Application of Response Surface Methodology for Preparation of ZnAC2/CAC Adsorbents for Hydrogen Sulfide (H2S) Capture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adsorbent Preparation
2.2. Characterization
2.3. H2S Adsorption Test
2.4. Regeneration of Adsorbents
2.5. Control Factors and Level Selection
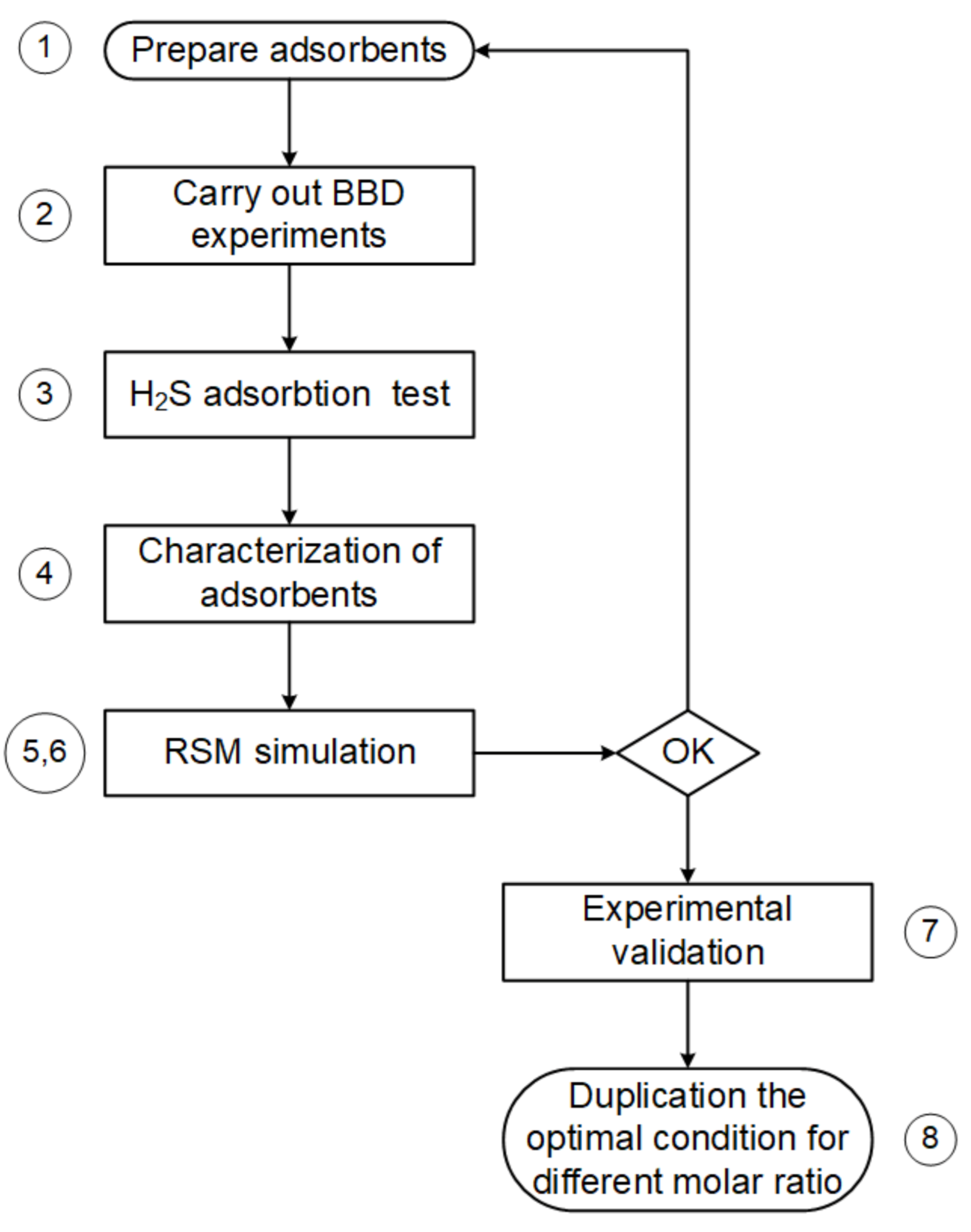
2.6. Steps for Process Parameter Optimization
3. Results and Discussion
3.1. Box–Behnken Model Evaluation
3.2. ANOVA
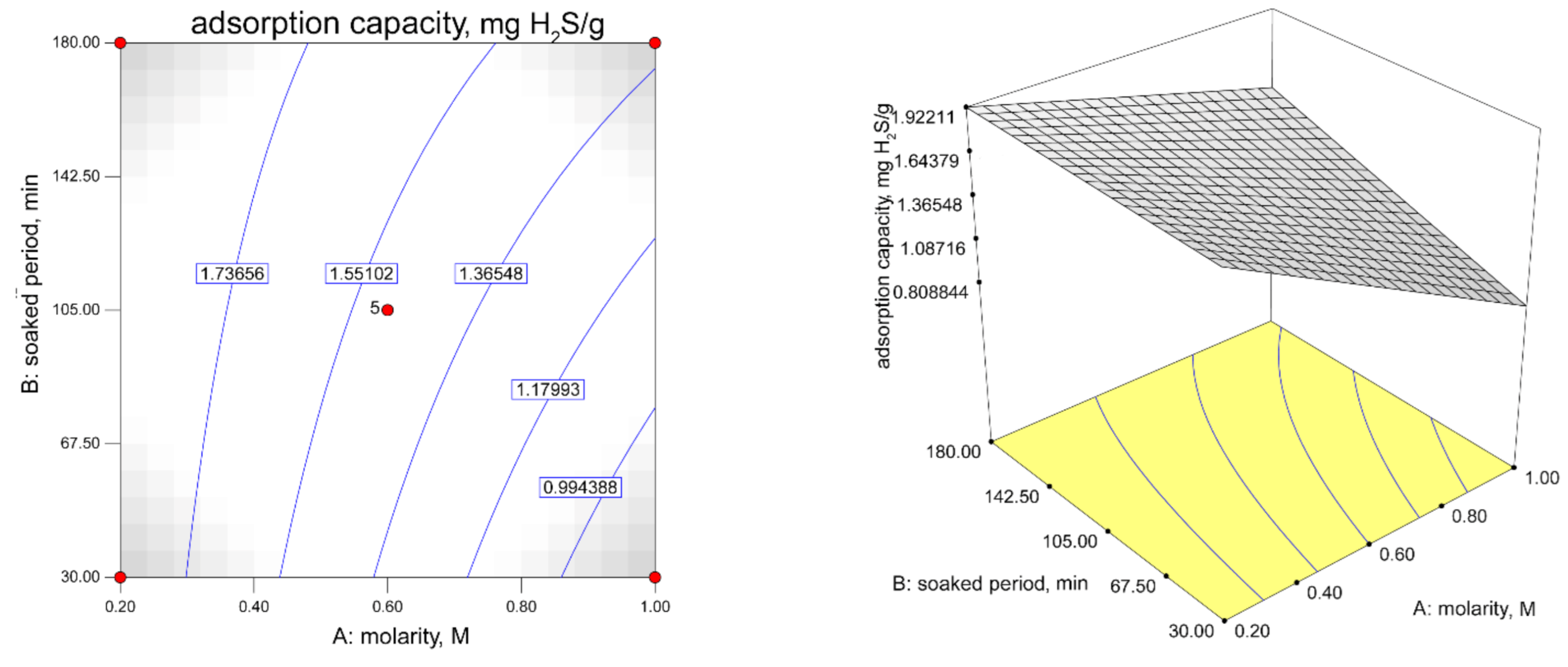
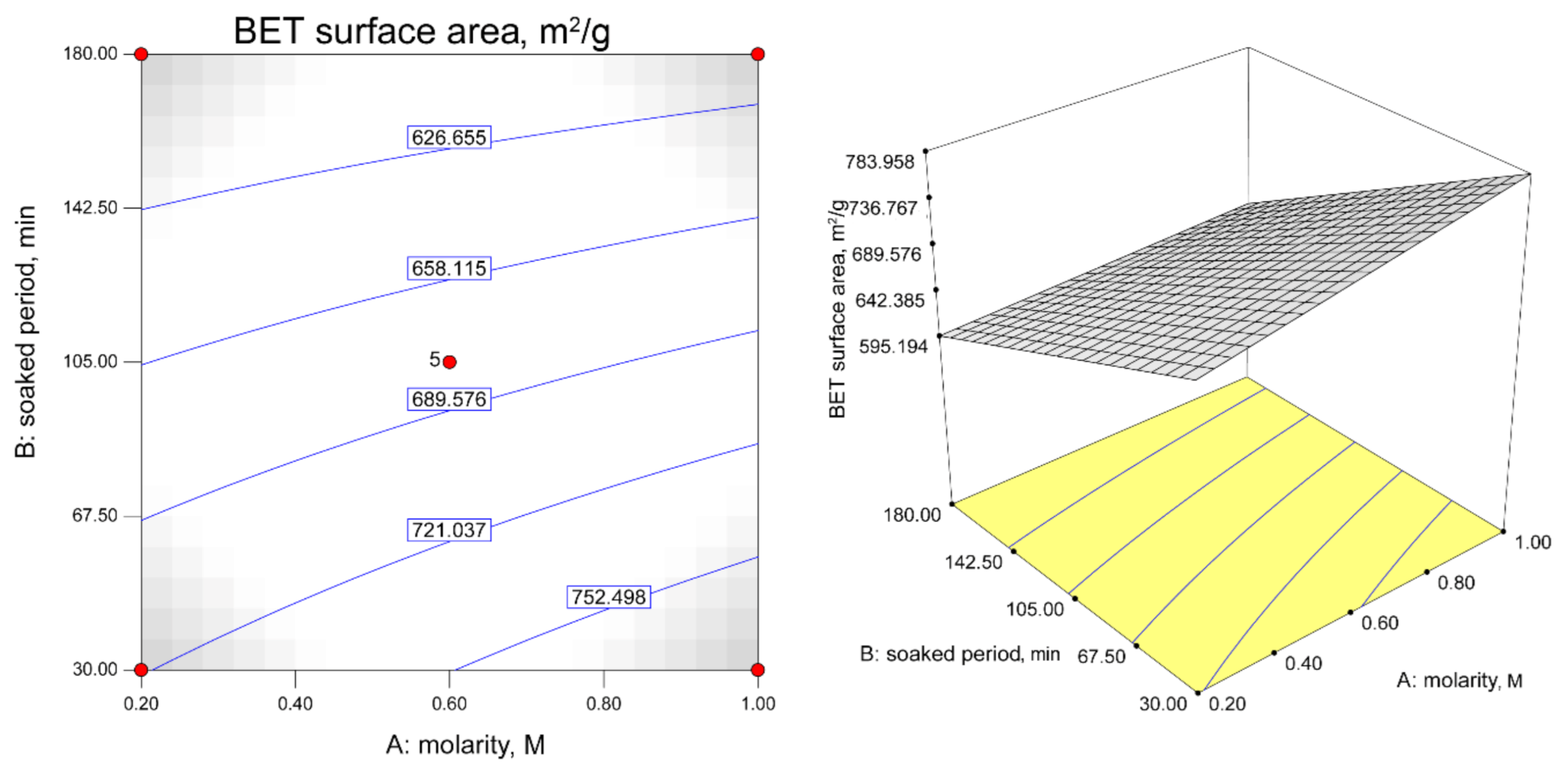
3.3. Contour Plots for H2S Adsorption Capacity and BET Surface Area Responses
3.4. Optimization and Validation
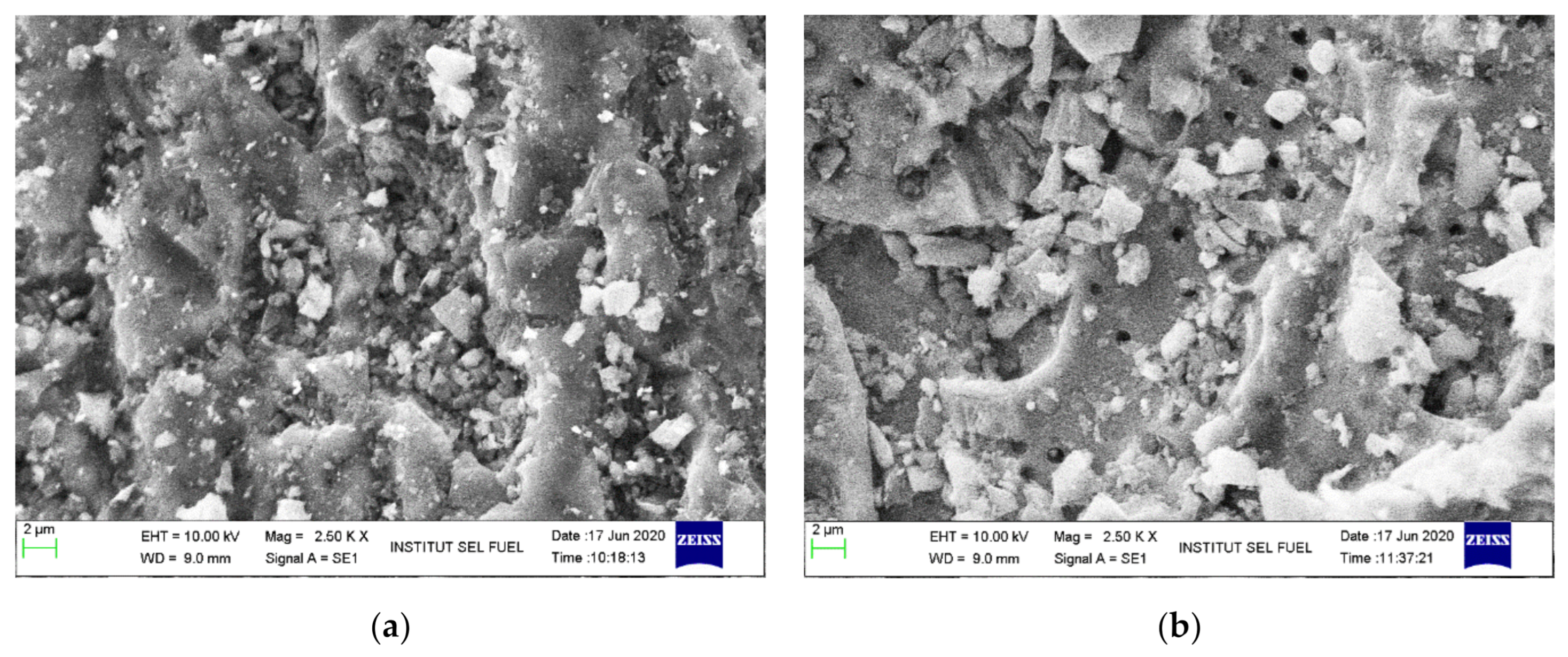
3.5. Adsorbent Characterization
3.6. Performance of Adsorption–Desorption Cycle
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature List
| Encoded value of an independent variable | |
| Actual value of an independent variable | |
| Actual value of a center point’s independent variable | |
| Phase shift value of an independent variable | |
| Y | Response variable |
| Model constant | |
| Linear coefficient | |
| Quadratic coefficient | |
| Interaction coefficient | |
| Statistical error | |
| N | Number of runs |
| k | Number of variables |
| CN | Number of center points |
| MSS | Mean square |
| MSF | Mean square of factors (interactions) |
| MSE | Mean square of errors |
| A | Molarity |
| B | Soaked period |
| C | Soaked temperature |
| Q | Adsorption capacity, mg H2S/g |
| R2 | Coefficient of determination |
| DF | Degree of freedom |
| Prob | Probability |
| PRESS | Predicted residual error sum of squares |
| C.V | Coefficient variation |
| F-value | Fisher’s variance ratio |
| Prob > F | Probability value |
| C | Outlet concentration |
| CO | Inlet concentration |
| BET | Brunauer–Emmett–Teller |
| SEM | Scanning electron microscopy |
| EDX | Energy dispersive X-ray analysis |
| CAC | Commercial coconut activated carbon |
| AC | Activated carbon |
References
- Sitthikhankaew, R.; Chadwick, D.; Assabumrungrat, S.; Laosiripojana, N. Effect of KI and KOH Impregnations over Activated Carbon on H2S Adsorption Performance at Low and High Temperatures. Sep. Sci. Technol. 2014, 49, 354–366. [Google Scholar] [CrossRef]
- Cornejo, C.; Wilkie, A.C. Greenhouse gas emissions and biogas potential from livestock in Ecuador. Energy Sustain. Dev. 2010, 14, 256–266. [Google Scholar] [CrossRef]
- Wongsapai, W.; Thienburanathum, P.; Rerkkriengkrai, P. Biogas situation and development in Thai Swine Farm. Renew. Energy Power Qual. J. 2008, 1, 222–226. [Google Scholar] [CrossRef]
- Tippayawong, N.; Promwungkwa, A.; Rerkkriangkrai, P. Long-term operation of a small biogas/diesel dual-fuel engine for on-farm electricity generation. Biosyst. Eng. 2007, 98, 26–32. [Google Scholar] [CrossRef]
- Tippayawong, N.; Thanompongchart, P. Biogas quality upgrade by simultaneous removal of CO2 and H2S in a packed column reactor. Energy 2010, 35, 4531–4535. [Google Scholar] [CrossRef]
- Arthur, R.; Baidoo, M.F.; Antwi, E. Biogas as a potential renewable energy source: A Ghanaian case study. Renew. Energy 2011, 36, 1510–1516. [Google Scholar] [CrossRef]
- Angelidaki, I.; Ellegaard, L.; Ahring, B.K. Applications of The Anaerobic Digestion Process. Adv. Biochem. Eng. BioTechnol. 2003, 82, 1–33. [Google Scholar]
- Balsamo, M.; Cimino, S.; de Falco, G.; Erto, A.; Lisi, L. ZnO-CuO Supported on Activated Carbon for H2S Removal at Room Temperature. Chem. Eng. J. 2016, 304, 399–407. [Google Scholar] [CrossRef]
- Siriwardane, I.W.; Udangawa, R.; de Silva, R.M.; Kumarasinghe, A.R.; Acres, R.G.; Hettiarachchi, A.; de Silva, K.M.N. Synthesis and Characterization of Nano Magnesium Oxide Impregnated Granular Activated Carbon Composite for H2S Removal Applications. Mater. Des. 2017, 136, 127–136. [Google Scholar] [CrossRef]
- Crisci, A.G.D.; Moniri, A.; Xu, Y. Hydrogen from hydrogen sulfide: Towards a more sustainable hydrogen economy. Int. J. Hydrog. Energy 2019, 44, 1299–1327. [Google Scholar] [CrossRef]
- Zulkefli, N.N.; Masdar, M.S.; Jahim, J.; Harianto, E.H. Overview of H2S Removal Technologies from Biogas Production. Int. J. Appl. Eng. Res. 2016, 11, 10060–10066. [Google Scholar]
- Palma, V.; Vaiano, V.; Barba, D.; Colozzi, M.; Palo, E.; Barbato, L.; Cortese, S. H2S Oxidative Decomposition for The Sim-ultaneous Production of Sulphur and Hydrogen. Chem. Eng. Trans. 2016, 52, 1201–1206. [Google Scholar]
- Bandosz, T.J. On the Adsorption/Oxidation of Hydrogen Sulfide on Activated Carbons at Ambient Temperatures. J. Colloid Interface Sci. 2002, 246, 1–20. [Google Scholar] [CrossRef]
- Sisani, E.; Cinti, G.; Discepoli, G.; Penchini, D.; Desideri, U.; Marmottini, F. Adsorptive Removal of H2S In Biogas Conditions for High Temperature Fuel Cell Systems. Int. J. Hydrog. Energy 2014, 39, 21753–21766. [Google Scholar] [CrossRef]
- Mescia, D.; Hernández, S.; Conoci, A.; Russo, N. MSW landfill biogas desulfurization. Int. J. Hydrog. Energy 2011, 36, 7884–7890. [Google Scholar] [CrossRef]
- Bamdad, H.; Hawboldt, K.; MacQuarrie, S. A review on common adsorbents for acid gases removal: Focus on biochar. Renew. Sustain. Energy Rev. 2018, 81, 1705–1720. [Google Scholar] [CrossRef]
- Khabazipour, M.; Mansoor, A. Removal of Hydrogen Sulfide from Gas Streams Using Porous Materials: A Review. Ind. Eng. Chem. Res. 2019, 58, 22133–22164. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Rouquerol, J.; Rouquerol, F. Adsorption by Powders and Porous Solids; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Galuszka, J.; Iaquaniello, G.; Ciambelli, P.; Palma, V.; Brancaccio, E. Membrane-Assisted Catalytic Cracking of Hydrogen Sulphide (H2S). In Membrane Reactors for Hydrogen Production Processes; Springer: London, UK, 2011; pp. 161–182. [Google Scholar]
- Basile, A.; De Falco, M.; Centi, G.; Iaquaniello, G. Membrane Reactor Engineering: Applications for a Greener Process Industry; John Wiley & Sons: Hoboken, NJ, USA, 2016; p. 344. [Google Scholar]
- Papurello, D.; Tomasi, L.; Silvestri, S.; Santarelli, M. Evaluation of the Wheeler-Jonas parameters for biogas trace compounds removal with activated carbons. Fuel Process. Technol. 2016, 152, 93–101. [Google Scholar] [CrossRef]
- Pelaez-Samaniego, M.R.; Perez, J.F.; Ayiania, M.; Garcia-Perez, T. Chars from wood gasification for removing H2S from biogas. Biomass Bioenergy 2020, 142, 105754. [Google Scholar] [CrossRef]
- Surra, E.; Nogueira, M.C.; Bernardo, M.; Lapa, N.; Esteves, I.; Fonseca, I. New adsorbents from maize cob wastes and anaerobic digestate for H2S removal from biogas. Waste Manag. 2019, 94, 136–145. [Google Scholar] [CrossRef]
- Shah, M.S.; Tsapatsis, M.; Siepmann, J.I. Hydrogen Sulfide Capture: From Absorption in Polar Liquids to Oxide, Zeolite, and Metal–Organic Framework Adsorbents and Membranes. Chem. Rev. 2017, 117, 9755–9803. [Google Scholar] [CrossRef] [PubMed]
- Ozekmekci, M.; Salkic, G.; Fellah, M.F. Use of zeolites for the removal of H2S: A mini-review. Fuel Process. Technol. 2015, 139, 49–60. [Google Scholar] [CrossRef]
- Kerr, G.T.; Johnson, G.C. Catalytic Oxidation of Hydrogen Sulfide to Sulfur Over A Crystalline Aluminosilicate. J. Phys. Chem. 1960, 64, 381–382. [Google Scholar] [CrossRef]
- Steijns, M.; Derks, F.; Verloop, A.; Mars, P. ChemInform Abstract: The Mechanism of the Catalytic Oxidation of Hydrogen Sulfide. II. Kinetics and Mechanism of Hydrogen Sulfide Oxidation Catalyzed by Sulfur. Chem. Inf. 1976, 7, 87–95. [Google Scholar] [CrossRef]
- Bashkova, S.; Baker, F.S.; Wu, X.; Armstrong, T.R.; Schwartz, V. Activated carbon catalyst for selective oxidation of hydrogen sulphide: On the influence of pore structure, surface characteristics, and catalytically-active nitrogen. Carbon 2007, 45, 1354–1363. [Google Scholar] [CrossRef]
- Micoli, L.; Bagnasco, G.; Turco, M. H2S removal from biogas for fuelling MCFCs: New adsorbing materials. Int. J. Hydrog. Energy 2014, 39, 1783–1787. [Google Scholar] [CrossRef]
- Phooratsamee, W.; Hussaro, K.; Teekasap, S.; Hirunlabh, J. Increasing Adsorption of Activated Carbon from Palm Oil Shell for Adsorb H2S From Biogas Production by Impregnation. Am. J. Environ. Sci. 2014, 10, 431–445. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, N.Y.M.; Masdar, M.S.; Isahak, W.N.R.W.; Nordin, D.; Husaini, T.; Majlan, E.H.; Wu, S.Y.; Rejab, S.A.M.; Lye, C.C. Impregnated carbon–ionic liquid as innovative adsorbent for H2/CO2 separation from biohydrogen. Int. J. Hydrog. Energy 2019, 44, 3414–3424. [Google Scholar] [CrossRef]
- Zulkefli, N.N.; Masdar, M.S.; Isahak, W.N.R.W.; Jahim, J.M.; Rejab, S.A.M.; Lye, C.C. Removal of hydrogen sulfide from a biogas mimic by using impregnated activated carbon adsorbent. PLoS ONE 2019, 14, e0211713. [Google Scholar] [CrossRef] [Green Version]
- Sidek, M.Z.; Cheah, Y.J.; Zulkefli, N.N.; Yusuf, N.M.; Isahak, W.N.R.W.; Sitanggang, R.; Masdar, M.S. Effect of impregnated activated carbon on carbon dioxide adsorption performance for biohydrogen purification. Mater. Res. Express 2018, 6, 015510. [Google Scholar] [CrossRef]
- Feng, Y.; Dou, J.; Tahmasebi, A.; Xu, J.; Li, X.; Yu, J.; Yin, F. Regeneration of Fe–Zn–Cu Sorbents Supported on Activated Lignite Char for the Desulfurization of Coke Oven Gas. Energy Fuels 2015, 29, 7124–7134. [Google Scholar] [CrossRef]
- Georgiadis, A.G.; Charisiou, N.D.; Goula, M.A. Removal of hydrogen sulfide from various industrial gases: A review of the most promising adsorbing materials. Catalysts 2020, 10, 521. [Google Scholar] [CrossRef]
- Hunter, G.B.J.; Hunter, J.S. Statistics for Experimenters; John Wiley and Sons: New York, NY, USA, 2005. [Google Scholar]
- Bruns, R.E.; Scarminio, I.S.; Neto, B.B. Statistical Design—Chemometrics; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Massart, D.L.; Vandeginste, B.G.M.; Buydens, L.M.C.; de Jong, S.; Lewi, P.J.; Smeyers-Verbeke, J. Handbook of Chemometrics and Qualimetrics: Part A; Elsevier: Amsterdam, The Netherlands, 1977. [Google Scholar]
- Tong, L.-I.; Chang, Y.-C.; Lin, S.-H. Determining the optimal re-sampling strategy for a classification model with imbalanced data using design of experiments and response surface methodologies. Expert Syst. Appl. 2011, 38, 4222–4227. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 2nd ed.; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2002. [Google Scholar]
- Papurello, D.; Iafrate, C.; Lanzini, A.; Santarelli, M. Trace compounds impact on SOFC performance: Experimental and modelling approach. Appl. Energy 2017, 208, 637–654. [Google Scholar] [CrossRef]
- Kupecki, J.; Papurello, D.; Lanzini, A.; Naumovich, Y.; Motylinski, K.; Blesznowski, M.; Santarelli, M. Numerical model of planar anode supported solid oxide fuel cell fed with fuel containing H2S operated in direct internal reforming mode (DIR-SOFC). Appl. Energy 2018, 230, 1573–1584. [Google Scholar] [CrossRef]
- Papurello, D.; Lanzini, A. SOFC single cells fed by biogas: Experimental tests with trace contaminants. Waste Manag. 2018, 72, 306–312. [Google Scholar] [CrossRef]
- Zulkefli, N.N.; Khaia, T.Z.; Nadaraja, S.; Venugopal, N.R.; Yusri, N.A.M.; Sofian, N.M.; Masdar, M.S. Capabilities dual chemical mixture (DCM) adsorbents through metal anchoring in H2S captured. Solid State Technol. 2020, 63, 181–191. [Google Scholar]
- Baş, D.; Boyacı, I.H. Modeling and optimization I: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, L.; Dilbaghi, N. Optimization of process variables for decolorization of Disperse Yellow 211 by Bacillus subtilis using Box–Behnken design. J. Hazard. Mater. 2009, 164, 1024–1029. [Google Scholar] [CrossRef]
- Wu, L.; Yick, K.-L.; Ng, S.-P.; Yip, J. Application of the Box–Behnken design to the optimization of process parameters in foam cup molding. Expert Syst. Appl. 2012, 39, 8059–8065. [Google Scholar] [CrossRef]
- Moradi, M.; Daryan, J.T.; Mohamadalizadeh, A. Response surface modeling of H2S conversion by catalytic oxidation reaction over catalysts based on SiC nanoparticles using Box−Behnken experimental design. Fuel Process. Technol. 2013, 109, 163–171. [Google Scholar] [CrossRef]
- Dong, C.H.; Xie, X.Q.; Wang, X.L.; Zhan, Y.; Yao, Y.J. Application of Box-Behnken design in optimisation for polysaccharides extraction from cultured mycelium of Cordyceps sinensis. Food Bioprod. Process. 2009, 87, 139–144. [Google Scholar] [CrossRef]
- Sidek, M.Z.; Masdar, M.S.; Nik Dir, N.M.H.; Amran, N.F.A.; Ajit Sing, S.K.D.; Wong, W.L. Integrasi Sistem Penulenan Biohidrogen dan Aplikasi Sel Fuel. J. Kejuruter. 2018, 1, 41–48. [Google Scholar]
- Arifina, R.A.; Hasana, H.A.; Kamarudina, N.H.N.; Ismailb, N.I. Synthesis of Mesoporous Silica for Ammonia Adsorption in Aqueous Solution. J. Kejuruter. 2018, 1, 59–64. [Google Scholar] [CrossRef]
- Nakamura, T.; Kawasaki, N.; Hirata, M.; Oida, Y.; Tanada, S. Adsorption of Hydrogen Sulfide by Zinc-Containing Activated carbon. Toxicol. Environ. Chem. 2002, 82, 93–98. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Al-Musawi, T.J.; Silva, M.F.; Panahi, A.H.; Havangi, M.; Mostafapur, F.K. Photocatalytic degradation of catechol using ZnO nanoparticles as catalyst: Optimizing the experimental parameters using the Box-Behnken statistical methodology and kinetic studies. Microchem. J. 2019, 147, 643–653. [Google Scholar] [CrossRef]
- Pimenta, C.D.; Silva, M.B.; de Morais Campos, R.L.; de Campos, W.R., Jr. Desirability and design of experiments applied to the optimization of the reduction of decarburization of the process heat treatment for steel wire SAE 51B35. Am. J. Theor. Appl. Stat. 2018, 7, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Feng, W.; Kwon, S.; Borguet, E.; Vidic, R. Adsorption of Hydrogen Sulfide onto Activated Carbon Fibers: Effect of Pore Structure and Surface Chemistry. Environ. Sci. Technol. 2005, 39, 9744–9749. [Google Scholar] [CrossRef]
- Keyvanloo, K.; Towfighi, J.; Sadrameli, S.; Mohamadalizadeh, A. Investigating the effect of key factors, their interactions and optimization of naphtha steam cracking by statistical design of experiments. J. Anal. Appl. Pyrolysis 2010, 87, 224–230. [Google Scholar] [CrossRef]
- Sen, R.; Swaminathan, T. Response surface modeling and optimization to elucidate and analyze the effects of inoculum age and size on surfactin production. Biochem. Eng. J. 2004, 21, 141–148. [Google Scholar] [CrossRef]
- Mohamadalizadeh, A.; Towfighi, J.; Rashidi, A.; Manteghian, M.; Mohajeri, A.; Arasteh, R. Nanoclays as nano adsorbent for oxidation of H2S into elemental sulfur. Korean J. Chem. Eng. 2011, 28, 1221–1226. [Google Scholar] [CrossRef]
- Isik-Gulsac, I. Investigation of Impregnated Activated Carbon Properties Used in Hydrogen Sulfide Fine Removal. Braz. J. Chem. Eng. 2016, 33, 1021–1030. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Jirsak, T.; Chaturvedi, S. Reaction of H2S with MgO(100) and Cu/MgO(100) surfaces: Band-gap size and chemical reactivity. J. Chem. Phys. 1999, 111, 8077–8087. [Google Scholar] [CrossRef]
- Preece, D.A.; Montgomery, D.C. Design and Analysis of Experiments. Int. Stat. Rev. 1978, 46, 120. [Google Scholar] [CrossRef] [Green Version]
- Primavera, A.; Trovarelli, A.; Andreussi, P.; Dolcetti, G. The effect of water in the low-temperature catalytic oxidation of hydrogen sulfide to sulfur over activated carbon. Appl. Catal. A Gen. 1998, 173, 185–192. [Google Scholar] [CrossRef]
- Bagreev, A.; Bandosz, T.J. H2S adsorption/oxidation on unmodified activated carbons: Importance of prehumidification. Carbon 2001, 39, 2303–2311. [Google Scholar] [CrossRef]
- Vinodhini, V.; Das, N. Packed bed column studies on Cr (VI) removal from tannery wastewater by neem sawdust. Desalination 2010, 264, 9–14. [Google Scholar] [CrossRef]








| H2S Removal Technologies | Strength | Weakness | Comments |
|---|---|---|---|
| Clauss process [10] |
|
|
|
| Adsorption [14] |
|
|
|
| Absorption (liquid solution) [24] |
|
|
|
| Biological [15,16,17,18] |
|
|
|
| Membrane [19,20] |
|
|
|
| Factors | Level | |||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| A | Molarity (M) | 0.2 | 0.6 | 1.0 |
| B | Soaked period (min) | 30 | 105 | 180 |
| C | Soaked temperature (°C) | 30 | 65 | 100 |
| No. of Run | ZnAc2 Molarity, M (A) | Soaked Period, min (B) | Soaked Temperature, °C (C) | Adsorption Capacity, mg H2S/g (Y1) | BET Surface Area, m2/g (Y2) |
|---|---|---|---|---|---|
| 1 | 0.20 | 105.00 | 30.00 | 1.81 | 842.74 |
| 2 | 1.00 | 105.00 | 30.00 | 2.03 | 584.01 |
| 3 | 0.60 | 105.00 | 65.00 | 0.47 | 544.52 |
| 4 | 0.60 | 30.00 | 30.00 | 0.67 | 765.23 |
| 5 | 1.00 | 105.00 | 100.00 | 0.56 | 757.80 |
| 6 | 0.20 | 30.00 | 65.00 | 1.75 | 692.65 |
| 7 | 0.60 | 105.00 | 65.00 | 1.84 | 737.64 |
| 8 | 1.00 | 180.00 | 65.00 | 1.47 | 698.38 |
| 9 | 1.00 | 30.00 | 65.00 | 0.58 | 825.52 |
| 10 | 0.60 | 30.00 | 100.0 | 2.37 | 198.31 |
| 11 | 0.60 | 180.00 | 30.00 | 1.36 | 694.21 |
| 12 | 0.60 | 105.00 | 65.00 | 1.48 | 726.12 |
| 13 | 0.20 | 180.00 | 65.00 | 2.11 | 612.05 |
| 14 | 0.60 | 105.00 | 65.00 | 2.23 | 473.72 |
| 15 | 0.60 | 180.00 | 100.00 | 1.70 | 485.28 |
| 16 | 0.20 | 105.00 | 100.00 | 2.14 | 555.00 |
| 17 | 0.60 | 105.00 | 65.00 | 0.89 | 731.88 |
| Source | Sum of Squares | DF | Mean Square | F Value | Prob > F | |
| Mean | 38.14 | 1 | 38.14 | Suggested | ||
| Linear | 1.56 | 3 | 0.52 | 1.36 | 0.30 | |
| 2FI | 1.35 | 3 | 0.45 | 1.25 | 0.34 | Suggested |
| Quadratic | 0.15 | 3 | 0.05 | 0.10 | 0.96 | |
| Cubic | 1.47 | 3 | 0.49 | 0.98 | 0.49 | Aliased |
| Residual | 2.00 | 4 | 0.50 | |||
| Total | 44.66 | 17 | 2.63 | |||
| lack of fit | ||||||
| Linear | 2.97 | 9 | 0.33 | 0.66 | 0.72 | |
| 2FI | 1.62 | 6 | 0.267 | 0.54 | 0.76 | Suggested |
| Quadratic | 1.47 | 3 | 0.49 | 0.98 | 0.49 | |
| Cubic | 0 | 0 | Aliased | |||
| Pure error | 2.00 | 4 | 0.50 | |||
| Source | Standard Deviation | R2 | Adjusted R2 | Predicted R2 | PRESS | |
| Linear | 0.62 | 0.24 | 0.06 | −0.29 | 8.41 | |
| 2FI | 0.60 | 0.45 | 0.11 | −0.58 | 10.29 | Suggested |
| Quadratic | 0.70 | 0.47 | −0.21 | −3.08 | 26.64 | |
| Cubic | 0.71 | 0.69 | −0.22 | + | Aliased |
| Source | Sum of Squares | DF | Mean Square | F-Value | F-Value from Table (p = 0.05) | Prob > F | |
|---|---|---|---|---|---|---|---|
| Model | 2.91 | 6 | 0.49 | 1.34 | 3.22 | 0.32 | Not significant |
| A | 1.26 | 1 | 1.26 | 3.48 | 4.96 | 0.09 | |
| B | 0.20 | 1 | 0.20 | 0.57 | 4.96 | 0.47 | |
| C | 0.099 | 1 | 0.099 | 0.27 | 4.96 | 0.61 | |
| AB | 0.070 | 1 | 0.070 | 0.19 | 4.96 | 0.67 | |
| AC | 0.82 | 1 | 0.82 | 2.26 | 4.96 | 0.16 | |
| BC | 0.46 | 1 | 0.46 | 1.29 | 4.96 | 0.28 | |
| Residual | 3.61 | 10 | 0.36 | 0.54 | 2.98 | 0.76 | |
| Lack of fit | 1.62 | 6 | 0.27 | Not significant | |||
| Pure error | 2.00 | 4 | 0.50 | ||||
| Cor total | 6.53 | 16 |
| Source | Result |
|---|---|
| Standard deviation | 0.60 |
| Mean | 1.50 |
| Coefficient variation (%), C.V | 40.14 |
| PRESS | 10.29 |
| R2 | 0.45 |
| Adjusted R2 | 0.11 |
| Prediction R2 | −0.58 |
| Adequate precision | 4.40 |
| Source | Sum of Squares | DF | Mean Square | F-Value | Prob > F | |
| Mean | 7,813,331 | 1 | 7,813,331 | |||
| Linear | 57,607.09 | 3 | 19,202.36 | 1.62 | 0.23 | |
| 2FI | 68,436.96 | 3 | 22,812.32 | 2.65 | 0.11 | Suggested |
| Quadratic | 10,032.71 | 3 | 3344.236 | 0.31 | 0.82 | |
| Cubic | 13,827.22 | 3 | 4609.073 | 0.30 | 0.83 | Aliased |
| Residual | 62,118.94 | 4 | 15,529.74 | |||
| Total | 8,025,354 | 17 | 472,079.7 | |||
| Lack-of-Fit Tests | ||||||
| Source | Squares | DF | Square | Value | Prob > F | |
| Linear | 92,296.88 | 9 | 10,255.21 | 0.66036 | 0.7227 | |
| 2FI | 23,859.93 | 6 | 3976.654 | 0.256067 | 0.9323 | Suggested |
| Quadratic | 13,827.22 | 3 | 4609.073 | 0.29679 | 0.8270 | |
| Cubic | 0 | 0 | Aliased | |||
| Pure error | 62,118.94 | 4 | 15,529.74 | |||
| Source | Standard Deviation | R2 | Adjusted R2 | Predicted R2 | PRESS | |
| Linear | 108.99 | 0.27 | 0.10 | −0.21 | 257,363.7 | |
| 2FI | 92.72 | 0.59 | 0.35 | 0.21 | 167,922.1 | Suggested |
| Quadratic | 104.16 | 0.64 | 0.18 | −0.50 | 318,296.4 | |
| Cubic | 124.62 | 0.71 | -0.17 | + | Aliased | |
| Source | Sum of Squares | DF | Mean Square | F-Value | F-Value from Table (p = 0.05) | Prob > F | |
|---|---|---|---|---|---|---|---|
| Model | 1.3 × 105 | 6 | 2.1 × 104 | 2.44 | 3.22 | 0.10 | Not significant |
| A | 3.3 × 103 | 1 | 3.3 × 103 | 0.39 | 4.96 | 0.55 | |
| B | 4.3 × 104 | 1 | 4.4 × 104 | 5.09 | 4.96 | 0.05 | |
| C | 1.0 × 104 | 1 | 1.0 × 104 | 1.22 | 4.96 | 0.30 | |
| AB | 541.25 | 1 | 541.25 | 0.06 | 4.96 | 0.81 | |
| AC | 5.3 × 104 | 1 | 5.3 × 104 | 6.19 | 4.96 | 0.03 | |
| BC | 1.5 × 104 | 1 | 1.5 × 104 | 1.70 | 4.96 | 0.22 | |
| Residual | 8.6 × 104 | 10 | 8.6 × 103 | 2.98 | |||
| Lack of fit | 2.4 × 104 | 6 | 4.0 × 103 | 0.26 | 0.93 | Not significant | |
| Pure error | 6.2 × 104 | 4 | 1.6 × 104 | ||||
| Cor total | 2.1 × 105 | 16 |
| Source | Result |
|---|---|
| Standard deviation | 92.72 |
| Mean | 677.94 |
| Coefficient variation (%) | 13.68 |
| PRESS | 16800 |
| R2 | 0.59 |
| Adjusted R2 | 0.35 |
| Prediction R2 | 0.21 |
| Adequate precision | 5.10 |
| Adsorbents | C | Ca | Na | K | Zn | O | S |
|---|---|---|---|---|---|---|---|
| CAC (F) | 81.41 | 3.87 | 0.25 | 6.93 | 0.21 | 6.35 | 0.98 |
| ZnAc2/CAC_O (F) | 85.75 | 0.75 | 0.02 | 0.43 | 7.72 | 5.14 | 0.19 |
| ZnAc2/CAC_O (E) | 79.04 | 0.49 | 0.00 | 0.62 | 5.14 | 8.56 | 6.15 |
| ZnAc2/CAC_O (D) | 87.21 | 0.63 | 0.00 | 0.39 | 6.01 | 5.53 | 0.23 |
| ZnAc2/CAC_N (F) | 89.24 | 0.88 | 0.00 | 0.38 | 5.28 | 3.98 | 0.24 |
| ZnAc2/CAC_N (E) | 86.04 | 0.37 | 0.00 | 0.52 | 2.84 | 4.81 | 5.42 |
| ZnAc2/CAC_N (D) | 89.66 | 0.67 | 0.00 | 0.24 | 4.97 | 4.16 | 0.30 |
| Adsorbents | BET Surface Area, m2/g | Total Pore Volume, m3/g (×10−7) | Vmicro/Vtotal (%) | Pore Size, Å |
|---|---|---|---|---|
| ZnAc2/CAC_O (F) | 713.81 | 3.49 | 0.78 | 19.33 |
| ZnAc2/CAC_O (E) | 649.56 | 2.92 | 0.74 | 18.04 |
| ZnAc2/CAC_N (F) | 717.41 | 3.48 | 0.77 | 19.26 |
| ZnAc2/CAC_N (E) | 656.75 | 2.94 | 0.74 | 17.93 |
| Cycle | Adsorption Capacity, mg H2S/g ZnAc2/CAC_O | Adsorption Capacity, mg H2S/g ZnAc2/CAC_N |
|---|---|---|
| 1 | 2.12 | 1.42 |
| 2 | 1.89 | 1.16 |
| 3 | 1.78 | 1.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulkefli, N.N.; Masdar, M.S.; Wan Isahak, W.N.R.; Abu Bakar, S.N.H.; Abu Hasan, H.; Mohd Sofian, N. Application of Response Surface Methodology for Preparation of ZnAC2/CAC Adsorbents for Hydrogen Sulfide (H2S) Capture. Catalysts 2021, 11, 545. https://doi.org/10.3390/catal11050545
Zulkefli NN, Masdar MS, Wan Isahak WNR, Abu Bakar SNH, Abu Hasan H, Mohd Sofian N. Application of Response Surface Methodology for Preparation of ZnAC2/CAC Adsorbents for Hydrogen Sulfide (H2S) Capture. Catalysts. 2021; 11(5):545. https://doi.org/10.3390/catal11050545
Chicago/Turabian StyleZulkefli, Nurul Noramelya, Mohd Shahbudin Masdar, Wan Nor Roslam Wan Isahak, Siti Nur Hatika Abu Bakar, Hassimi Abu Hasan, and Nabilah Mohd Sofian. 2021. "Application of Response Surface Methodology for Preparation of ZnAC2/CAC Adsorbents for Hydrogen Sulfide (H2S) Capture" Catalysts 11, no. 5: 545. https://doi.org/10.3390/catal11050545







