Photocatalytic Oxidation of Chlorantraniliprole, Imidacloprid, Pirimicarb, Thiamethoxam and Their Main Photoreaction InterMediates as Impacted by Water Matrix Composition under UVA-LED Exposure
Abstract
:1. Introduction
2. Results and Discussion
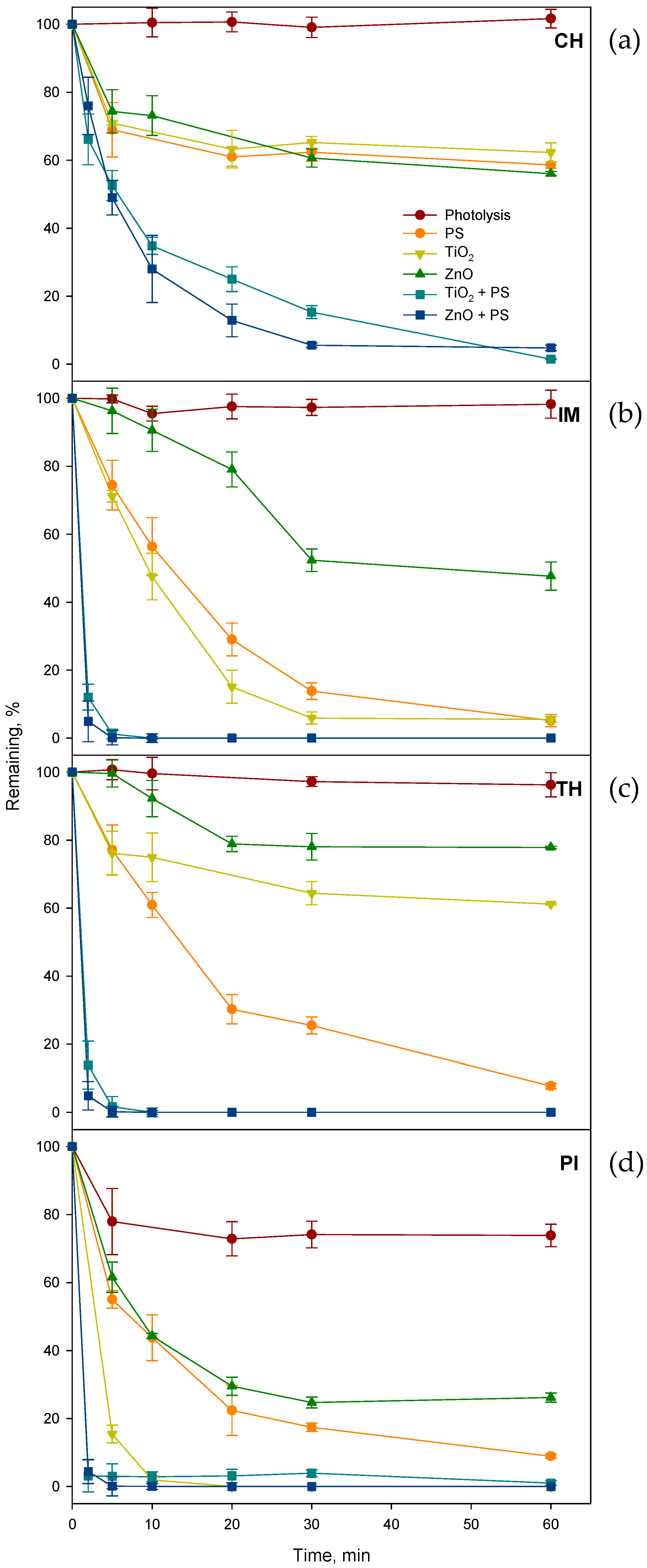
2.1. Assessment of the Optimum Catalysts Loading for the Photocatalytic Degradation in Pure Water
2.2. Photocatalytic Activity in Real Matrices
3. Materials and Methods
3.1. Reagents
3.2. Water Sources
3.3. Photochemical Experiments
3.4. Analytical Measurements
3.5. Determination of Kinetic Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ternes, T.; Joss, A.; Oehlmann, J. Occurrence, fate, removal and assessment of emerging contaminants in water in the water cycle (from wastewater to drinking water). Water Res. 2015, 72, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment technologies for emerging contaminants in wastewater treatment plants: A review. Sci. Total Environ. 2021, 753, 141990. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ok, Y.S.; Kim, K.H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596–597, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Lado Ribeiro, A.R.; Moreira, N.F.F.; Li Puma, G.; Silva, A.M.T. Impact of water matrix on the removal of micropollutants by advanced oxidation technologies. Chem. Eng. J. 2019, 363, 155–173. [Google Scholar] [CrossRef] [Green Version]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Rizzo, L.; Malato, S.; Antakyali, D.; Beretsou, V.G.; Đolić, M.B.; Gernjak, W.; Heath, E.; Ivancev-Tumbas, I.; Karaolia, P.; Lado Ribeiro, A.R.; et al. Consolidated vs new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Sci. Total Environ. 2019, 655, 986–1008. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Rasul, M.G.; Brown, R.; Hashib, M.A. Influence of parameters on the heterogeneous photocatalytic degradation of pesticides and phenolic contaminants in wastewater: A short review. J. Environ. Manag. 2011, 92, 311–330. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, S.; Martins, P.M.; Lanceros-Méndez, S.; Kühn, K.; Cuniberti, G. Reusability of photocatalytic TiO2 and ZnO nanoparticles immobilized in poly(vinylidene difluoride)-co-trifluoroethylene. Appl. Surf. Sci. 2016, 384, 497–504. [Google Scholar] [CrossRef]
- Fenoll, J.; Garrido, I.; Hellín, P.; Flores, P.; Vela, N.; Navarro, S. Photocatalytic oxidation of pirimicarb in aqueous slurries containing binary and ternary oxides of zinc and titanium. J. Photochem. Photobiol. A Chem. 2015, 298, 24–32. [Google Scholar] [CrossRef]
- Vaya, D.; Surolia, P.K. Semiconductor based photocatalytic degradation of pesticides: An overview. Environ. Technol. Innov. 2020, 20, 101128. [Google Scholar] [CrossRef]
- Reddy, P.V.L.; Kim, K.H. A review of photochemical approaches for the treatment of a wide range of pesticides. J. Hazard. Mater. 2015, 285, 325–335. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Al-Kaabi, M.A.; Ashfaq, M.Y.; Da’na, D.A. Produced water characteristics, treatment and reuse: A review. J. Water Process. Eng. 2019, 28, 222–239. [Google Scholar] [CrossRef]
- Gomez-Ruiz, B.; Ribao, P.; Diban, N.; Rivero, M.J.; Ortiz, I.; Urtiaga, A. Photocatalytic degradation and mineralization of perfluorooctanoic acid (PFOA) using a composite TiO2−rGO catalyst. J. Hazard. Mater. 2018, 344, 950–957. [Google Scholar] [CrossRef] [Green Version]
- Sousa, M.A.; Gonçalves, C.; Pereira, J.H.O.S.; Vilar, V.J.P.; Boaventura, R.A.R.; Alpendurada, M.F. Photolytic and TiO2-assisted photocatalytic oxidation of the anxiolytic drug lorazepam (Lorenin® pills) under artificial UV light and natural sunlight: A comparative and comprehensive study. Sol. Energy 2013, 87, 219–228. [Google Scholar] [CrossRef]
- Sousa, M.A.; Lacina, O.; Hrádková, P.; Pulkrabová, J.; Vilar, V.J.P.; Gonçalves, C.; Boaventura, R.A.R.; Hajšlová, J.; Alpendurada, M.F. Lorazepam photofate under photolysis and TiO2-assisted photocatalysis: Identification and evolution profiles of by-products formed during phototreatment of a WWTP effluent. Water Res. 2013, 47, 5584–5593. [Google Scholar] [CrossRef]
- Tapia-Tlatelpa, T.; Buscio, V.; Trull, J.; Sala, V. Performance analysis and methodology for replacing conventional lamps by optimized LED arrays for photocatalytic processes. Chem. Eng. Res. Des. 2020, 156, 456–468. [Google Scholar] [CrossRef]
- Zawadzki, P.; Kudlek, E.; Dudziak, M. Kinetics of the Photocatalytic Decomposition of Bisphenol A on Modified Photocatalysts. J. Ecol. Eng. 2018, 19, 260–268. [Google Scholar] [CrossRef]
- Jo, W.K.; Tayade, R.J. New generation energy-efficient light source for photocatalysis: LEDs for environmental applications. Ind. Eng. Chem. Res. 2014, 53, 2073–2084. [Google Scholar] [CrossRef]
- Matafonova, G.; Batoev, V. Recent advances in application of UV light-emitting diodes for degrading organic pollutants in water through advanced oxidation processes: A review. Water Res. 2018, 132, 177–189. [Google Scholar] [CrossRef]
- Davididou, K.; Nelson, R.; Monteagudo, J.M.; Durán, A.; Expósito, A.J.; Chatzisymeon, E. Photocatalytic degradation of bisphenol-A under UV-LED, blacklight and solar irradiation. J. Clean. Prod. 2018, 203, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, J.P.; Achari, G.; Langford, C.H. Design and evaluation of a UV LED Photocatalytic Reactor Using Anodized TiO2 Nanotubes. Water Environ. Res. 2016, 88, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Hossaini, H.; Moussavi, G.; Farrokhi, M. The investigation of the LED-activated FeFNS-TiO2 nanocatalyst for photocatalytic degradation and mineralization of organophosphate pesticides in water. Water Res. 2014, 59, 130–144. [Google Scholar] [CrossRef]
- Muramoto, Y.; Kimura, M.; Nouda, S. Development and future of ultraviolet light-emitting diodes: UV-LED will replace the UV lamp. Semicond. Sci. Technol. 2014, 29, 084004. [Google Scholar] [CrossRef] [Green Version]
- Tokode, O.; Prabhu, R.; Lawton, L.A.; Robertson, P.K.J. UV LED source for heterogeneous photocatalysis. In Environmental Photochemistry Part III, 1st ed.; Bahnemann, D.W., Robertson, P.K.J., Eds.; Springer: Heidelberg, Germany, 2015; pp. 159–179. [Google Scholar] [CrossRef] [Green Version]
- Eskandarian, M.R.; Choi, H.; Fazli, M.; Rasoulifard, M.H. Effect of UV-LED wavelengths on direct photolytic and TiO2 photocatalytic degradation of emerging contaminants in water. Chem. Eng. J. 2016, 300, 414–422. [Google Scholar] [CrossRef]
- Chen, D.H.; Ye, X.; Li, K. Oxidation of PCE with a UV LED photocatalytic reactor. Chem. Eng. Technol. 2005, 28, 95–97. [Google Scholar] [CrossRef]
- Bowker, C.; Sain, A.; Shatalov, M.; Ducoste, J. Microbial UV fluence-response assessment using a novel UV-LED collimated beam system. Water Res. 2011, 45, 2011–2019. [Google Scholar] [CrossRef]
- Khan, M.A. AlGaN multiple quantum well based deep UV LEDs and their applications. Phys. Status Solidi A Appl. Res. 2006, 203, 1764–1770. [Google Scholar] [CrossRef]
- Aliste, M.; Garrido, I.; Pérez-Lucas, G.; Flores, P.; Hellín, P.; Navarro, S.; Fenoll, J. Appraisal of water matrix on the removal of fungicide residues by heterogeneous photocatalytic treatment using UV-LED lamp as light source. Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bousmaha, M.; Bezzerrouk, M.A.; Kharroubi, B.; Akriche, A.; Naceur, R.; Hattabi, I.; Sandjak-Eddine, K. Enhanced photocatalysis by depositing ZnO thin film in the inner wall of glass tube. Optik 2019, 183, 727–731. [Google Scholar] [CrossRef]
- Hossaini, H.; Moussavi, G.; Farrokhi, M. Oxidation of diazinon in cns-ZnO/LED photocatalytic process: Catalyst preparation, photocatalytic examination, and toxicity bioassay of oxidation by-products. Sep. Purif. Technol. 2017, 174, 320–330. [Google Scholar] [CrossRef]
- Aliste, M.; Pérez-Lucas, G.; Garrido, I.; Fenoll, J.; Navarro, S. Mobility of insecticide residues and main intermediates in a clay-loam soil, and impact of leachate components on their photocatalytic degradation. Chemosphere 2021, 274, 129965. [Google Scholar] [CrossRef]
- Aliste, M.; Garrido, I.; Flores, P.; Hellín, P.; Pérez-Lucas, G.; Navarro, S.; Fenoll, J. Photocatalytic degradation of four insecticides and their main generated transformation products in soil and pepper crop irrigated with reclaimed agro-wastewater under natural sunlight. J. Hazard. Mater. 2021, 414, 125603. [Google Scholar] [CrossRef]
- Herrmann, J.M. Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Aliste, M.; Pérez-Lucas, G.; Vela, N.; Garrido, I.; Fenoll, J.; Navarro, S. Solar-driven photocatalytic treatment as sustainable strategy to remove pesticide residues from leaching water. Environ. Sci. Pollut. Res. Int. 2020, 27, 7222–7233. [Google Scholar] [CrossRef]
- Herrmann, J.M. Photocatalysis fundamentals revisited to avoid several misconceptions. Appl. Catal. B Environ. 2010, 99, 461–468. [Google Scholar] [CrossRef]
- Pirisi, F.M.; Cabras, P.; Garau, V.L.; Melis, M.; Secchi, E. Photodegradation of Pesticides. Photolysis Rates and Half-Life of Pirimicarb and Its Metabolites in Reactions in Water and in Solid Phase. J. Agric. Food Chem. 1996, 44, 2417–2422. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Matzek, L.W.; Carter, K.E. Activated persulfate for organic chemical degradation: A review. Chemosphere 2016, 151, 178–188. [Google Scholar] [CrossRef]
- Yang, Q.; Ma, Y.; Chen, F.; Yao, F.; Sun, J.; Wang, S.; Yi, K.; Hou, L.; Li, X.; Wang, D. Recent advances in photo-activated sulfate radical-advanced oxidation process (SR-AOP) for refractory organic pollutants removal in water. Chem. Eng. J. 2019, 378, 122149. [Google Scholar] [CrossRef]
- Quiroz, M.A.; Bandala, E.R.; Martínez-Huitle, C.A. Advanced Oxidation Processes (AOPs) for removal of pesticides from aqueous media. In Pesticides–Formulations, Effects, Fate; Stoytcheva, M., Ed.; InTechOpen: London, UK, 2011; pp. 685–705. Available online: https://www.intechopen.com/books/pesticides-formulations-effects-fate/advanced-oxidation-processes-aops-for-removal-of-pesticides-from-aqueous-media (accessed on 25 February 2021).
- Vela, N.; Fenoll, J.; Garrido, I.; Pérez-Lucas, G.; Flores, P.; Hellín, P.; Navarro, S. Reclamation of agro-wastewater polluted with pesticide residues using sunlight activated persulfate for agricultural reuse. Sci. Total Environ. 2019, 660, 923–930. [Google Scholar] [CrossRef]
- Fenoll, J.; Garrido, I.; Cava, J.; Hellín, P.; Flores, P.; Navarro, S. Photometabolic pathways of chlorantraniliprole in aqueous slurries containing binary and ternary oxides of Zn and Ti. Chem. Eng. J. 2015, 264, 720–727. [Google Scholar] [CrossRef]
- Aliste, M.; Garrido, I.; Flores, P.; Hellín, P.; Vela, N.; Navarro, S.; Fenoll, J. Reclamation of agro-wastewater polluted with thirteen pesticides by solar photocatalysis to reuse in irrigation of greenhouse lettuce grown. J. Environ. Manag. 2020, 266, 110565. [Google Scholar] [CrossRef] [PubMed]
- Fenoll, J.; Hellín, P.; Martinez, C.M.; Flores, P.; Navarro, S. Semiconductor-sensitized photodegradation of s-triazine and chloroacetanilide herbicides in leaching water using TiO2 and ZnO as catalyst under natural sunlight. J. Photochem. Photobiol. A Chem. 2012, 238, 81–87. [Google Scholar] [CrossRef]
- Fenoll, J.; Hellín, P.; Martinez, C.M.; Flores, P.; Navarro, S. Semiconductor oxides-sensitized photodegradation of fenamiphos in leaching water under natural sunlight. Appl. Catal. B. 2012, 115–116, 31–37. [Google Scholar] [CrossRef]
- Armstrong, D.A.; Huie, R.E.; Koppenol, W.H.; Lymar, S.V.; Merényi, G.; Neta, P.; Ruscic, B.; Stanbury, D.M.; Steenken, S.; Wardman, P. Standard electrode potentials involving radicals in aqueous solution: Inorganic radicals (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1139–1150. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Zarkadis, A.K.; Albanis, T.A. Photodegradation of selected herbicides in various natural waters and soils under environmental conditions. J. Environ. Qual. 2001, 30, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Drosos, M.; Frimmel, F.H. Inhibitory effect of NOM in photocatalysis process: Explanation and resolution. Chem. Eng. J. 2018, 334, 968–975. [Google Scholar] [CrossRef]
- Liqing, Z.; Guoguang, L.; Dezhi, S.; Kun, Y. Hydrolysis of thiamethoxam. Bull. Environ. Contam. Toxicol. 2006, 76, 942–949. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. 2016, 22, 1050–1064. [Google Scholar] [CrossRef] [Green Version]
- Fenoll, J.; Vela, N.; Garrido, I.; Pérez-Lucas, G.; Navarro, S. Abatement of spinosad and indoxacarb residues in pure water by photocatalytic treatment using binary and ternary oxides of Zn and Ti. Environ. Sci. Pollut. Res. 2014, 21, 12143–12153. [Google Scholar] [CrossRef]
- Fenoll, J.; Hellín, P.; Martínez, C.M.; Flores, P.; Navarro, S. Determination of 48 pesticides and their main metabolites in water samples by employing sonication and liquid chromatography-tandem mass spectrometry. Talanta 2011, 85, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Fenoll, J.; Garrido, I.; Hellín, P.; Flores, P.; Navarro, S. Photodegradation of neonicotinoid insecticides in water by semiconductor oxides. Environ. Sci. Pollut. Res. 2015, 22, 15055–15066. [Google Scholar] [CrossRef] [PubMed]
- Boesten, J.J.T.I.; Aden, K.; Beigel, C.; Beulke, S.; Dust, M.; Dyson, J.S.; Fomsgaard, I.S.; Jones, R.L.; Karlsson, S.; van der Linden, A.M.A.; et al. Guidance Document on Estimating Persistence and Degradation Kinetics from Environmental Fate Studies on Pesticides in EU Registration. The Final Report of the Work Group on Degradation Kinetics of FOCUS (FOrum for the Co-ordination of Pesticide Fate Models and Their USe). EC Document Reference Sanco/10058/2005 Version 2.0. Brussels, 2006. Available online: https://esdac.jrc.ec.europa.eu/public_path/projects_data/focus/dk/docs/finalreportFOCDegKinetics.pdf (accessed on 18 January 2021).

| Water | Chlorantraniliprole | Imidacloprid | Pirimicarb | Thiamethoxam | ||||
|---|---|---|---|---|---|---|---|---|
| kapp | t1/2 | kapp | t1/2 | kapp | t1/2 | kapp | t1/2 | |
| DW | 0.065 | 11 | 1.027 | 0.7 | 1.721 | 0.4 | 0.951 | 0.7 |
| IW | 0.007 | 105 | 0.053 | 13 | 0.123 | 6 | 0.049 | 14 |
| LW-H | 0.000 | 1733 | 0.004 | 165 | 0.004 | 173 | 0.002 | 289 |
| LW-L | 0.004 | 161 | 0.025 | 28 | 0.020 | 35 | 0.020 | 35 |
| WWTPe | 0.004 | 193 | 0.024 | 29 | 0.054 | 13 | 0.023 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aliste, M.; Garrido, I.; Pérez-Lucas, G.; Navarro, S.; Fenoll, J. Photocatalytic Oxidation of Chlorantraniliprole, Imidacloprid, Pirimicarb, Thiamethoxam and Their Main Photoreaction InterMediates as Impacted by Water Matrix Composition under UVA-LED Exposure. Catalysts 2021, 11, 609. https://doi.org/10.3390/catal11050609
Aliste M, Garrido I, Pérez-Lucas G, Navarro S, Fenoll J. Photocatalytic Oxidation of Chlorantraniliprole, Imidacloprid, Pirimicarb, Thiamethoxam and Their Main Photoreaction InterMediates as Impacted by Water Matrix Composition under UVA-LED Exposure. Catalysts. 2021; 11(5):609. https://doi.org/10.3390/catal11050609
Chicago/Turabian StyleAliste, Marina, Isabel Garrido, Gabriel Pérez-Lucas, Simón Navarro, and José Fenoll. 2021. "Photocatalytic Oxidation of Chlorantraniliprole, Imidacloprid, Pirimicarb, Thiamethoxam and Their Main Photoreaction InterMediates as Impacted by Water Matrix Composition under UVA-LED Exposure" Catalysts 11, no. 5: 609. https://doi.org/10.3390/catal11050609







