SnS2/TiO2 Nanocomposites for Hydrogen Production and Photodegradation under Extended Solar Irradiation
Abstract
:1. Introduction
2. Results
2.1. Characterization of Materials
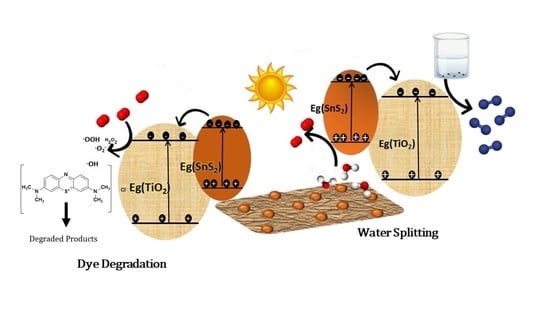
2.2. Photocatalytic Hydrogen Evolution
2.3. Photocatalytic Degradation
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Synthesis
Titanium Dioxide
SnS2-Embedded TiO2 Nanocomposite
3.2.2. Characterization
3.2.3. Photocatalytic Hydrogen Evolution
3.2.4. Photocatalytic Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ran, J.; Zhang, J.; Yu, J.; Jaroniec, M.; Qiao, S.Z. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 2014, 43, 7787–7812. [Google Scholar] [CrossRef]
- Nguyen, P.D.; Duong, T.M.; Tran, P.D. Current progress and challenges in engineering viable artificial leaf for solar water splitting. J. Sci. Adv. Mater. Devices 2017, 2, 399–417. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Fu, X.; Wang, C.; Ni, M.; Leung, M.K.H.; Wang, X.; Fu, X. Hydrogen production over titania-based photocatalysts. ChemSusChem 2010, 3, 681–694. [Google Scholar] [CrossRef]
- Yin, Y.; Jin, Z.; Hou, F. Enhanced solar water-splitting efficiency using core/sheath heterostructure CdS/TiO2 nanotube arrays. Nanotechnology 2007. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Hong, S.C. Kinetic study for photocatalytic degradation of volatile organic compounds in air using thin film TiO2 photocatalyst. Appl. Catal. B Environ. 2002, 35, 305–315. [Google Scholar] [CrossRef]
- Rasalingam, S.; Peng, R.; Koodali, R.T. Removal of Hazardous Pollutants from Wastewaters: Applications of TiO2-SiO2 Mixed Oxide Materials Shivatharsiny. J. Nanomater. 2014, 2014, 617405. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarti, S.; Dutta, B.K. Photocatalytic degradation of model textile dyes in wastewater using ZnO as semiconductor catalyst. J. Hazard. Mater. 2004, 112, 269–278. [Google Scholar] [CrossRef]
- Mugunthan, E.; Saidutta, M.B.; Jagadeeshbabu, P.E. Photocatalytic degradation of diclofenac using TiO2–SnO2 mixed oxide catalysts. Environ. Technol. 2019, 40, 929–941. [Google Scholar] [CrossRef]
- Kong, C.; Min, S.; Lu, G. Dye-sensitized NiSx catalyst decorated on graphene for highly efficient reduction of water to hydrogen under visible light irradiation. ACS Catal. 2014, 4, 2763–2769. [Google Scholar] [CrossRef]
- Pirashanthan, A.; Murugathas, T.; Mariappan, K.; Ravirajan, P.; Velauthapillai, D.; Yohi, S. A multifunctional ruthenium based dye for hybrid nanocrystalline titanium dioxide/poly(3-hexylthiophene) solar cells. Mater. Lett. 2020, 274, 127997. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, X.; Zeng, X.; Li, Y.; Zheng, L.; Wan, C. Enhanced photocatalytic activity of TiO2 nanoparticles using SnS2/RGO hybrid as co-catalyst: DFT study and photocatalytic mechanism. J. Alloys Compd. 2016, 685, 774–783. [Google Scholar] [CrossRef]
- Jafari, T.; Moharreri, E.; Amin, A.S.; Miao, R.; Song, W.; Suib, S.L. Photocatalytic water splitting—The untamed dream: A review of recent advances. Molecules 2016, 21, 900. [Google Scholar] [CrossRef] [PubMed]
- Pirashanthan, A.; Murugathas, T.; Robertson, N.; Ravirajan, P.; Velauthapillai, D. A Quarterthiophene-Based Dye as an Efficient. Polymers 2019, 11, 1752. [Google Scholar] [CrossRef] [Green Version]
- Eidsvåg, H.; Bentouba, S.; Vajeeston, P.; Yohi, S.; Velauthapillai, D. TiO2 as a Photocatalyst for Water Splitting—An Experimental and Theoretical Review. Molecules 2021, 26, 1687. [Google Scholar] [CrossRef] [PubMed]
- Rajaramanan, T.; Natarajan, M.; Ravirajan, P.; Senthilnanthanan, M.; Velauthapillai, D. Ruthenium (Ru) Doped Titanium Dioxide (P25) electrode for dye sensitized solar cells. Energies 2020, 13, 1532. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhou, X.; Xing, Y. In situ thermal-assisted loading of monodispersed Pt nanoclusters on CdS nanoflowers for efficient photocatalytic hydrogen evolution. Appl. Surf. Sci. 2020, 506, 144933. [Google Scholar] [CrossRef]
- Shanmugaratnam, S.; Rasalingam, S. Transition Metal Chalcogenide (TMC) Nanocomposites for Environmental Remediation Application over Extended Solar Irradiation. Intech 2019. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Zhang, W.; Xiao, X. Preparation of titanium dioxide/tungsten disulfide composite photocatalysts with enhanced photocatalytic activity under visible light. Korean J. Chem. Eng. 2016, 33, 107–113. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, J.; Sun, H.; Ng, Y.H.; Zhang, K.Q.; Lai, Y. MoS2 Quantum Dots@TiO2 Nanotube Arrays: An Extended-Spectrum-Driven Photocatalyst for Solar Hydrogen Evolution. ChemSusChem 2018, 11, 1708–1721. [Google Scholar] [CrossRef]
- Meng, Z.; Oh, W. Photodegradation of Organic Dye by CoS2 and Carbon(C60, Graphene, CNT)/TiO2 Composite Sensitizer. Chin. J. Catal. 2012, 33, 1495–1501. [Google Scholar] [CrossRef]
- Kajana, T.; Velauthapillai, D.; Shivatharsiny, Y.; Ravirajan, P.; Yuvapragasam, A.; Senthilnanthanan, M. Structural and photoelectrochemical characterization of heterostructured carbon sheet/Ag2MoO4-SnS/Pt photocapacitor. J. Photochem. Photobiol. A Chem. 2020, 401, 112784. [Google Scholar] [CrossRef]
- Chen, T.Y.; Chang, Y.H.; Hsu, C.L.; Wei, K.H.; Chiang, C.Y.; Li, L.J. Comparative study on MoS2 and WS2 for electrocatalytic water splitting. Int. J. Hydrog. Energy 2013, 38, 12302–12309. [Google Scholar] [CrossRef]
- Ali, A.; Oh, W.C. Photocatalytic performance of CoS2-graphene-TiO2 ternary composites for reactive black B (RBB) degradation. J. Korean Ceram. Soc. 2017, 54, 308–313. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. MoS2 nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, K.; Xie, J.; Li, M.; Li, X. In situ one-pot fabrication of g-C3N4 nanosheets/NiS cocatalyst heterojunction with intimate interfaces for efficient visible light photocatalytic H2 generation. Appl. Surf. Sci. 2018, 430, 208–217. [Google Scholar] [CrossRef]
- Shanmugaratnam, S.; Velauthapillai, D.; Ravirajan, P.; Christy, A.A.; Shivatharsiny, Y. CoS2/TiO2 in nanocomposites for hydrogen production under UV irradiation. Materials 2019, 12, 3882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, Z.; Chetia, T.R.; Vardhaman, A.K.; Barpuzary, D.; Sastri, C.V.; Qureshi, M. Visible light assisted photocatalytic hydrogen generation and organic dye degradation by CdS-metal oxide hybrids in presence of graphene oxide. RSC Adv. 2012, 2, 12122–12128. [Google Scholar] [CrossRef]
- Sun, B.; Liu, A.; Li, J.; Wang, J.; Wang, S. Development of novel highly stable synergistic quaternary photocatalyst for the efficient hydrogen evolution reaction. Appl. Surf. Sci. 2020, 510, 145498. [Google Scholar] [CrossRef]
- Zhu, W.; Yang, Y.; Ma, D.; Wang, H.; Zhang, Y.; Hu, H. Controlled growth of flower-like SnS2 hierarchical structures with superior performance for lithium-ion battery applications. Ionics 2014, 21, 19–26. [Google Scholar] [CrossRef]
- Mondal, C.; Ganguly, M.; Pal, J.; Roy, A.; Jana, J.; Pal, T. Morphology controlled synthesis of SnS2 nanomaterial for promoting photocatalytic reduction of aqueous Cr(VI) under visible light. Langmuir 2014, 30, 4157–4164. [Google Scholar] [CrossRef]
- Gaur, R.; Jeevanandam, P. Synthesis of SnS2 nanoparticles and their application as photocatalysts for the reduction of Cr(VI). J. Nanosci. Nanotechnol. 2018, 18, 165–177. [Google Scholar] [CrossRef]
- Feng, J.; Chen, J.; Geng, B.; Feng, H.; Li, H.; Yan, D.; Zhuo, R.; Cheng, S.; Wu, Z.; Yan, P. Two-dimensional hexagonal SnS2 nanoflakes: Fabrication, characterization, and growth mechanism. Appl. Phys. A Mater. Sci. Process. 2011, 103, 413–419. [Google Scholar] [CrossRef]
- Geng, H.; Su, Y.; Wei, H.; Xu, M.; Wei, L.; Yang, Z.; Zhang, Y. Controllable synthesis and photoelectric property of hexagonal SnS2 nanoflakes by Triton X-100 assisted hydrothermal method. Mater. Lett. 2013, 111, 204–207. [Google Scholar] [CrossRef]
- Rasalingam, S.; Wu, C.M.; Koodali, R.T. Modulation of pore sizes of titanium dioxide photocatalysts by a facile template free hydrothermal synthesis method: Implications for photocatalytic degradation of rhodamine B. ACS Appl. Mater. Interfaces 2015, 7, 4368–4380. [Google Scholar] [CrossRef]
- Rangappa, A.P.; Kumar, D.P.; Gopannagari, M.; Reddy, D.A.; Hong, Y.; Kim, Y.; Kim, T.K. Highly efficient hydrogen generation in water using 1D CdS nanorods integrated with 2D SnS2 nanosheets under solar light irradiation. Appl. Surf. Sci. 2020, 508, 144803. [Google Scholar] [CrossRef]
- Zong, X.; Han, J.; Ma, G.; Yan, H.; Wu, G.; Li, C. Photocatalytic H2 evolution on CdS loaded with WS2 as cocatalyst under visible light irradiation. J. Phys. Chem. C 2011, 115, 12202–12208. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Peng, S. Tunable photodeposition of MoS2 onto a composite of reduced graphene oxide and CdS for synergic photocatalytic hydrogen generation. J. Phys. Chem. C 2014, 118, 19842–19848. [Google Scholar] [CrossRef]
- Chang, K.; Mei, Z.; Wang, T.; Kang, Q.; Ouyang, S.; Ye, J. MoS2/graphene cocatalyst for efficient photocatalytic H2 evolution under visible light irradiation. ACS Nano 2014, 8, 7078–7087. [Google Scholar] [CrossRef]
- Yuan, Y.; Lu, H.; Ji, Z.; Zhong, J.; Ding, M.; Chen, D.; Li, Y.; Tu, W.; Cao, D.; Yu, Z.; et al. Enhanced visible-light-induced hydrogen evolution from water in a noble-metal-free system catalyzed by ZnTCPP-MoS2/TiO2 assembly. Chem. Eng. J. 2015, 275, 8–16. [Google Scholar] [CrossRef]
- Liu, E.; Chen, J.; Ma, Y.; Feng, J.; Jia, J.; Fan, J.; Hu, X. Fabrication of 2D SnS2 /g-C3N4 heterojunction with enhanced H2 evolution during photocatalytic water splitting. J. Colloid Interface Sci. 2018, 524, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Xue, X.; Zhang, W.; Li, X.; Liu, J.; Yang, S.; Hu, Y.; Chen, R.; Yan, Y.; Zhu, G.; et al. Well-designed Te/SnS2/Ag artificial nanoleaves for enabling and enhancing visible-light driven overall splitting of pure water. Nano Energy 2017, 39, 539–545. [Google Scholar] [CrossRef]
- Yu, J.; Xu, C.Y.; Ma, F.X.; Hu, S.P.; Zhang, Y.W.; Zhen, L. Monodisperse SnS2 nanosheets for high-performance photocatalytic hydrogen generation. ACS Appl. Mater. Interfaces 2014, 6, 22370–22377. [Google Scholar] [CrossRef] [PubMed]
- Barba-Nieto, I.; Christoforidis, K.C.; Fernández-García, M.; Kubacka, A. Promoting H2 photoproduction of TiO2-based materials by surface decoration with Pt nanoparticles and SnS2 nanoplatelets. Appl. Catal. B Environ. 2020, 277, 119246. [Google Scholar] [CrossRef]







| Material | Preparation Method | Amount of Hydrogen Produced | Ligtht Source | Sacrificial Agent | |
|---|---|---|---|---|---|
| CdS on WS2 | Impregnation–sulfidation | 0.198 mmolh−1 | Visible | Latic acid | [36] |
| Dye-sensitized NiSx on graphene | In situ chemical deposition | 0.34 mmolh−1 | - | - | [9] |
| MoS2 on RGO and CdS | Photoreduction | 0.099 mmolh−1 | Visible | Latic acid | [37] |
| MoS2 on graphene | Hydrothermal | 1.80 mmolh−1 | Visible | Na2S-Na2S2O3 | [38] |
| MoS2 QDs on TiO2 NTA | Electrodeposition | 0.065 mmolcm−2h−1 0.053 mmolcm−2h−1 0.016 mmolcm−2h−1 | UV Visible NIR | - | [19] |
| ZnTCPP-MoS2 on TiO2 | Hydrothermal | 0.010 mmolh−1 | - | triethanolamine (TEOA) | [39] |
| 10 wt. % CoS2 on TiO2 | Hydrothermal | 2.55 mmolg−1 | UV | Methanol | [26] |
| 2D SnS2 on g-C3N4 | Hydrothermal | 0.972 mmolh−1g−1 | Visible | TEOA and H2Pt2Cl6.6H2O | [40] |
| Te/SnS2/Ag | Hydrothermal | 0.332 mmolh−1 | UV–visible | - | [41] |
| SnS2 nanosheets | Solvothermal | 1.06 mmolh−1g−1 | UV–visible | Na2S Na2S2O3 | [42] |
| CdS on SnS2 | Hydrothermal | 20.2 mmolh−1g−1 | UV–visible | Latic acid | [35] |
| Pt nanoparticles on oxide, | Hydrothermal | 10.0 mmolh−1g−1 | UV | [43] | |
| Pt nanoparticles on SnS2 nanoplatelets, and | 9.0 mmolh−1g−1 | ||||
| Pt nanoparticles on SnS2 and oxide | 3.0 mmolh−1g−1 | ||||
| 10 wt. % SnS2 on TiO2 nanocomposite | Hydrothermal | 0.195 mmolg−1 | UV–visible | Methanol |
| Sample | Amount of Hydrogen Evolved (µmolg−1) | Rate Constant of Photodegradation Reaction (×10−4 s−1) | Calculated BandGap Value (eV) |
|---|---|---|---|
| ST-100 | 0.00 | 1.955 ± 0.185 | 1.890 |
| ST-20 | 142.35 | 2.768 ± 0.181 | 1.980 |
| ST-15 | 171.30 | 4.415 ± 0.258 | 2.005 |
| ST-10 | 195.55 | 3.948 ± 0.110 | 2.015 |
| ST-5 | 28.25 | 2.661 ± 0.388 | 2.250 |
| ST-0 | 89.20 | 2.745 ± 0.513 | 3.100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shanmugaratnam, S.; Selvaratnam, B.; Baride, A.; Koodali, R.; Ravirajan, P.; Velauthapillai, D.; Shivatharsiny, Y. SnS2/TiO2 Nanocomposites for Hydrogen Production and Photodegradation under Extended Solar Irradiation. Catalysts 2021, 11, 589. https://doi.org/10.3390/catal11050589
Shanmugaratnam S, Selvaratnam B, Baride A, Koodali R, Ravirajan P, Velauthapillai D, Shivatharsiny Y. SnS2/TiO2 Nanocomposites for Hydrogen Production and Photodegradation under Extended Solar Irradiation. Catalysts. 2021; 11(5):589. https://doi.org/10.3390/catal11050589
Chicago/Turabian StyleShanmugaratnam, Sivagowri, Balaranjan Selvaratnam, Aravind Baride, Ranjit Koodali, Punniamoorthy Ravirajan, Dhayalan Velauthapillai, and Yohi Shivatharsiny. 2021. "SnS2/TiO2 Nanocomposites for Hydrogen Production and Photodegradation under Extended Solar Irradiation" Catalysts 11, no. 5: 589. https://doi.org/10.3390/catal11050589