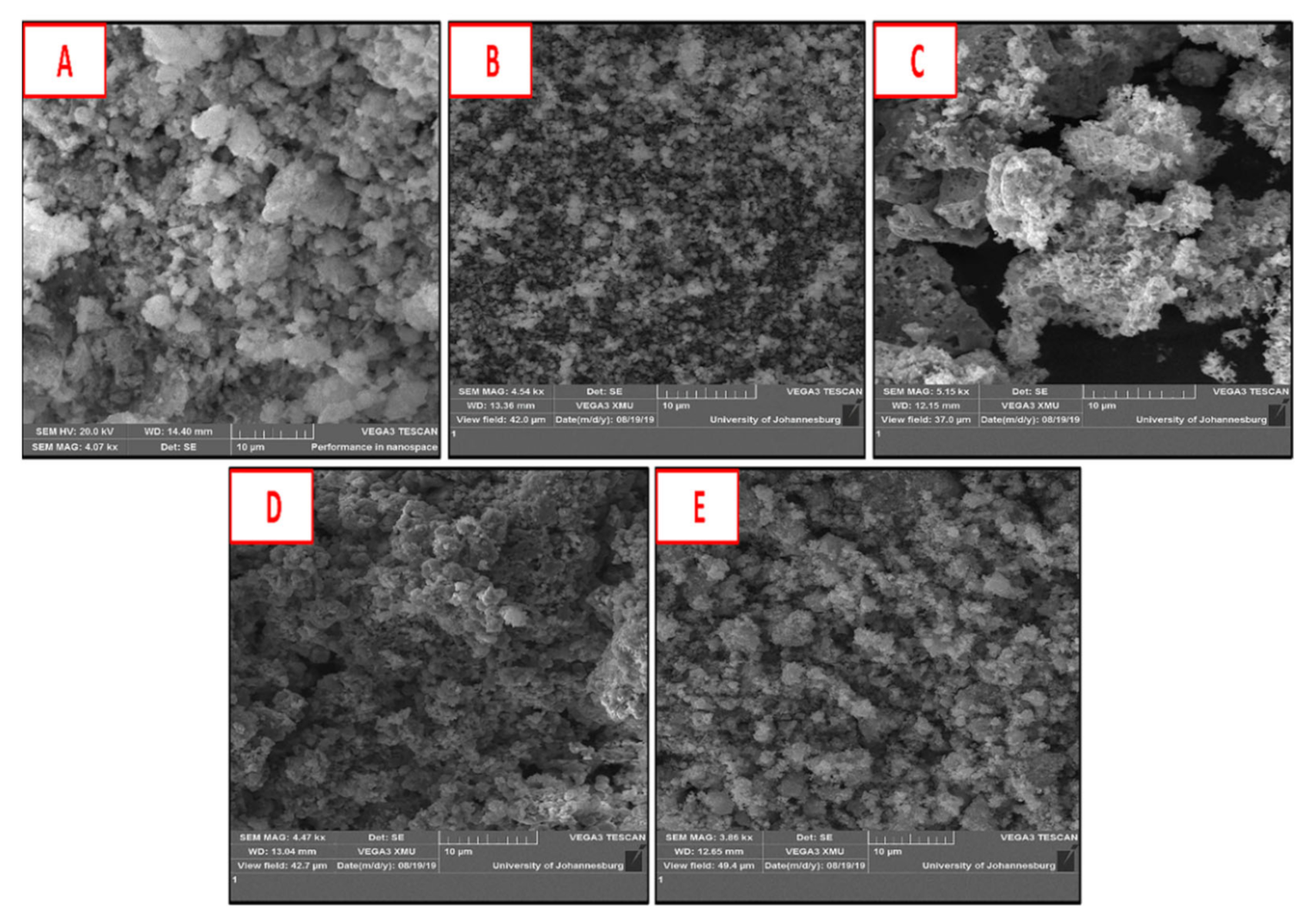
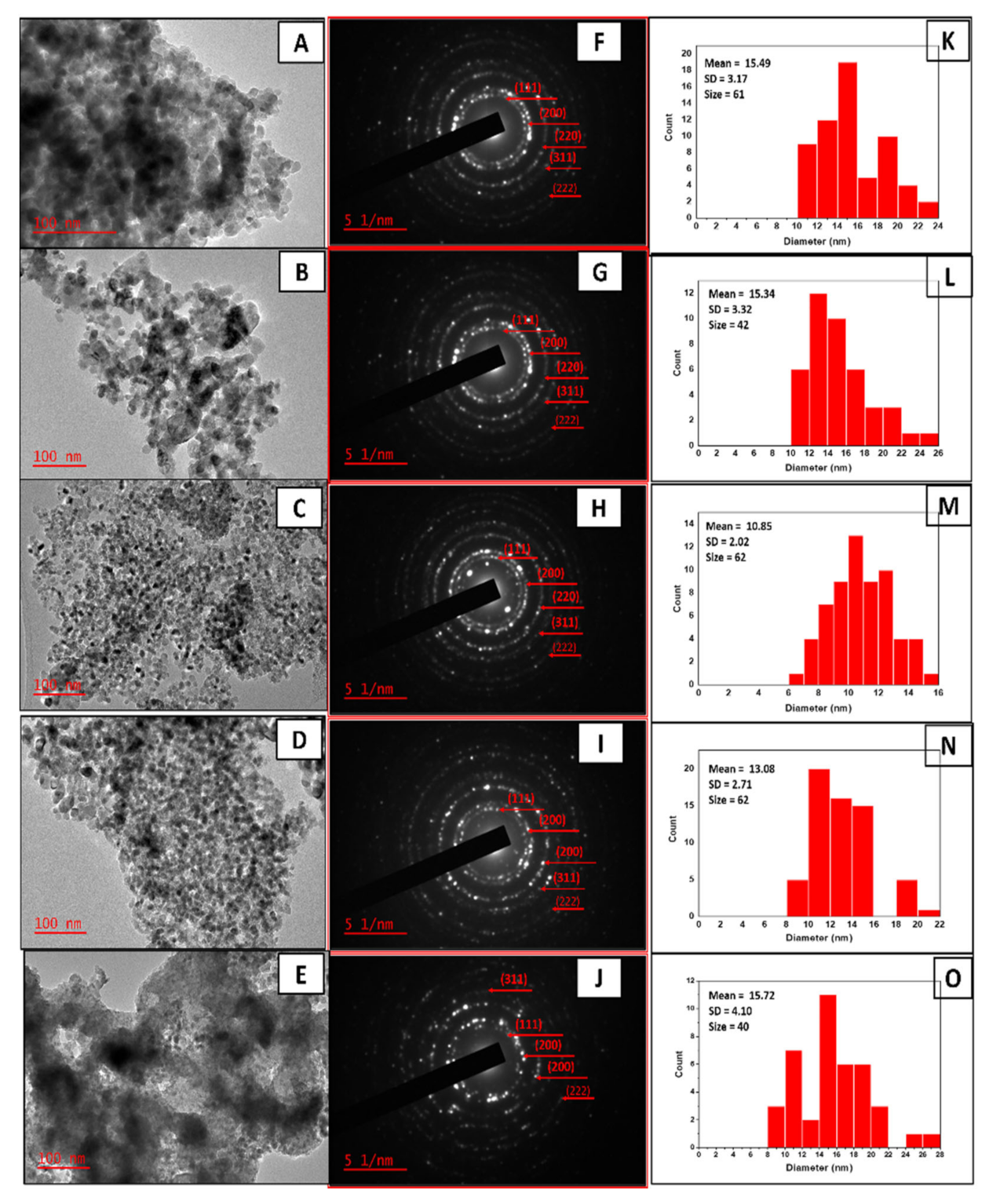
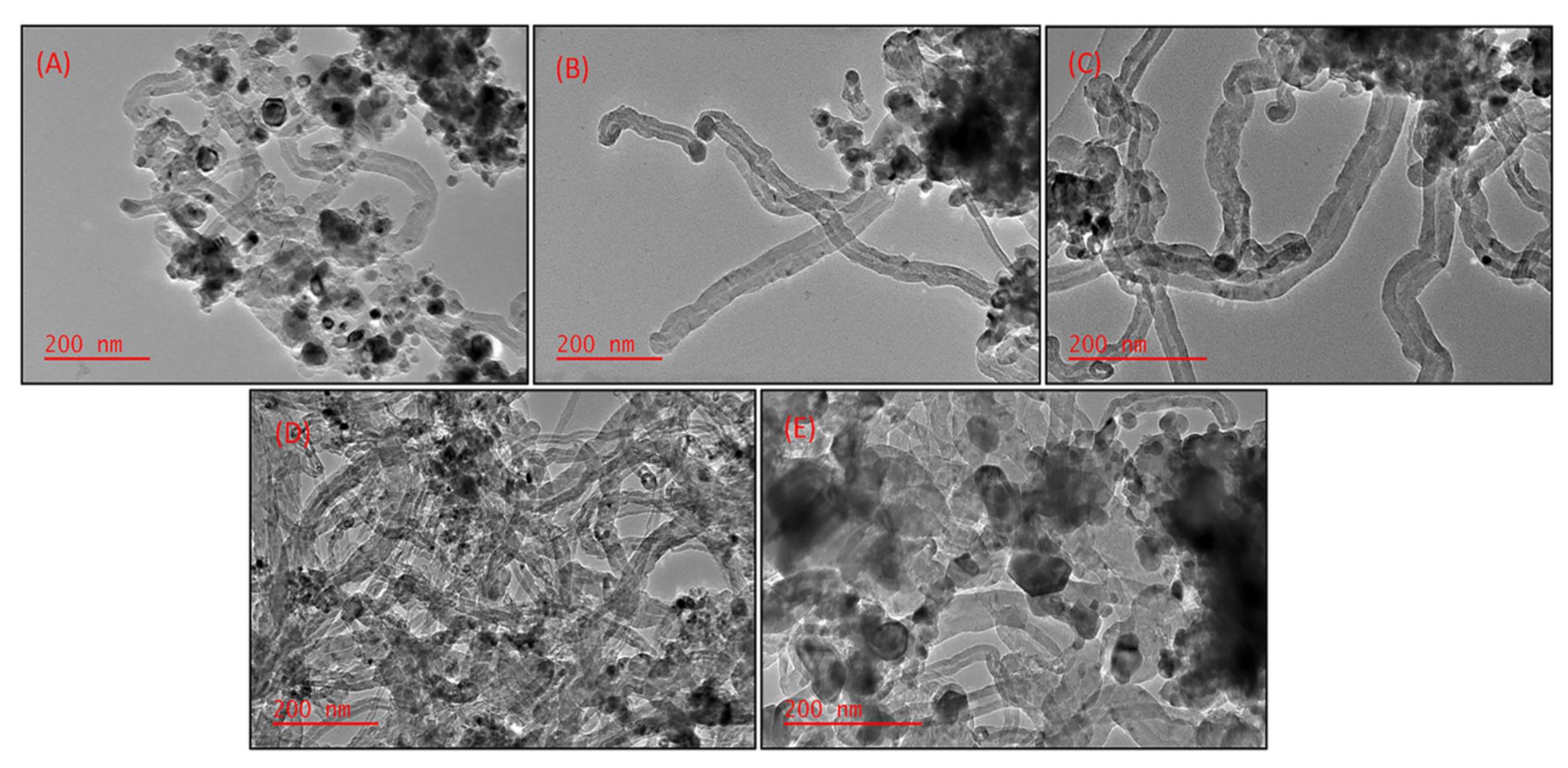
2.2. Effect of Different Supports on the Morphology of CNTs
2.3. Effect of Different Supports on the Yield of CNTs
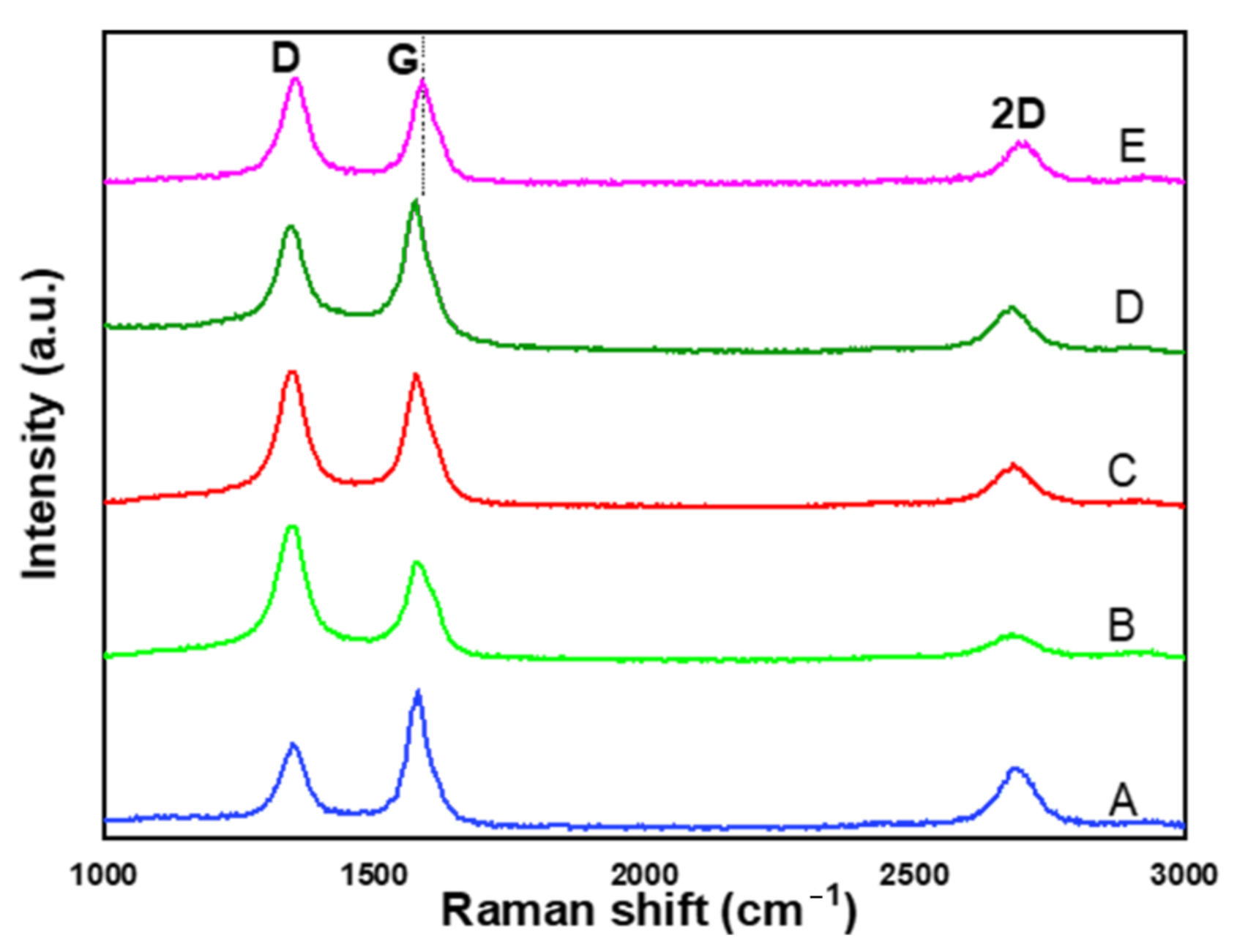
2.4. Effect of Different Supports on the Purity and Graphitization Degree of CNTs
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Synthesis of CNTs
3.4. Characterization of Catalysts
3.5. Characterization of CNTs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gahleitner, M.; Paulik, C. Polypropylene and other Polyolefins. In Brydson’s Plastics Materials; Elsevier Ltd.: Kidlington, UK, 2017; pp. 279–309. ISBN 9780323358248. [Google Scholar]
- Maddah, H.A. Polypropylene as a promising plastics: A Review. Am. J. Polym. Sci. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Posch, D.W. Polyolefins. In Applied Plastics Engineering Handbook; Elsevier Inc.: Kidlington, UK, 2017; pp. 27–53. ISBN 9780323390408. [Google Scholar]
- Singh, N.; Hui, D.; Singh, R.; Ahuja, I.; Feo, L.; Fraternali, F. Recycling of plastic solid waste: A state of art review and future applications. Compos. Part B Eng. 2017, 115, 409–422. [Google Scholar] [CrossRef]
- Bajad, G.S.; Tiwari, S.K.; Vijayakumar, R. Synthesis and characterization of CNTs using polypropylene waste as precursor. Mater. Sci. Eng. B 2015, 194, 68–77. [Google Scholar] [CrossRef]
- Gong, J.; Liu, J.; Wan, D.; Chen, X.; Wen, X.; Mijowska, E.; Jiang, Z.; Wang, Y.; Tang, T. Catalytic carbonization of polypropylene by the combined catalysis of activated carbon with Ni2O3 into carbon nanotubes and its mechanism. Appl. Catal. A Gen. 2012, 449, 112–120. [Google Scholar] [CrossRef]
- Nahil, M.A.; Wu, C.; Williams, P.T. Influence of metal addition to Ni-based catalysts for the co-production of carbon nanotubes and hydrogen from the thermal processing of waste polypropylene. Fuel Process. Technol. 2015, 130, 46–53. [Google Scholar] [CrossRef]
- Cai, N.; Li, X.; Xia, S.; Sun, L.; Hu, J.; Bartocci, P.; Fantozzi, F.; Williams, P.T.; Yang, H.; Chen, H. Pyrolysis-catalysis of different waste plastics over Fe/Al2O3 catalyst: High-value hydrogen, liquid fuels, carbon nanotubes and possible reaction mechanisms. Energy Convers. Manag. 2021, 229, 113794. [Google Scholar] [CrossRef]
- Deng, J.; You, Y.; Sahajwalla, V.; Joshi, R.K. Transforming waste into carbon-based nanomaterials. Carbon 2016, 96, 105–115. [Google Scholar] [CrossRef]
- Raman and TGA Study of Carbon Nanotubes Synthesized Over Mo/Fe Catalyst on Aluminium Oxide, Calcium Carbonate and Magnesium Oxide Support. Chem. Sci. Trans. 2013, 2, 1160–1173. [CrossRef] [Green Version]
- Yeoh, W.-M.; Lee, K.-Y.; Chai, S.-P.; Lee, K.-T.; Mohamed, A.R. Effective synthesis of carbon nanotubes via catalytic decomposition of methane: Influence of calcination temperature on metal–support interaction of Co–Mo/MgO catalyst. J. Phys. Chem. Solids 2013, 74, 1553–1559. [Google Scholar] [CrossRef]
- Yao, D.; Wu, C.; Yang, H.; Zhang, Y.; Nahil, M.A.; Chen, Y.; Williams, P.T.; Chen, H. Co-production of hydrogen and carbon nanotubes from catalytic pyrolysis of waste plastics on Ni-Fe bimetallic catalyst. Energy Convers. Manag. 2017, 148, 692–700. [Google Scholar] [CrossRef]
- Munnik, P.; De Jongh, P.E.; De Jong, K.P. Recent Developments in the Synthesis of Supported Catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef]
- Shah, K.A.; Malik, M.A.; Andrabi, S.M.A.; Shah, M.A. A Study of Catalyst Preparation Methods for Synthesis of Carbon Nanotubes. Chem. Sci. Trans. 2016, 5, 1–7. [Google Scholar] [CrossRef]
- Lobiak, E.; Shlyakhova, E.; Bulusheva, L.; Plyusnin, P.; Shubin, Y.; Okotrub, A. Ni–Mo and Co–Mo alloy nanoparticles for catalytic chemical vapor deposition synthesis of carbon nanotubes. J. Alloys Compd. 2015, 621, 351–356. [Google Scholar] [CrossRef]
- Kubička, D.; Horáček, J.; Setnička, M.; Bulánek, R.; Zukal, A.; Kubičková, I. Effect of support-active phase interactions on the catalyst activity and selectivity in deoxygenation of triglycerides. Appl. Catal. B Environ. 2014, 145, 101–107. [Google Scholar] [CrossRef]
- Li, G.; Tan, S.; Song, R.; Tang, T. Synergetic Effects of Molybdenum and Magnesium in Ni–Mo–Mg Catalysts on the One-Step Carbonization of Polystyrene into Carbon Nanotubes. Ind. Eng. Chem. Res. 2017, 56, 11734–11744. [Google Scholar] [CrossRef]
- Ashik, U.; Daud, W.W.; Hayashi, J.-I. A review on methane transformation to hydrogen and nanocarbon: Relevance of catalyst characteristics and experimental parameters on yield. Renew. Sustain. Energy Rev. 2017, 76, 743–767. [Google Scholar] [CrossRef]
- Ahmad, S.; Liao, Y.; Hussain, A.; Zhang, Q.; Ding, E.-X.; Jiang, H.; Kauppinen, E.I. Systematic investigation of the catalyst composition effects on single-walled carbon nanotubes synthesis in floating-catalyst CVD. Carbon 2019, 149, 318–327. [Google Scholar] [CrossRef]
- Najafishirtari, S.; Guglieri, C.; Marras, S.; Scarpellini, A.; Brescia, R.; Prato, M.; Righi, G.; Franchini, A.; Magri, R.; Manna, L.; et al. Metal-support interaction in catalysis: The influence of the morphology of a nano-oxide domain on catalytic activity. Appl. Catal. B Environ. 2018, 237, 753–762. [Google Scholar] [CrossRef]
- Doustan, F.; Pasha, M.A. Growth of carbon nanotubes over Fe-Co and Ni-Co catalysts supported on different phases of TiO2 substrate by thermal CVD. Full-Nanotub. Carbon Nanostruct. 2016, 24, 25–33. [Google Scholar] [CrossRef]
- Zhuo, C.; Levendis, Y.A. Upcycling waste plastics into carbon nanomaterials: A review. J. Appl. Polym. Sci. 2014, 131, 1–14. [Google Scholar] [CrossRef]
- Bajad, G.; Guguloth, V.; Vijayakumar, R.P.; Bose, S. Conversion of plastic waste into CNTs using Ni/Mo/MgO catalyst—An optimization approach by mixture experiment. Full-Nanotub. Carbon Nanostruct. 2015, 24, 162–169. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.; Hu, L.; Qian, W.-Z.; Luo, G.-H.; Wei, F. Synthesis of thin-walled carbon nanotubes from methane by changing the Ni/Mo ratio in a Ni/Mo/MgO catalyst. New Carbon Mater. 2008, 23, 319–325. [Google Scholar] [CrossRef]
- Modekwe, H.U.; Mamo, M.A.; Daramola, M.O.; Moothi, K. Catalytic Performance of Calcium Titanate for Catalytic Decomposition of Waste Polypropylene to Carbon Nanotubes in a Single-Stage CVD Reactor. Catalysts 2020, 10, 1030. [Google Scholar] [CrossRef]
- Liu, H.; Seaton, N. Determination of the connectivity of porous solids from nitrogen sorption measurements—III. Solids containing large mesopores. Chem. Eng. Sci. 1994, 49, 1869–1878. [Google Scholar] [CrossRef]
- Sing, K.S.; Williams, R.T. Physisorption Hysteresis Loops and the Characterization of Nanoporous Materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Li, M.; Song, J.; Yue, F.; Pan, F.; Yan, W.; Hua, Z.; Li, L.; Yang, Z.; Li, L.; Wen, G.; et al. Complete Hydrodesulfurization of Dibenzothiophene via Direct Desulfurization Pathway over Mesoporous TiO2-Supported NiMo Catalyst Incorporated with Potassium. Catalysts 2019, 9, 448. [Google Scholar] [CrossRef] [Green Version]
- De Boer, J.; Heuvel, A.V.D.; Linsen, B. Studies on pore systems in catalysts IV. The two causes of reversible hysteresis. J. Catal. 1964, 3, 268–273. [Google Scholar] [CrossRef]
- Subbotin, A.N.; Subbotina, I.R.; Golosman, E.Z. Hysteresis phenomena in heterogeneous exothermal catalytic reactions and methods for decreasing the overheating of catalyst nanoclusters. Mendeleev Commun. 2015, 25, 216–218. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z.; Kadier, A.; Takriff, M.S.; Hassan, N.S.M. One-pot sol–gel synthesis of Ni/TiO2 catalysts for methane decomposition into COx free hydrogen and multiwalled carbon nanotubes. Int. J. Hydrogen Energy 2017, 42, 16495–16513. [Google Scholar] [CrossRef]
- Zhuang, Z.; Giles, S.A.; Zheng, J.; Jenness, G.R.; Caratzoulas, S.; Vlachos, D.G.; Yan, Y. Nickel supported on nitrogen-doped carbon nanotubes as hydrogen oxidation reaction catalyst in alkaline electrolyte. Nat. Commun. 2016, 7, 10141. [Google Scholar] [CrossRef] [Green Version]
- Nairan, A.; Zou, P.; Liang, C.; Liu, J.; Wu, D.; Liu, P.; Yang, C. NiMo Solid Solution Nanowire Array Electrodes for Highly Efficient Hydrogen Evolution Reaction. Adv. Funct. Mater. 2019, 29, 1903747. [Google Scholar] [CrossRef]
- Fan, X.; Liu, D.; Zhao, Z.; Li, J.; Liu, J. Influence of Ni/Mo ratio on the structure-performance of ordered mesoporous Ni-Mo-O catalysts for oxidative dehydrogenation of propane. Catal. Today 2020, 339, 67–78. [Google Scholar] [CrossRef]
- Méndez, F.J.; Bravo-Ascención, G.; González-Mota, M.; Puente-Lee, I.; Bokhimi, X.; Klimova, T.E. NiMo catalysts supported on Al, Nb, Ti or Zr-containing MCM-41 for dibenzothiophene hydrodesulfurization. Catal. Today 2020, 349, 217–227. [Google Scholar] [CrossRef]
- Wang, J.; Shen, B.; Lan, M.; Kang, D.; Wu, C. Carbon nanotubes (CNTs) production from catalytic pyrolysis of waste plastics: The influence of catalyst and reaction pressure. Catal. Today 2020, 351, 50–57. [Google Scholar] [CrossRef]
- Damyanova, S.; Spojakina, A.; Jiratova, K. Effect of mixed titania-alumina supports on the phase composition of NiMo/TiO2Al2O3 catalysts. Appl. Catal. A Gen. 1995, 125, 257–269. [Google Scholar] [CrossRef]
- González-Cortés, S.L.; Aray, I.; Rodulfo-Baechler, S.M.A.; Lugo, C.A.; Del Castillo, H.L.; Loaiza-Gil, A.; Imbert, F.E.; Figueroa, H.; Pernía, W.; Rodríguez, A.; et al. On the structure and surface properties of NiO/MgO–La2O3 catalyst: Influence of the support composition and preparation method. J. Mater. Sci. 2007, 42, 6532–6540. [Google Scholar] [CrossRef]
- Salinas, D.A.; Marchena, C.L.; Pierella, L.B.; Pecchi, G. Catalytic oxidation of 2-(methylthio)-benzothiazole on alkaline earth titanates, ATiO3 (A = Ca, Sr, Ba). Mol. Catal. 2017, 438, 76–85. [Google Scholar] [CrossRef]
- Shen, Y.; Lua, A.C. Sol–gel synthesis of Ni and Ni supported catalysts for hydrogen production by methane decomposition. RSC Adv. 2014, 4, 42159–42167. [Google Scholar] [CrossRef]
- Ni, L.; Kuroda, K.; Zhou, L.-P.; Kizuka, T.; Ohta, K.; Matsuishi, K.; Nakamura, J. Kinetic study of carbon nanotube synthesis over Mo/Co/MgO catalysts. Carbon 2006, 44, 2265–2272. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhang, Y.; Nahil, M.A.; Williams, P.T.; Wu, C. Development of Ni- and Fe- based catalysts with different metal particle sizes for the production of carbon nanotubes and hydrogen from thermo-chemical conversion of waste plastics. J. Anal. Appl. Pyrolysis 2017, 125, 32–39. [Google Scholar] [CrossRef]
- Takenaka, S.; Ishida, M.; Serizawa, M.; Tanabe, A.E.; Otsuka, K. Formation of Carbon Nanofibers and Carbon Nanotubes through Methane Decomposition over Supported Cobalt Catalysts. J. Phys. Chem. B 2004, 108, 11464–11472. [Google Scholar] [CrossRef]
- Wang, B.; Yang, Y.; Li, L.-J.; Chen, Y. Effect of different catalyst supports on the (n,m) selective growth of single-walled carbon nanotube from Co–Mo catalyst. J. Mater. Sci. 2009, 44, 3285–3295. [Google Scholar] [CrossRef]
- Cao, A.; Xu, C.; Liang, J.; Wu, D.; Wei, B. X-ray diffraction characterization on the alignment degree of carbon nanotubes. Chem. Phys. Lett. 2001, 344, 13–17. [Google Scholar] [CrossRef]
- Che, B.D.; Nguyen, B.Q.; Nguyen, L.-T.T.; Nguyen, H.T.; Nguyen, V.Q.; Van Le, T.; Nguyen, N.H. The impact of different multi-walled carbon nanotubes on the X-band microwave absorption of their epoxy nanocomposites. Chem. Central J. 2015, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Lehman, J.H.; Terrones, M.; Mansfield, E.; Hurst, K.E.; Meunier, V. Evaluating the characteristics of multiwall carbon nanotubes. Carbon 2011, 49, 2581–2602. [Google Scholar] [CrossRef]
- Xu, X.; Huang, S.; Yang, Z.; Zou, C.; Jiang, J.; Shang, Z. Controllable synthesis of carbon nanotubes by changing the Mo content in bimetallic Fe–Mo/MgO catalyst. Mater. Chem. Phys. 2011, 127, 379–384. [Google Scholar] [CrossRef]
- Mansfield, E.; Kar, A.; Hooker, S.A. Applications of TGA in quality control of SWCNTs. Anal. Bioanal. Chem. 2010, 396, 1071–1077. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Douvartzides, S.L.; Siakavelas, G.I.; Tzounis, L.; Sebastian, V.; Stolojan, V.; Hinder, S.J.; Baker, M.A.; Polychronopoulou, K.; Goula, M.A. The Relationship between Reaction Temperature and Carbon Deposition on Nickel Catalysts Based on Al2O3, ZrO2 or SiO2 Supports during the Biogas Dry Reforming Reaction. Catalysts 2019, 9, 676. [Google Scholar] [CrossRef] [Green Version]
- Dresselhaus, M.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Yusof, Y.; Zaidi, M.I.; Johan, M.R. Enhanced Structural, Thermal, and Electrical Properties of Multiwalled Carbon Nanotubes Hybridized with Silver Nanoparticles. J. Nanomater. 2016, 2016, 6141496. [Google Scholar] [CrossRef]
- Liu, H.; Yan, L.; Chang, Y.; Fang, H.; Zhang, T. Spectral Deconvolution and Feature Extraction with Robust Adaptive Tikhonov Regularization. IEEE Trans. Instrum. Meas. 2013, 62, 315–327. [Google Scholar] [CrossRef]
- Pelech, I.; Narkiewicz, U.; Kaczmarek, A.; Jedrzejewska, A. Preparation and characterization of multi-walled carbon nano-tubes grown on transition metal catalysts. Polish J. Chem. Technol. 2014, 16, 117–122. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.; Feng, J.; Liu, J.; Jiang, Z.; Chen, X.; Mijowska, E.; Wen, X.; Tang, T. Catalytic carbonization of polypropylene into cup-stacked carbon nanotubes with high performances in adsorption of heavy metallic ions and organic dyes. Chem. Eng. J. 2014, 248, 27–40. [Google Scholar] [CrossRef]
- Gong, J.; Liu, J.; Chen, X.; Jiang, Z.; Wen, X.; Mijowska, E.; Tang, T. Converting real-world mixed waste plastics into porous carbon nanosheets with excellent performance in the adsorption of an organic dye from wastewater. J. Mater. Chem. A 2015, 3, 341–351. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Tao, X.; Xu, J.; Huang, W.; Luo, J.; Luo, Z.; Li, T.; Liu, F.; Bao, Y.; et al. Mass production of high-quality multi-walled carbon nanotube bundles on a Ni/Mo/MgO catalyst. Carbon 2005, 43, 295–301. [Google Scholar] [CrossRef]
- Das, R.; Hamid, S.B.A.; Ali, E.; Ramakrishna, S.; Yongzhi, W. Carbon Nanotubes Characterization by X-ray Powder Diffraction—A Review. Curr. Nanosci. 2014, 11, 23–35. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Conant, G.C.; Ou, R.; Beerntsen, B.T. Cloning and characterization of the peptidoglycan recognition protein genes in the mosquito, Armigeres subalbatus (Diptera: Culicidae). J. Med Èntomol. 2012, 49, 656–671. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Kharisov, B.I. Variations of interlayer spacing in carbon nanotubes. RSC Adv. 2014, 4, 30807–30815. [Google Scholar] [CrossRef]
- Modekwe, H.; Mamo, M.; Moothi, K.; Daramola, M. Synthesis of bimetallic NiMo/MgO catalyst for catalytic conversion of waste plastics (polypropylene) to carbon nanotubes (CNTs) via chemical vapour deposition method. Mater. Today Proc. 2021, 38, 549–552. [Google Scholar] [CrossRef]
- Iloy, R.A.; Jalama, K. Effect of Operating Temperature, Pressure and Potassium Loading on the Performance of Silica-Supported Cobalt Catalyst in CO2 Hydrogenation to Hydrocarbon Fuel. Catalysts 2019, 9, 807. [Google Scholar] [CrossRef] [Green Version]



| Catalyst | BET Surface Area (m2g−1) | Pore Volume (cm3g−1) | Pore Size (nm) | NiO Crystallite Size (nm) |
|---|---|---|---|---|
| NiMo | 72.5 | 0.347 | 12.0 | 7.7 |
| NiMo/MgO | 56.6 | 0.267 | 12.0 | 11.8 |
| NiMo/CaO | 16.0 | 0.085 | 14.3 | 6.3 |
| NiMo/TiO2 | 27.5 | 0.094 | 9.0 | 5.8 |
| NiMo/CaTiO3 | 19.4 | 0.129 | 22.3 | 11.3 |
| Catalyst | Weight (%) | |||||
|---|---|---|---|---|---|---|
| Ni | Mo | Mg | Ca | Ti | O | |
| NiMo/MgO | 44.51 | 10.53 | 16.76 | - | - | 28.20 |
| NiMo/CaO | 44.59 | 10.97 | - | 15.51 | - | 28.93 |
| NiMo/TiO2 | 44.40 | 10.50 | - | - | 16.01 | 29.09 |
| NiMo/CaTiO3 | 41.52 | 9.55 | - | 15.02 | 14.29 | 19.62 |
| NiMo | 44.90 | 9.00 | - | - | - | 46.10 |
| Sample | CNTs Yield (%) | CNTs Average Outer Diameter (nm) | d(002) (nm) | Intensity Ratio IG/ID | Intensity Ratio ID/IG | I(002) (a.u.) |
|---|---|---|---|---|---|---|
| CNTNiMo | 18.4 | 25.08 ± 7.538 | 0.335 | 0.93 | 1.17 | 4814 |
| CNTNiMo/MgO | 33.3 | 25.54 ± 6.397 | 0.339 | 0.99 | 1.01 | 13,802 |
| CNTNiMo/CaO | 31.0 | 19.36 ± 3.897 | 0.338 | 1.00 | 1.00 | 12,499 |
| CNTNiMo/TiO2 | 37.0 | 22.08 ± 3.448 | 0.339 | 1.07 | 0.93 | 4871 |
| CNTNiMo/CaTiO3 | 40.0 | 24.01 ± 4.890 | 0.340 | 1.25 | 0.80 | 4744 |
| Catalysts | Ni Average Particle Size of Spent Catalyst (nm) | Ni Crystallite Size of Spent Catalyst (nm) |
|---|---|---|
| NiMo/MgO | 15.8–26.0 | 11.08 |
| NiMo/CaO | 6.0–13.0 | 7.02 |
| NiMo/TiO2 | 13.0–20.0 | 6.10 |
| NiMo/CaTiO3 | 10.6–17.7 | 10.52 |
| NiMo | 22.0–42.0 | 12.65 |
| Catalyst | Ni (mol) | Mo (mol) | Support (mol) | Corresponding CNTs Notation |
|---|---|---|---|---|
| NiMo | 5 | 0.1 | - | CNTNiMo |
| NiMo/MgO | 5 | 0.1 | 1 | CNTNiMo/MgO |
| NiMo/CaO | 5 | 0.1 | 1 | CNTNiMo/CaO |
| NiMo/TiO2 | 5 | 0.1 | 1 | CNTNiMo/TiO2 |
| NiMo/CaTiO3 | 5 | 0.1 | 1 | CNTNiMo/CaTiO3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modekwe, H.U.; Mamo, M.A.; Moothi, K.; Daramola, M.O. Effect of Different Catalyst Supports on the Quality, Yield and Morphology of Carbon Nanotubes Produced from Waste Polypropylene Plastics. Catalysts 2021, 11, 692. https://doi.org/10.3390/catal11060692
Modekwe HU, Mamo MA, Moothi K, Daramola MO. Effect of Different Catalyst Supports on the Quality, Yield and Morphology of Carbon Nanotubes Produced from Waste Polypropylene Plastics. Catalysts. 2021; 11(6):692. https://doi.org/10.3390/catal11060692
Chicago/Turabian StyleModekwe, Helen Uchenna, Messai Adenew Mamo, Kapil Moothi, and Michael Olawale Daramola. 2021. "Effect of Different Catalyst Supports on the Quality, Yield and Morphology of Carbon Nanotubes Produced from Waste Polypropylene Plastics" Catalysts 11, no. 6: 692. https://doi.org/10.3390/catal11060692