Polymetallic Group 4 Complexes: Catalysts for the Ring Opening Polymerisation of rac-Lactide
Abstract
:1. Introduction
2. Results and Discussion
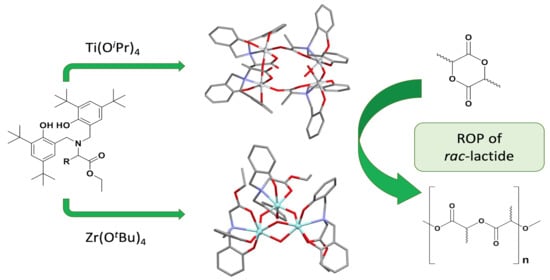
2.1. Synthesis of Ti and Zr Clusters
2.2. Catalytic Activity in the ROP of rac-Lactide
3. Materials and Methods
3.1. General Considerations
3.2. General Procedure for the Synthesis of Ti ABP Complexes 4–6
3.2.1. Data for 4
3.2.2. Data for 5
3.2.3. Data for 6
3.3. Synthesis of Zr ABP Complexes
3.3.1. Data for 7
3.3.2. Data for 8
3.4. General Procedure for the Polymerisation of rac-Lactide
3.5. Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sauer, A.; Kapelski, A.; Fliedel, C.; Dagorne, S.; Kol, M.; Okuda, J. Structurally well-defined group 4 metal complexes as initiators for the ring-opening polymerization of lactide monomers. Dalton Trans. 2013, 42, 9007–9023. [Google Scholar] [CrossRef]
- Thomas, C.M. Stereocontrolled ring-opening polymerization of cyclic esters: Synthesis of new polyester microstructures. Chem. Soc. Rev. 2010, 39, 165–173. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Karroonnirun, O. Ring-opening polymerization of lactides catalyzed by natural amino-acid based zinc catalysts. Inorg. Chem. 2010, 49, 2360–2371. [Google Scholar] [CrossRef]
- Daneshmand, P.; Jiménez-Santiago, J.L.; Aragon-Alberti, M.; Schaper, F. Catalytic-Site-Mediated Chain-End Control in the Polymerization of rac-Lactide with Copper Iminopyrrolide Complexes. Organometallics 2018, 37, 1751–1759. [Google Scholar] [CrossRef]
- Coates, G.W. Precise Control of Polyolefin Stereochemistry Using Single-Site Metal Catalysts. Chem. Rev. 2000, 100, 1223–1252. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Han, F.; Zhang, W.; Ma, W.; Deng, Q.; Song, W.; Yan, H.; Dong, G. Preparation of zirconium and hafnium complexes containing chiral N atoms from asymmetric tertiary amine ligands, and their catalytic properties for polymerization of rac-lactide. Catal. Sci. Technol. 2017, 7, 1394–1403. [Google Scholar] [CrossRef]
- Chmura, A.J.; Davidson, M.G.; Frankis, C.J.; Jones, M.D.; Lunn, M.D. Highly active and stereoselective zirconium and hafnium alkoxide initiators for solvent-free ring-opening polymerization of rac-lactide. Chem. Commun. 2008, 11, 1293–1295. [Google Scholar] [CrossRef]
- Chmura, A.J.; Chuck, C.J.; Davidson, M.G.; Jones, M.D.; Lunn, M.D.; Bull, S.D.; Mahon, M.F. A germanium alkoxide supported by a C3-symmetric ligand for the stereoselective synthesis of highly heterotactic polylactide under solvent-free conditions. Angew. Chem. Int. Ed. 2007, 46, 2280–2283. [Google Scholar] [CrossRef] [PubMed]
- Spassky, N.; Wisniewski, M.; Pluta, C.; Borgne, A.L. Highly stereoelective polymerization of rac-(D,L)-lactide with a chiral schiff’s base/aluminium alkoxide initiator. Macromol. Chem. Phys. 1996, 197, 2627–2637. [Google Scholar] [CrossRef]
- Radano, C.P.; Baker, G.L.; Smith, M.R. Stereoselective Polymerization of a Racemic Monomer with a Racemic Catalyst: Direct Preparation of the Polylactic Acid Stereocomplex from Racemic Lactide. J. Am. Chem. Soc. 2000, 122, 1552–1553. [Google Scholar] [CrossRef]
- Ovitt, T.M.; Coates, G.W. Stereochemistry of Lactide Polymerization with Chiral Catalysts: New Opportunities for Stereocontrol Using Polymer Exchange Mechanisms. J. Am. Chem. Soc. 2002, 124, 1316–1326. [Google Scholar] [CrossRef]
- Chmura, A.J.; Davidson, M.G.; Jones, M.D.; Lunn, M.D.; Mahon, M.F.; Johnson, A.F.; Khunkamchoo, P.; Roberts, S.L.; Wong, S.S.F. Group 4 Complexes with Aminebisphenolate Ligands and Their Application for the Ring Opening Polymerization of Cyclic Esters. Macromolecules 2006, 39, 7250–7257. [Google Scholar] [CrossRef]
- Nomura, N.; Ishii, R.; Akakura, M.; Aoi, K. Stereoselective Ring-Opening Polymerization of Racemic Lactide Using Aluminum-Achiral Ligand Complexes: Exploration of a Chain-End Control Mechanism. J. Am. Chem. Soc. 2002, 124, 5938–5939. [Google Scholar] [CrossRef] [PubMed]
- Zelikoff, A.L.; Kopilov, J.; Goldberg, I.; Coates, G.W.; Kol, M. New facets of an old ligand: Titanium and zirconium complexes of phenylenediamine bis(phenolate) in lactide polymerisation catalysis. Chem. Commun. 2009, 6804–6806. [Google Scholar] [CrossRef] [PubMed]
- Kirk, S.M.; Kociok-Köhn, G.; Jones, M.D. Zirconium vs Aluminum Salalen Initiators for the Production of Biopolymers. Organometallics 2016, 35, 3837–3843. [Google Scholar] [CrossRef]
- Arnold, P.L.; Buffet, J.C.; Blaudeck, R.P.; Sujecki, S.; Blake, A.J.; Wilson, C. C3-symmetric lanthanide tris(alkoxide) complexes formed by preferential complexation and their stereoselective polymerization of rac-lactide. Angew. Chem. Int. Ed. 2008, 47, 6033–6036. [Google Scholar] [CrossRef] [PubMed]
- Jianming, R.; Anguo, X.; Hongwei, W.; Hailin, Y. Review—Recent development of ring-opening polymerization of cyclic esters using aluminum complexes. Des. Monomers Polym. 2013, 17, 345–355. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Zhao, Y.; Santoro, O.; Elsegood, M.R.J.; Bedwell, E.V.; Zahra, K.; Walton, A.; Redshaw, C. Use of titanocalix[4]arenes in the ring opening polymerization of cyclic esters. Catal. Sci. Technol. 2020, 10, 1619–1639. [Google Scholar] [CrossRef]
- Liu, Y.; Dawe, L.N.; Kozak, C.M. Bimetallic and trimetallic zinc amino-bis(phenolate) complexes for ring-opening polymerization of rac-lactide. Dalton Trans. 2019, 48, 13699–13710. [Google Scholar] [CrossRef]
- Nie, K.; Fang, L.; Yao, Y.; Zhang, Y.; Shen, Q.; Wang, Y. Synthesis and characterization of amine-bridged bis(phenolate)lanthanide alkoxides and their application in the controlled polymerization of rac-lactide and rac-beta-butyrolactone. Inorg. Chem. 2012, 51, 11133–11143. [Google Scholar] [CrossRef]
- Su, C.-K.; Chuang, H.-J.; Li, C.-Y.; Yu, C.-Y.; Ko, B.-T.; Chen, J.-D.; Chen, M.-J. Oxo-Bridged Bimetallic Group 4 Complexes Bearing Amine-Bis(benzotriazole phenolate) Derivatives as Bifunctional Catalysts for Ring-Opening Polymerization of Lactide and Copolymerization of Carbon Dioxide with Cyclohexene Oxide. Organometallics 2014, 33, 7091–7100. [Google Scholar] [CrossRef]
- Degée’, P.; Dubois, P.; Jérôme, R.; Jacobsen, S.; Fritz, H.-G. New catalysis for fast bulk ring-opening polymerization of lactide monomers. Macromol. Symp. 1999, 144, 289–302. [Google Scholar] [CrossRef]
- Jeffery, B.J.; Whitelaw, E.L.; Garcia-Vivo, D.; Stewart, J.A.; Mahon, M.F.; Davidson, M.G.; Jones, M.D. Group 4 initiators for the stereoselective ROP of rac-beta-butyrolactone and its copolymerization with rac-lactide. Chem. Commun. 2011, 47, 12328–12330. [Google Scholar] [CrossRef]
- Gendler, S.; Segal, S.; Goldberg, I.; Goldschmidt, Z.; Kol, M. Titanium and zirconium complexes of dianionic and trianionic amine-phenolate-type ligands in catalysis of lactide polymerization. Inorg. Chem. 2006, 45, 4783–4790. [Google Scholar] [CrossRef]
- Cols, J.E.; Taylor, C.E.; Gagnon, K.J.; Teat, S.J.; McIntosh, R.D. Well-defined Ti4 pre-catalysts for the ring-opening polymerisation of lactide. Dalton Trans. 2016, 45, 17729–17738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cols, J.E.P.; Hill, V.G.; Williams, S.K.; McIntosh, R.D. Aggregated initiators: Defining their role in the ROP of rac-lactide. Dalton Trans. 2018, 47, 10626–10635. [Google Scholar] [CrossRef]
- Redshaw, C.; Tang, Y. Tridentate ligands and beyond in group IV metal a-olefin homo-/co-polymerization catalysis. Chem. Soc. Rev. 2012, 41, 4484–4510. [Google Scholar] [CrossRef]
- Fazekas, E.; Jenkins, D.T.; Forbes, A.A.; Gallagher, B.; Rosair, G.M.; McIntosh, R.D. Amino acid-derived bisphenolate palladium complexes as C-C coupling catalysts. 2021. submitted manuscript. [Google Scholar]
- Padmanabhan, S.; Katoa, S.; Nomura, K. Synthesis and Structure of Titanatranes Containing Tetradentate Trianionic Donor Ligands of the Type [(O-2,4-R2C6H2-6-CH2)2(OCH2CH2)]N3- and Their Use in Catalysis for Ethylene Polymerization. Organometallics 2007, 26, 1616–1626. [Google Scholar] [CrossRef]
- Tshuva, E.Y.; Goldberg, I.; Kol, M.; Goldschmidt, Z. Living polymerization and block copolymerization of alpha-olefins by an amine bis(phenolate) titanium catalyst. Chem. Commun. 2001, 20, 2120–2121. [Google Scholar] [CrossRef]
- Olmstead, M.M.; Power, P.P.; Viggiano, M. New class of sigma-bonded aliphatic aza-macrocyclic complexes of transition metals: Synthesis and X-ray crystal structures of nitrogen-bridged [(TiN4C12H24)2] and the oxo-bridged species [(N4C12H25)TiOTi(N4C12H25)]. J. Am. Chem. Soc. 1983, 105, 2927–2928. [Google Scholar] [CrossRef]
- Ugrinova, V.; Ellis, G.A.; Brown, S.N. Remarkable thermodynamic stability toward hydrolysis of tripodal titanium alkoxides. Chem. Commun. 2004, 468–469. [Google Scholar] [CrossRef]
- Nielson, A.J.; Shen, C.; Waters, J.M. Molecular engineering of coordination pockets in chloro-tris-phenoxo complexes of titanium(IV). Polyhedron 2006, 25, 2039–2054. [Google Scholar] [CrossRef]
- Barroso, S.; Madeira, F.; Calhorda, M.J.; Ferreira, M.J.; Duarte, M.T.; Martins, A.M. Toward the understanding of radical reactions: Experimental and computational studies of titanium(III) diamine bis(phenolate) complexes. Inorg. Chem. 2013, 52, 9427–9439. [Google Scholar] [CrossRef] [PubMed]
- Bernardinelli, G.; Seidel, T.M.; Kundig, E.P.; Prins, L.J.; Kolarovic, A.; Mba, M.; Pontini, M.; Licini, G. Stereoselective dimerization of racemic C3-symmetric Ti(IV) amine triphenolate complexes. Dalton Trans. 2007, 16, 1573–1576. [Google Scholar] [CrossRef] [PubMed]
- Kremer, A.B.; Mehrkhodavandi, P. Dinuclear catalysts for the ring opening polymerization of lactide. Coord. Chem. Rev. 2019, 380, 35–57. [Google Scholar] [CrossRef]
- Liang, L.C.; Chien, C.C.; Chen, M.T.; Lin, S.T. Zirconium and hafnium complexes containing N-alkyl-substituted amine biphenolate ligands: Unexpected ligand degradation and divergent complex constitutions governed by N-alkyls. Inorg. Chem. 2013, 52, 7709–7716. [Google Scholar] [CrossRef]
- Toupance, T.; Dubberley, S.R.; Rees, N.H.; Tyrrell, B.R.; Mountford, P. Zirconium Complexes of Diamine−Bis(phenolate) Ligands: Synthesis, Structures, and Solution Dynamics. Organometallics 2002, 21, 1367–1382. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, Y.; Yuan, D.; Yao, Y.; Shen, Q. Zirconium complexes stabilized by amine-bridged bis(phenolato) ligands as precatalysts for intermolecular hydroamination reactions. Dalton Trans. 2015, 44, 20352–20360. [Google Scholar] [CrossRef]
- Chmura, A.J.; Davidson, M.G.; Jones, M.D.; Lunn, M.D.; Mahon, M.F. Group 4 complexes of amine bis(phenolate)s and their application for the ring opening polymerisation of cyclic esters. Dalton Trans. 2006, 7, 887–889. [Google Scholar] [CrossRef]
- Davidson, M.G.; Doherty, C.L.; Johnson, A.L.; Mahon, M.F. Isolation and characterisation of transition and main group metal complexes supported by hydrogen-bonded zwitterionic polyphenolic ligands. Chem. Commun. 2003, 15, 1832–1833. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhao, H.; Wang, L.; Sun, J.; Zhang, Y.; Cao, Z. Immortal ring-opening polymerization of lactides with super high monomer to catalyst ratios initiated by zirconium and titanium complexes containing multidentate amino-bis(phenolate) ligands. New J. Chem. 2017, 41, 5669–5677. [Google Scholar] [CrossRef]
- Hu, M.; Wang, M.; Zhu, H.; Zhang, L.; Zhang, H.; Sun, L. Preparation and structures of enantiomeric dinuclear zirconium and hafnium complexes containing two homochiral N atoms, and their catalytic property for polymerization of rac-lactide. Dalton Trans. 2010, 39, 4440–4446. [Google Scholar] [CrossRef] [Green Version]
- Whitelaw, E.L.; Jones, M.D.; Mahon, M.F.; Kociok-Kohn, G. Novel Ti(IV) and Zr(IV) complexes and their application in the ring-opening polymerisation of cyclic ester. Dalton Trans. 2009, 41, 9020–9025. [Google Scholar] [CrossRef] [PubMed]
- Bisz, E.; Białek, M.; Zarychta, B. Synthesis, characterization and catalytic properties for olefin polymerization of two new dimeric zirconium(IV) complexes having diamine-bis(phenolate) and chloride ligands. Appl. Catal. A 2015, 503, 26–33. [Google Scholar] [CrossRef]
- Forder, T.R.; Mahon, M.F.; Davidson, M.G.; Woodman, T.; Jones, M.D. Synthesis and characterisation of unsymmetrical Zr(IV) amine tris(phenolate) complexes and their application in ROP of rac-LA. Dalton Trans. 2014, 43, 12095–12099. [Google Scholar] [CrossRef] [PubMed]
- Hormnirun, P.; Edward, L.M.; Vernon, C.G.; Robert, I.P.; White, A.J.P. Study of ligand substituent effects on the rate and stereoselectivity of lactide polymerization using aluminum salen-type initiators. Proc. Natl. Acad. Sci. USA 2006, 103, 15343–15348. [Google Scholar]
- Pang, X.; Duan, R.; Li, X.; Chen, X. Bimetallic salen-aluminum complexes: Synthesis, characterization and their reactivity with rac-lactide and ε-caprolactone. Polym. Chem. 2014, 5, 3894–3900. [Google Scholar] [CrossRef]
- Chmura, A.J.; Cousins, D.M.; Davidson, M.G.; Jones, M.D.; Lunn, M.D.; Mahon, M.F. Robust chiral zirconium alkoxide initiators for the room-temperature stereoselective ring-opening polymerisation of rac-lactide. Dalton Trans 2008, 11, 1437–1443. [Google Scholar] [CrossRef]
- McKeown, P.; Davidson, M.G.; Lowe, J.P.; Mahon, M.F.; Thomas, L.H.; Woodman, T.J.; Jones, M.D. Aminopiperidine based complexes for lactide polymerisation. Dalton Trans. 2016, 45, 5374–5387. [Google Scholar] [CrossRef] [Green Version]
- Mandal, M.; Ramkumar, V.; Chakraborty, D. Salen complexes of zirconium and hafnium: Synthesis, structural characterization and polymerization studies. Polym. Chem. 2019, 10, 3444–3460. [Google Scholar] [CrossRef]
- Dubois, P.; Jacobs, C.; Jéróme, R.; Teyssié, P. Macromolecular Engineering of Polylactones and Polylactides. 4. Mechanism and Kinetics of Lactide Homopolymerization by Aluminum Isopropoxide. Macromolecules 1991, 24, 2266–2270. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; Streek, J.v.d. Mercury: Visualization and analysis of crystal structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef] [Green Version]









| Entry | Cat. | [M]:[LA] | T (°C) | Time (h) | Conversion (%) b | Pi c | Mn(calc) d | Mn(obs) | Đ |
|---|---|---|---|---|---|---|---|---|---|
| 1 a | 4 | 1:100 | 130 | 24 | 31 | - | 4600 | - | - |
| 2 a | 5 | 1:100 | 130 | 24 | 41 | - | 6000 | - | - |
| 3 a | 6 | 1:100 | 130 | 24 | 73 | - | 10,600 | - | - |
| 4 | 6 | 1:200 | 130 | 48 | 88 | 0.51 | 22,900 | 3200 | 1.2 |
| 5 | 6 | 1:200 | 130 | 48 | 91 | 0.54 | 25,400 | 5800 | 1.3 |
| 6 a | 6 | 1:100 | 100 | 24 | 10 | - | 1600 | - | - |
| 7 a | 7 | 1:100 | 130 | 24 | 94 | - | 13,700 | - | - |
| 8 a | 7 | 1:100 | 100 | 48 | 94 | 0.59 | 13,700 | - | - |
| 9 | 7 | 1:200 | 100 | 48 | 97 | - | 27,200 | 5000 | 1.5 |
| 10 | 8 | 1:100 | 130 | 24 | 95 | 0.54 | 14,000 | 4200 | 1.4 |
| 11 | 8 | 1:100 | 100 | 24 | 93 | 0.55 | 13,500 | 4200 | 1.2 |
| 12 | 8 | 1:200 | 100 | 48 | 96 | 0.62 | 26,900 | 10,800 | 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jenkins, D.T.; Fazekas, E.; Patterson, S.B.H.; Rosair, G.M.; Vilela, F.; McIntosh, R.D. Polymetallic Group 4 Complexes: Catalysts for the Ring Opening Polymerisation of rac-Lactide. Catalysts 2021, 11, 551. https://doi.org/10.3390/catal11050551
Jenkins DT, Fazekas E, Patterson SBH, Rosair GM, Vilela F, McIntosh RD. Polymetallic Group 4 Complexes: Catalysts for the Ring Opening Polymerisation of rac-Lactide. Catalysts. 2021; 11(5):551. https://doi.org/10.3390/catal11050551
Chicago/Turabian StyleJenkins, David T., Eszter Fazekas, Samuel B. H. Patterson, Georgina M. Rosair, Filipe Vilela, and Ruaraidh D. McIntosh. 2021. "Polymetallic Group 4 Complexes: Catalysts for the Ring Opening Polymerisation of rac-Lactide" Catalysts 11, no. 5: 551. https://doi.org/10.3390/catal11050551