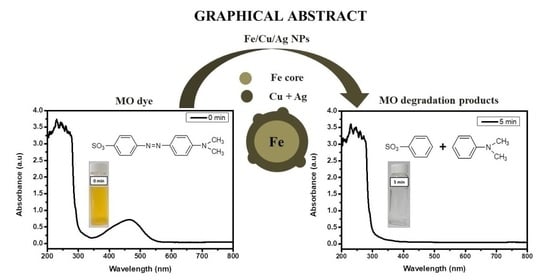
Degradation Kinetics of Methyl Orange Dye in Water Using Trimetallic Fe/Cu/Ag Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
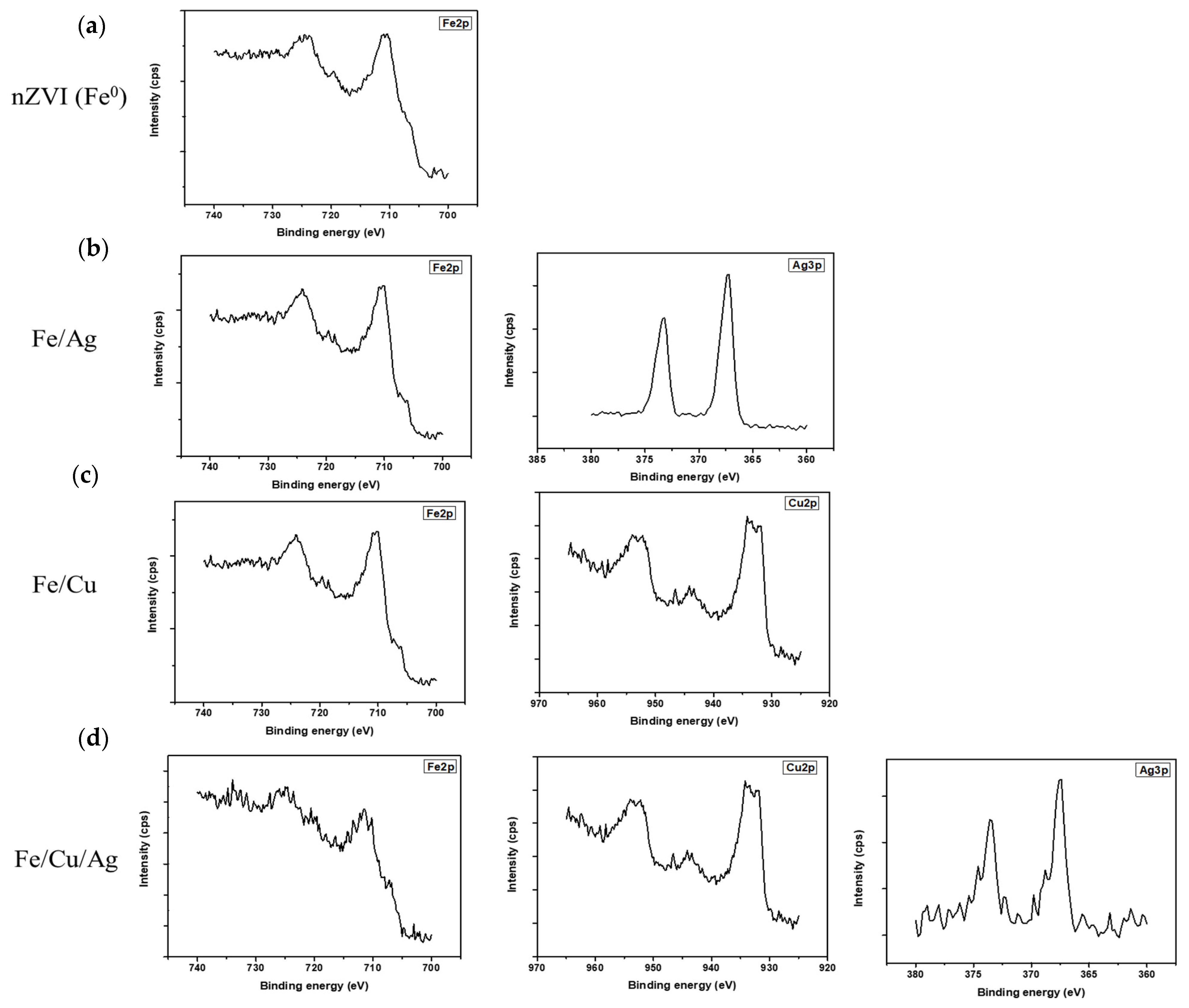
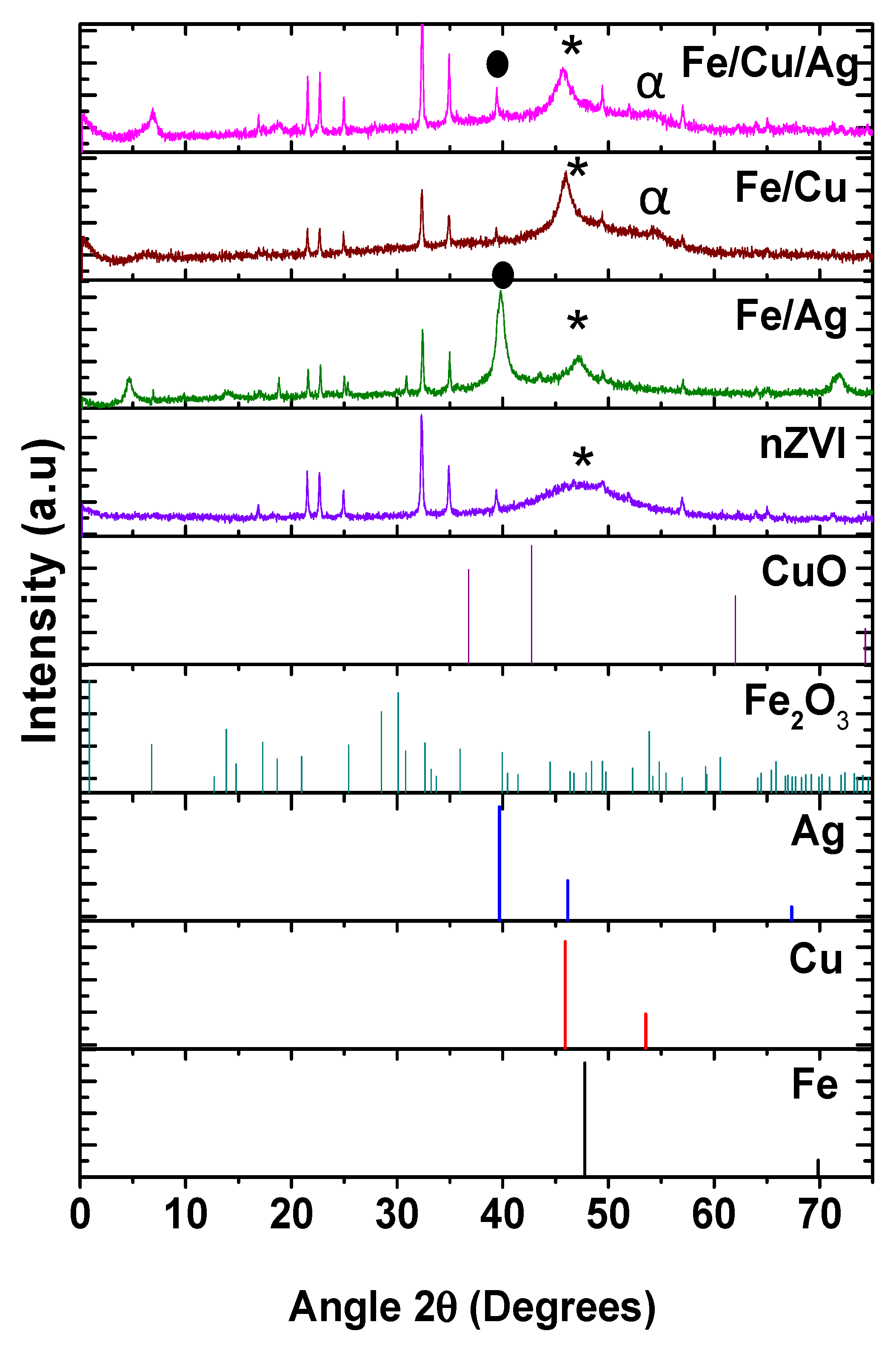
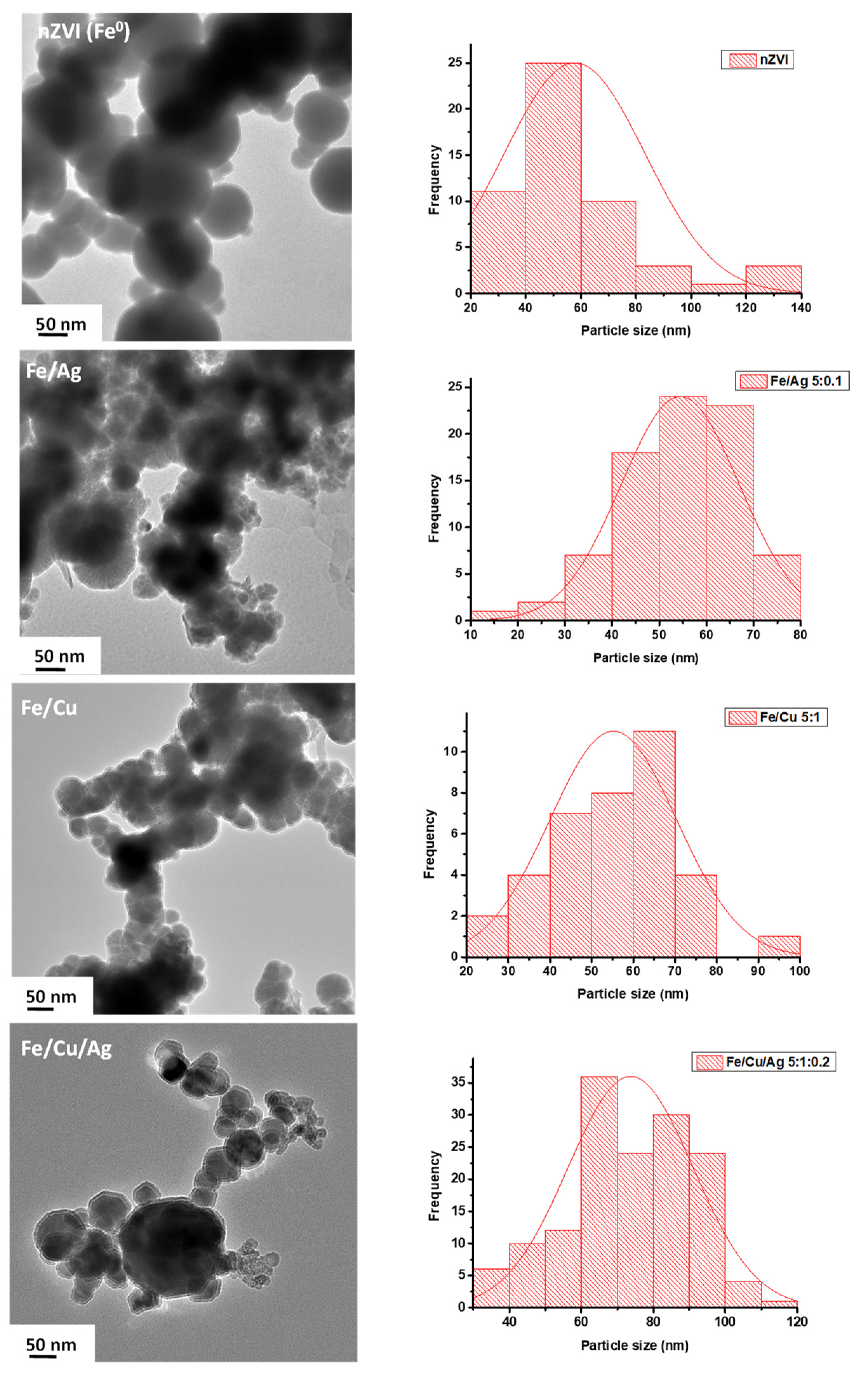
2.1. Material Characterization
2.2. Performance of the Nanoparticles in Methyl Orange Degradation
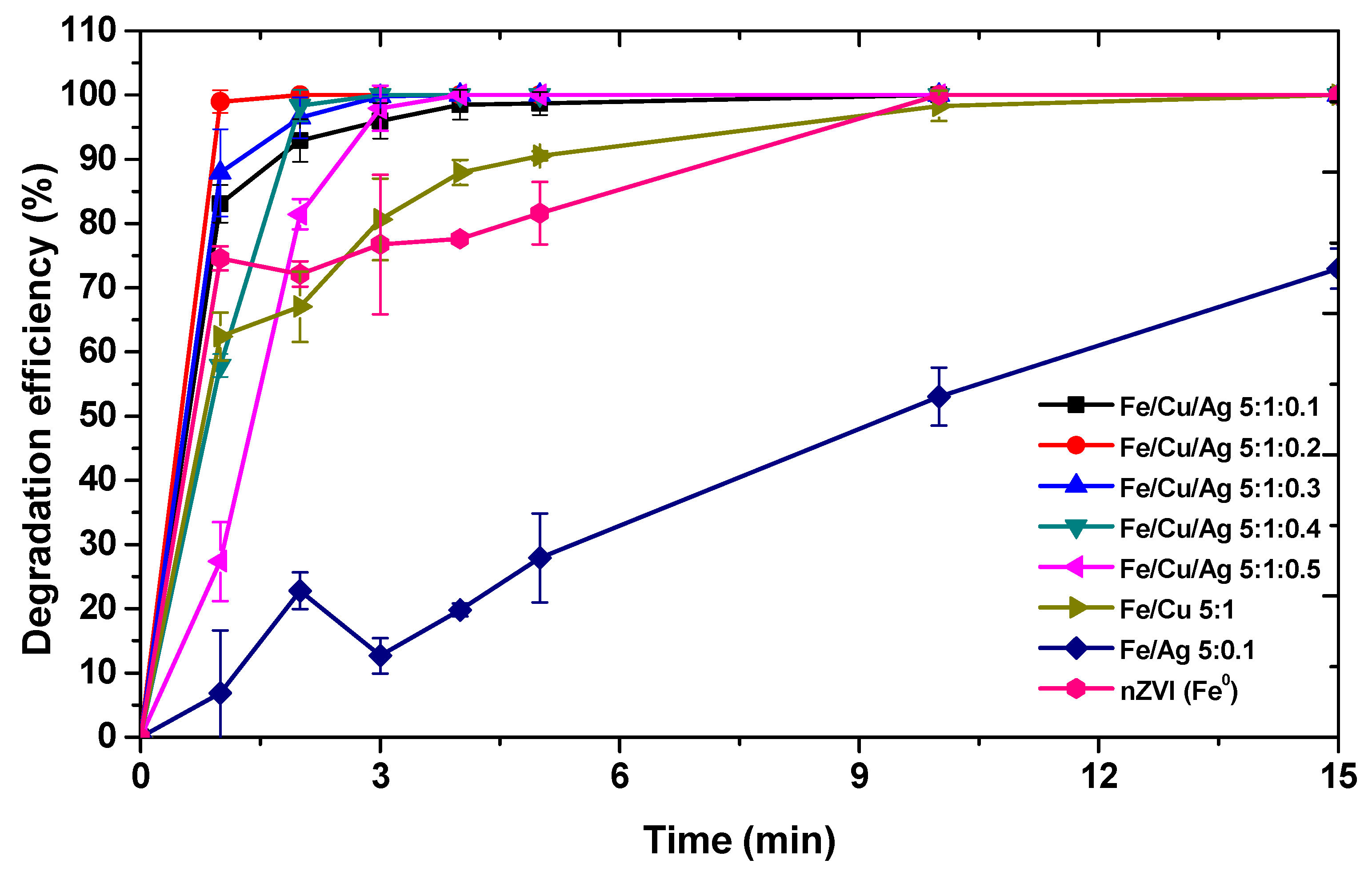
2.2.1. Effect of Catalyst on the Degradation of MO Dye
2.2.2. Parametric Tests on MO Dye Using Fe/Cu/Ag Nanoparticles
Effect of pH
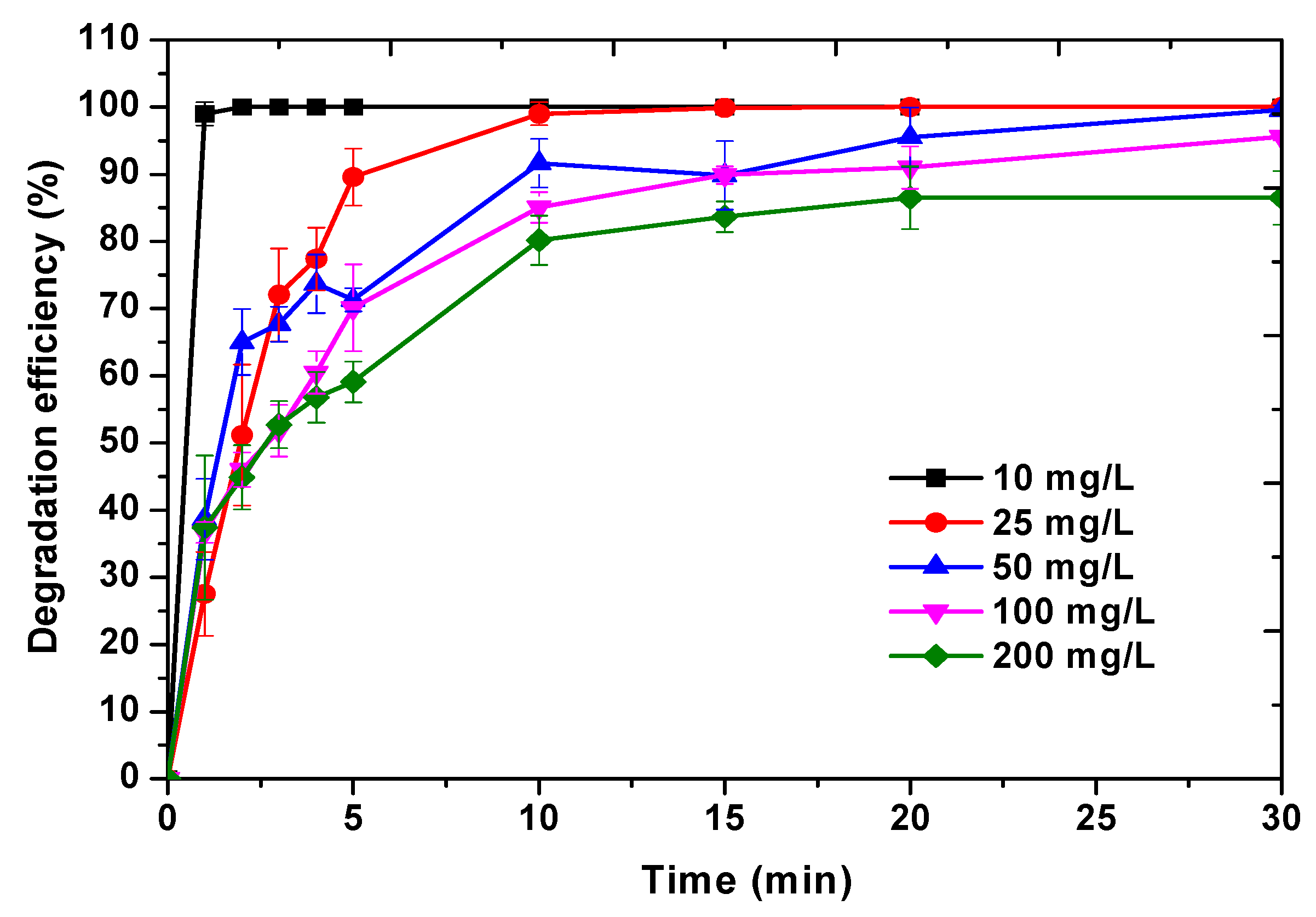
Effect of Initial MO Dye Concentration
Effect of Initial Fe/Cu/Ag Nanoparticle Dosage
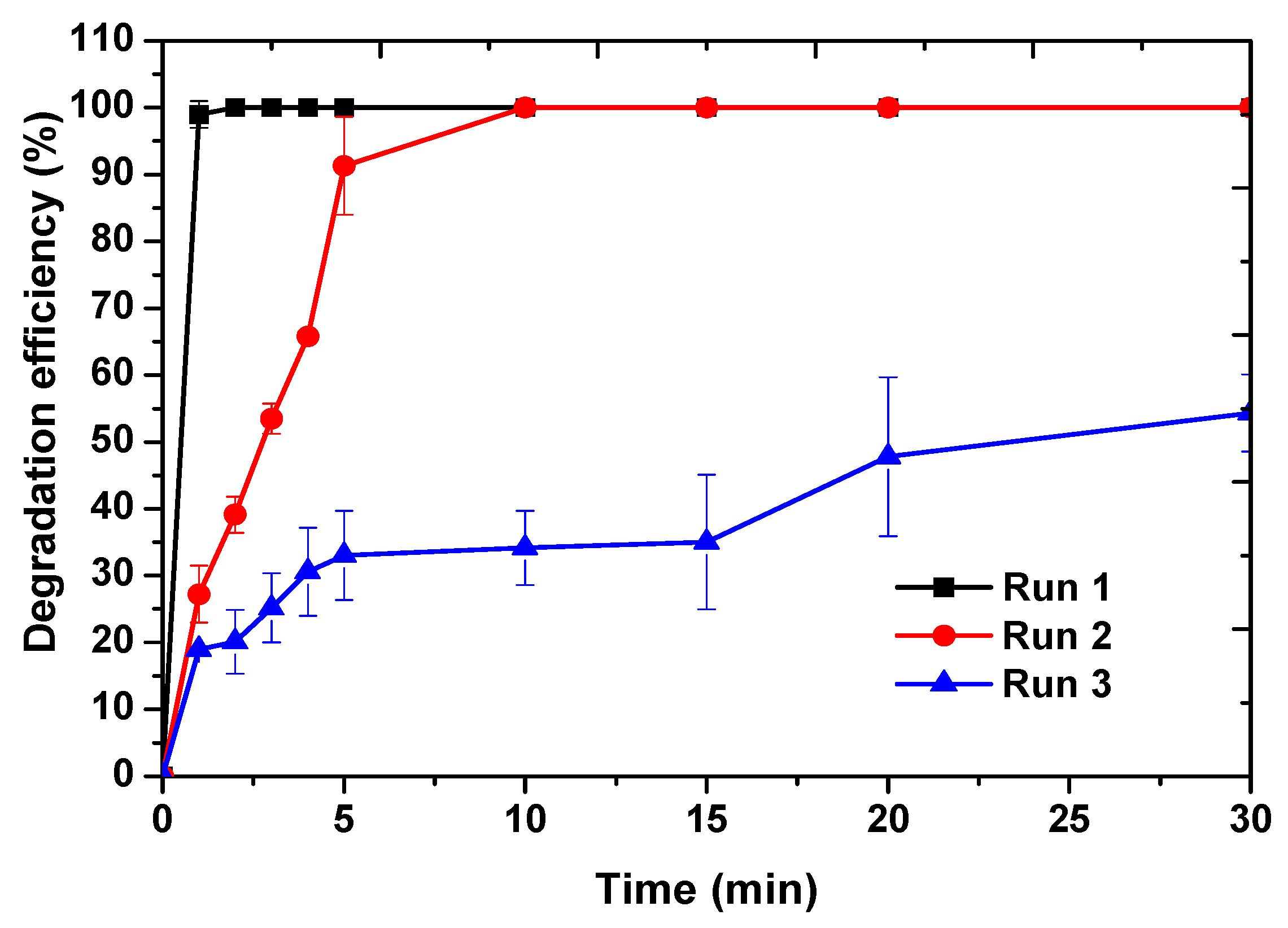
2.3. Catalyst Reusability Studies
2.4. Reaction Kinetics of the Degradation of Methyl Orange
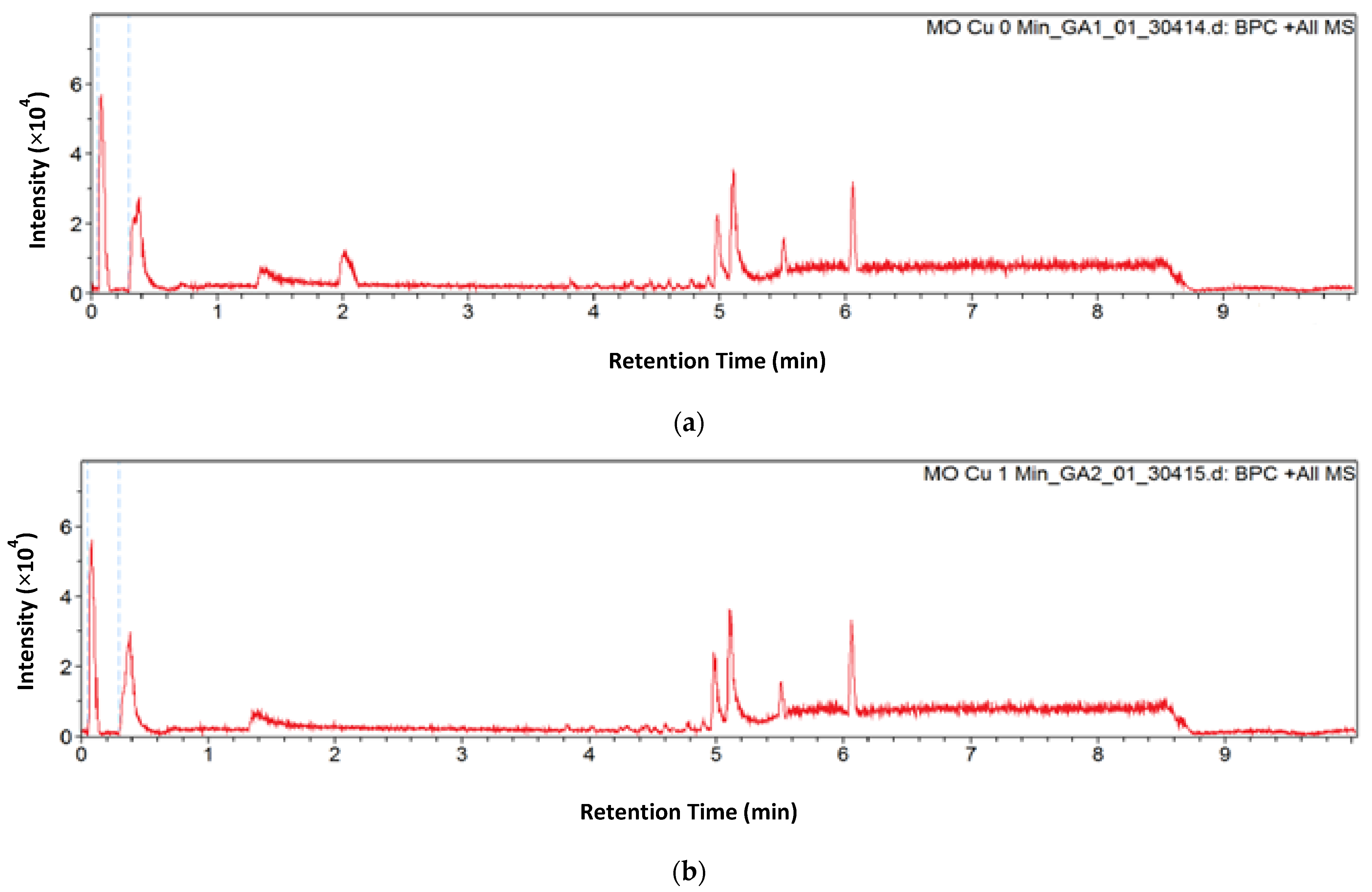
2.5. Degradation Products and Pathway
3. Materials and Methods
3.1. Materials
3.2. Synthesis of the Nanoparticles
3.3. Degradation Studies and Sample Analysis
3.4. Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- El-Sayed, E.M.; Elkady, M.F.; El-Latif, M.M.A. Biosynthesis and characterization of zerovalent iron nanoparticles and its application in azo dye degradation. Indian J. Chem. Technol. 2017, 24, 541–547. [Google Scholar]
- Ahuja, N.; Chopra, A.K.; Ansari, A.A. Removal of Colour from aqueous solutions by using zero valent iron nanoparticles. IOSR J. Environ. Sci. Toxicol. Food Technol. 2016, 10, 4–14. [Google Scholar]
- Nandhini, N.T.; Rajeshkumar, S.; Mythili, S. The possible mechanism of eco-friendly synthesized nanoparticles on hazardous dyes degradation. Biocatal. Agric. Biotechnol. 2019, 19, 101–138. [Google Scholar] [CrossRef]
- Gupta, V.K.; Jain, R.; Nayak, A.; Agarwal, S.; Shrivastava, M. Removal of the hazardous dye- Tartrazine by photodegradation on titanium dioxide surface. Mater. Sci. Eng. C 2011, 31, 1062–1067. [Google Scholar] [CrossRef]
- Liu, H.L.; Chiou, Y.R. Optimal decolorization efficiency of Reactive Red 239 by UV/TiO2 photocatalytic process coupled with response surface methodology. Chem. Eng. J. 2005, 112, 173–179. [Google Scholar] [CrossRef]
- Asouhidou, D.D.; Triantafyllidis, K.S.; Lazaridis, N.K.; Matis, K.A.; Kim, S.S.; Pinnavaia, T.J. Sorption of reactive dyes from aqueous solutions by ordered hexagonal and disordered mesoporous carbons. Microp. Mesop. Mater. 2009, 117, 257–267. [Google Scholar] [CrossRef]
- Alver, E.; Metin, A.Ü. Anionic dye removal from aqueous solutions using modified zeolite: Adsorption kinetics and isotherm studies. Chem. Eng. J. 2012, 200–202, 59–67. [Google Scholar] [CrossRef]
- Xingu-Contreras, E.; García-Rosales, G.; Cabral-Prietoa, A.; García-Sosa, I. Degradation of methyl orange using iron boride nanoparticles supported in a natural zeolite. Environ. Nanotechnol. Monit. Manag. 2017, 7, 121–129. [Google Scholar] [CrossRef]
- Rahman, N.; Abedin, Z.; Hossain, M.A. Rapid degradation of azo dyes using nano-scale zero valent iron. J. Environ. Sci. 2014, 10, 157–163. [Google Scholar] [CrossRef]
- Freyria, F.S.; Esposito, S.; Armandi, M.; Deorsola, F.; Garrone, E.; Bonelli, B. Role of pH in the Aqueous Phase Reactivity of Zerovalent Iron Nanoparticles with Acid Orange 7, a Model Molecule of Azo Dyes. J. Nanomater. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Raman, C.D.; Kanmani, S. Textile dye degradation using nano zero valent iron: A review. J. Environ. Manag. 2016, 177, 341–355. [Google Scholar] [CrossRef]
- Chatterjee, S.; Lim, S.R.; Woo, S.H. Removal of Reactive Black 5 by zero-valent iron modified with various surfactants. Chem. Eng. J. 2010, 160, 27–32S. [Google Scholar] [CrossRef]
- Lai, B.; Zhou, Y.X.; Yang, P. Passivation of sponge iron and GAC in Fe0/GAC mixed-potential corrosion reactor. Ind. Eng. Chem. Res. 2012, 51, 7777–7785. [Google Scholar] [CrossRef]
- Yuan, Y.; Yuan, D.; Zhang, Y.; Lai, B. Exploring the mechanism and kinetics of Fe-Cu-Ag trimetallic particles for p-nitrophenol reduction. Chemosphere 2017, 186, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Thakur, R.; Malwal, H.; Manuja, A.; Chaudhury, A. Biosynthesis of biocompatible and recyclable silver/iron and gold/iron coreshell nanoparticles for water purification technology. Biocatal. Agric. Biotechnol. 2018, 14, 189–197. [Google Scholar] [CrossRef]
- Bokare, A.D.; Chikare, R.C.; Rode, C.V.; Paknikar, K.M. Effect of surface chemistry of Fe-Ni nanoparticles on mechanistic pathways of azo dye degradation. Environ. Sci. Technol. 2007, 41, 7437–7443. [Google Scholar] [CrossRef]
- Smuleac, V.; Varma, R.; Sikdar, S.; Bhattacharyya, D. Green Synthesis of Fe and Fe/Pd bimetallic nanoparticles in membranes for reductive degradation of chlorinated organics. J. Memb. Sci. 2011, 379, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Kirichenko, O.; Kapustin, G.; Nissenbaum, V.; Mishin, I.; Kustov, L. Evaluation of stability of silica-supported Fe-Pd and Fe-Pt nanoparticles in aerobic conditions using thermal analysis. J. Therm. Anal. Calorim. 2014, 118, 749–758. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, H.; Zhao, G. Iron-copper bimetallic nanoparticles embedded within ordered mesoporous carbon as effective and stable heterogeneous Fenton catalyst for the degradation of organic contaminants. Appl. Catal. B Environ. 2015, 164, 396–406. [Google Scholar] [CrossRef]
- Ruíz-Baltazar, A.; Esparza, A.R.; Pérez, R.; Rosas, G. Structural characterization of Fe-Ag bimetallic nanoparticles synthesized by chemical reduction. Int. Res. J. Pure Appl. Chem. 2014, 4, 263–269. [Google Scholar] [CrossRef]
- Mossa Hosseini, S.; Ataie-Ashtiani, B.; Kholghi, M. Nitrate reduction by nano-Fe/Cu particles in packed column. Desalination 2011, 276, 214–221. [Google Scholar] [CrossRef]
- Li, T.; Farrell, J. Reductive Dechlorination of trichloroethene and carbon tetrachloride using iron and palladized-iron cathodes. Environ. Sci. Technol. 2000, 34, 173–179. [Google Scholar] [CrossRef]
- Bransfield, S.J.; Cwiertny, D.M.; Livi, K.; Fairbrother, D.H. Influence of transition metal additives and temperature on the rate of organohalide reduction by granular iron: Implications for reaction mechanisms. Appl. Catal. B Environ. 2007, 76, 348–356. [Google Scholar] [CrossRef]
- El-shafei, M.M.; Hamdy, A.; Hefny, M.M. Zero-valent iron nanostructures: Synthesis, characterization and application. J. Environ. Biotechnol. 2018, 7, 1–10. [Google Scholar]
- Zhang, Z.; Ji, Y.; Li, J.; Zhong, Z.; Su, F. Synergistic effect in bimetallic copper–silver (CuxAg) nanoparticles enhances silicon conversion in Rochow reaction. RSC Adv. 2015, 5, 54364–54371. [Google Scholar] [CrossRef]
- Mahmoud, A.S.; Ismail, A.; Mostafa, M.K.; Mahmoud, M.S.; Ali, W.; Shawky, A.M. Isotherm and kinetic studies for heptachlor removal from aqueous solution using Fe/Cu nanoparticles, artificial intelligence, and regression analysis. Sep. Sci. Technol. 2020, 55, 684–696. [Google Scholar] [CrossRef]
- Al-Namil, D.S.; El Khoury, E.; Patra, D. Solid-state green synthesis of Ag NPs: Higher temperature harvests larger Ag NPs but smaller size has better catalytic reduction reaction. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, J.; Chang, Y.Y.; Koduru, J.R.; Yang, J.K. Potential degradation of methylene blue (MB) by nano metallic particles: A kinetic study and possible mechanism of MB degradation. Environ. Eng. Res. 2018, 23, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Desalegn, B.; Megharaj, M.; Chen, Z.; Naidu, R. Green synthesis of zero valent iron nanoparticle using mango peel extract and surface characterization using XPS and GC-MS. Heliyon 2019, 5, e01750. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, C.; Hussain, I.; Li, C.; Li, J.; Sun, X.; Shen, J.; Han, W.; Wang, L. Iron–copper bimetallic nanoparticles supported on hollow mesoporous silica spheres: The effect of Fe/Cu ratio on heterogeneous Fenton degradation of a dye. RSC Adv. 2016, 6, 54623–54635. [Google Scholar] [CrossRef]
- Zhan, S.; Li, C.; Tian, H.; Ma, C.; Liu, H.; Luo, J.; Li, M. Synthesis, characterization and dye removal behavior of core–shell–shell Fe3O4/Ag/Polyoxometalates ternary nanocomposites. Nanomaterials 2019, 9, 1255. [Google Scholar] [CrossRef] [Green Version]
- Miyama, T.; Yonezawa, Y. Photoinduced formation and aggregation of silver nanoparticles at the surface of carboxymethyl cellulose films. J. Nano Res. 2004, 6, 457–465. [Google Scholar] [CrossRef]
- Han, L.; Xue, S.; Zhao, S.; Yan, Y.; Qian, L.; Chen, M. Biochar supported nanoscale iron particles for the efficient removal of methyl orange dye in aqueous solutions. PLoS ONE 2015, 10, e0132067. [Google Scholar] [CrossRef]
- Luo, S.; Yang, S.; Wang, X.; Sun, C. Reductive degradation of tetrabromobisphenol A over iron–silver bimetallic nanoparticles under ultrasound radiation. Chemosphere 2010, 79, 672–678. [Google Scholar] [CrossRef]
- Tengku Sallehudin, T.A.; Abu Seman, M.N.; Tuan Chik, S.M.S. Preparation and characterization silver nanoparticle embedded polyamide nanofiltration (NF) membrane. MATEC Web Conf. 2018, 150, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Akbari, B.; Tavandashti, M.P.; Zandrahimi, M. Particle size characterization of nanoparticles–A practical approach. Iran. J. Mater. Sci. Eng. 2011, 8, 48–56. [Google Scholar]
- Wang, J.; Liu, C.; Li, J.; Luo, R.; Hu, X.; Sun, X.; Shen, J.; Han, W.; Wang, L. In-situ incorporation of iron-copper bimetallic particles in electrospun carbon nanofibers as an efficient Fenton catalyst. Appl. Catal. B Environ. 2017, 207, 316–325. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Tong, L.; Li, J.; Luo, R.; Qi, J.; Li, Y.; Wang, L. Iron–copper bimetallic nanoparticles supported on hollow mesoporous silica spheres: An effective heterogeneous Fenton catalyst for orange II degradation. RSC Adv. 2015, 5, 69593–69605. [Google Scholar] [CrossRef]
- Falicov, L.M.; Somorjai, G.A. Correlation between catalytic activity and bonding and coordination number of atoms and molecules on transition metal surfaces: Theory and experimental evidence. Proc. Natl. Acad. Sci. USA 1985, 82, 2207–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Sun, G.; Cao, T. Surface chemistry of group IB metals and related oxides. Chem. Soc. Rev. 2017, 46, 1977–2000. [Google Scholar] [CrossRef]
- Bond, G. Periodic variations in the catalytic properties of metals: The influence of solid state parameters on adsorption and catalysis. Platin. Met. Rev. 1968, 12, 100–105. [Google Scholar]
- Ren, G.Q.; Pei, G.X.; Ren, Y.J.; Liu, K.P.; Chen, Z.Q.; Yang, J.Y.; Su, Y.; Liu, X.Y.; Li, W.Z.; Zhang, T. Effect of group IB metals on the dehydrogenation of propane to propylene over anti-sintering Pt/MgAl2O4. J. Catal. 2018, 366, 115–126. [Google Scholar] [CrossRef]
- Ghauch, A.; Assi, H.A.; Baydoun, H.; Tuqan, A.M.; Bejjani, A. Fe0-based trimetallic systems for the removal of aqueous diclofenac: Mechanism and kinetics. Chem. Eng. J. 2011, 172, 1033–1044. [Google Scholar] [CrossRef]
- Zin, M.T.; Borja, J.; Hinode, H.; Kurniawan, W. Synthesis of Bimetallic Fe/Cu nanoparticles with different copper loading ratios. Int. J. Chem. Nucl. Metall. Mater. Eng. 2013, 7, 669–673. [Google Scholar]
- Wang, T.; Su, J.; Jin, X.; Chen, Z.; Megharaj, M.; Naidu, R. Functional clay supported bimetallic nZVI/Pd nanoparticles used for removal of methyl orange from aqueous solution. J. Hazard. Mater. 2013, 262, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.; Lin, P.Y. FePt nanoparticles as heterogeneous Fenton-like catalysts for hydrogen peroxide decomposition and the decolorization of methylene blue. J. Nano Res. 2012, 14, 956. [Google Scholar] [CrossRef]
- Fathima, J.B.; Pugazhendhi, A.; Oves, M.; Venis, R. Synthesis of eco-friendly copper nanoparticles for augmentation of catalytic degradation of organic dyes. J. Mol. Liq. 2018, 260, 1–8. [Google Scholar] [CrossRef]
- Li, P.; Song, Y.; Wang, S.; Tao, Z.; Yu, S.; Liu, Y. Enhanced decolorization of methyl orange using zero-valent copper nanoparticles under assistance of hydrodynamic cavitation. Ultrason. Sonochem. 2015, 22, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.G.; Shyamala, R. Photocatalytic activity of SnO2–a-Fe2O3 composite mixtures: Exploration of number of active sites, turnover number and turnover frequency. Mater. Chem. Front. 2018, 2, 796–806. [Google Scholar] [CrossRef]
- Zhou, J.; Duan, X.; Ye, L.; Zheng, J.; Li, M.M.J.; Tsang, S.C.E.; Yuan, Y. Enhanced chemoselective hydrogenation of dimethyl oxalate to methyl glycolate over bimetallic Ag–Ni/SBA-15 catalysts. Appl. Catal. A Gen. 2015, 505, 344–353. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Wang, F.; Deng, L.; Ren, J.; Jiao, Z.; Zhou, G. Effect of surface composition and structure of the mesoporous Ni/KIT-6 catalyst on catalytic hydrodeoxygenation performance. Catalysts 2019, 9, 889. [Google Scholar] [CrossRef] [Green Version]
- Bernard, P.; Stelmachowski, P.; Broś, P.; Makowski, W.; Kotarba, A. Demonstration of the influence of specific surface area on reaction rate in heterogeneous catalysis. J. Chem. Educ. 2021, 98, 935–940. [Google Scholar] [CrossRef]
- Kondrat, S.S.; van Bokhoven, J.A. A perspective on counting catalytic active sites and rates of reaction using X-ray spectroscopy. Top. Catal. 2019, 62, 1218–1227. [Google Scholar] [CrossRef] [Green Version]
- Weckhuysen, B.M. Determining the active site in a catalytic process: Operando spectroscopy is more than a buzzword. Phys. Chem. Chem. Phys. 2003, 5, 4351–4360. [Google Scholar] [CrossRef]
- Valiyeva, G.G.; Bavasso, I.; Palma, L.D.; Hajiyeva, S.R.; Ramazanov, M.A.; Hajiyeva, F.V. Synthesis of Fe/Ni bimetallic nanoparticles and application to the catalytic removal of nitrates from water. Nanomaterials 2019, 9, 1130. [Google Scholar] [CrossRef] [Green Version]
- Sui, C.; Xing, L.H.; Cai, X.; Wang, Y.; Zhou, Q.; Li, M. Co-supported CeO2 nanoparticles for CO catalytic oxidation: Effects of different synthesis methods on catalytic performance. Catalysts 2020, 10, 243. [Google Scholar] [CrossRef] [Green Version]
- Sarathy, V.; Tratnyek, P.G.; Nurmi, J.T.; Baer, D.R.; Amonette, J.E.; Chun, C.L.; Penn, R.L.; Reardon, E.J. Aging of iron nanoparticles in aqueous solution: Effects on structure and reactivity. J. Phys. Chem. C 2008, 112, 2286–2293. [Google Scholar] [CrossRef]
- Shih, Y.H.; Tso, C.P.; Tung, L.Y. Rapid degradation of Methyl Orange with nanoscale zerovalent iron particles. J. Environ. Eng. Manag. 2010, 20, 137–143. [Google Scholar]
- Chen, Z.X.; Jin, X.Y.; Chen, Z.; Megharaj, M.; Naidu, R. Removal of methyl orange from aqueous solution using bentonite-supported nanoscale zero-valent iron. J. Colloid Interface Sci. 2011, 363, 601–607. [Google Scholar] [CrossRef]
- Shen, Z.; Liu, D.; Dong, X.; Shi, J.; Ma, Y.; Fan, J.; Zhang, L. Nitrate reduction using iron and copper bimetallic nanoparticles supported by chelating resin: Effect of solution chemistry, mechanism, and regeneration. J. Environ. Eng. 2020, 146, 04020011. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous catalyst deactivation and regeneration: A review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Gao, S.; Zhang, G.; Zhang, X. Enhanced adsorption of phosphate from aqueous solution by nanostructured iron(III)–copper(II) binary oxides. Chem. Eng. J. 2014, 235, 124–131. [Google Scholar] [CrossRef]
- Sharif, H.M.A.; Mahmood, A.; Cheng, H.Y.; Djellabi, R.; Ali, J.; Jiang, W.L.; Wang, S.S.; Haider, M.R.; Mahmood, N.; Wang, A.J. Fe3O4 nanoparticles coated with EDTA and Ag nanoparticles for the catalytic reduction of organic dyes from wastewater. ACS Appl. Nano Mater. 2019, 2, 5310–5319. [Google Scholar] [CrossRef]
- Navalon, S.; Alvaro, M.; Garcia, H. Heterogeneous Fenton catalysts based on clays, silicas and zeolites. Appl. Catal. B Environ. 2010, 99, 1–26. [Google Scholar] [CrossRef]
- Corbett, J.F. Pseudo first-order kinetics. J. Chem. Educ. 1972, 49, 663. [Google Scholar] [CrossRef]
- Lin, Y.T.; Weng, C.H.; Chen, F.Y. Effective removal of AB24 dye by nano/micro-size zero-valent iron. Sep. Purif. Technol. 2008, 64, 26–30. [Google Scholar] [CrossRef]
- Lai, B.; Chen, Z.; Zhou, Y.; Yang, P.; Wang, J.; Chen, Z. Removal of high concentration p-nitrophenol in aqueous solution by zero valent iron with ultrasonic irradiation (US-ZVI). J. Hazard. Mater. 2013, 250–251, 220–228. [Google Scholar] [CrossRef]
- Herath, H.M.D.R.; Shaw, P.N.; Cabot, P.; Hewavitharana, A.K. Effect of ionization suppression by trace impurities in mobile phase water on the accuracy of quantification by high-performance liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 1502–1506. [Google Scholar] [CrossRef]
- Waters Corporation US. Controlling Contamination in LC/MS Systems Best Practices. Available online: https://www.waters.com/webassets/cms/support/docs/715001307d_cntrl_cntm.pdf (accessed on 2 May 2020).
- Xie, S.; Huang, P.; Kruzic, J.J.; Zeng, X.; Qian, H. A highly efficient degradation mechanism of methyl orange using Fe-based metallic glass powders. Sci. Rep. 2016, 6, 21947. [Google Scholar] [CrossRef] [Green Version]
- Sarvari, H.; Goharshadi, E.K.; Samiee, S.; Ashraf, N. Removal of methyl orange from aqueous solutions by ferromagnetic Fe/Ni nanoparticles. Phys. Chem. Res. 2018, 6, 433–446. [Google Scholar]
- Lai, B.; Ji, Q.; Yuan, Y.; Yuan, D.; Zhou, Y.; Wang, J. Degradation of ultrahigh concentration pollutant by Fe/Cu bimetallic system at high operating temperature. Korean J. Chem. Eng. 2016, 33, 207–215. [Google Scholar] [CrossRef]
- Shafi, P.M.; Bose, A.C. Impact of crystalline defects and size on X-ray line broadening: A phenomenological approach for tetragonal SnO2 nanocrystals. AIP Adv. 2015, 5, 057137. [Google Scholar] [CrossRef]








| Sample | Element | Peak Binding Energy (eV) | Atomic % |
|---|---|---|---|
| nZVI (Fe0) | C | 288.4 | 41.75 |
| O | 529.6 | 53.59 | |
| Fe | 713.9 | 3.47 | |
| Fe/Ag | C | 287.9 | 40.96 |
| O | 528.6 | 51.42 | |
| Fe | 713.6 | 6.20 | |
| Ag | 371.07 | 1.42 | |
| Fe/Cu | C | 288.4 | 41.99 |
| O | 533.6 | 59.96 | |
| Fe | 713.9 | 3.32 | |
| Cu | 940.9 | 2.73 | |
| Fe/Cu/Ag | C | 283.6 | 50.5 |
| O | 533.6 | 45.58 | |
| Fe | 713.6 | 1.68 | |
| Ag | 376.3 | 0.22 | |
| Cu | 936.3 | 2.02 |
| Catalyst | Degradation Efficiency (%) | SBET b (m2/g) | Number of Active Sites (Moles) | TON a | TOF a (min−1) |
|---|---|---|---|---|---|
| nZVI (Fe0) | 72.13 | 47,092 | 3.60 × 10−5 | 3.0631 | 0.2042 |
| Fe/Cu 5:1 | 67.06 | 69,893 | 3.66 × 10−5 | 2.7949 | 0.1863 |
| Fe/Ag 5:0.1 | 22.79 | 51,952 | 3.64 × 10−5 | 0.9561 | 0.0637 |
| Fe/Cu/Ag 5:1:0.1 | 92.92 | 92,690 | 3.70 × 10−5 | 3.8345 | 0.2556 |
| Fe/Cu/Ag 5:1:0.2 | 100.00 | 104,420 | 3.74 × 10−5 | 4.0879 | 0.2725 |
| Fe/Cu/Ag 5:1:0.3 | 96.51 | 381,328 | 3.77 × 10−5 | 3.9095 | 0.2606 |
| Fe/Cu/Ag 5:1:0.4 | 98.36 | 397,073 | 3.80 × 10−5 | 3.9498 | 0.2633 |
| Fe/Cu/Ag 5:1:0.5 | 81.44 | 388,790 | 3.84 × 10−5 | 3.2428 | 0.2162 |
| Parameters | R2 | kobs (min−1) | |
|---|---|---|---|
| pH | 3 a | 0.897 | 0.7177 |
| 6 | 0.996 | 0.3930 | |
| 9 | 0.873 | 0.2353 | |
| Nanoparticle dosage (mg) | 4 | 0.934 | 0.1371 |
| 7 | 0.987 | 0.2408 | |
| 10 a | 0.865 | 0.6663 | |
| Initial dye concentrations (mg/L) | 10 a | 0.865 | 0.6663 |
| 25 | 0.995 | 0.4576 | |
| 50 | 0.901 | 0.1798 | |
| 100 | 0.964 | 0.1714 | |
| 200 | 0.941 | 0.1420 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kgatle, M.; Sikhwivhilu, K.; Ndlovu, G.; Moloto, N. Degradation Kinetics of Methyl Orange Dye in Water Using Trimetallic Fe/Cu/Ag Nanoparticles. Catalysts 2021, 11, 428. https://doi.org/10.3390/catal11040428
Kgatle M, Sikhwivhilu K, Ndlovu G, Moloto N. Degradation Kinetics of Methyl Orange Dye in Water Using Trimetallic Fe/Cu/Ag Nanoparticles. Catalysts. 2021; 11(4):428. https://doi.org/10.3390/catal11040428
Chicago/Turabian StyleKgatle, Masaku, Keneiloe Sikhwivhilu, Gebhu Ndlovu, and Nosipho Moloto. 2021. "Degradation Kinetics of Methyl Orange Dye in Water Using Trimetallic Fe/Cu/Ag Nanoparticles" Catalysts 11, no. 4: 428. https://doi.org/10.3390/catal11040428