Eucalyptol: A Bio-Based Solvent for the Synthesis of O,S,N-Heterocycles. Application to Hiyama Coupling, Cyanation, and Multicomponent Reactions
Abstract
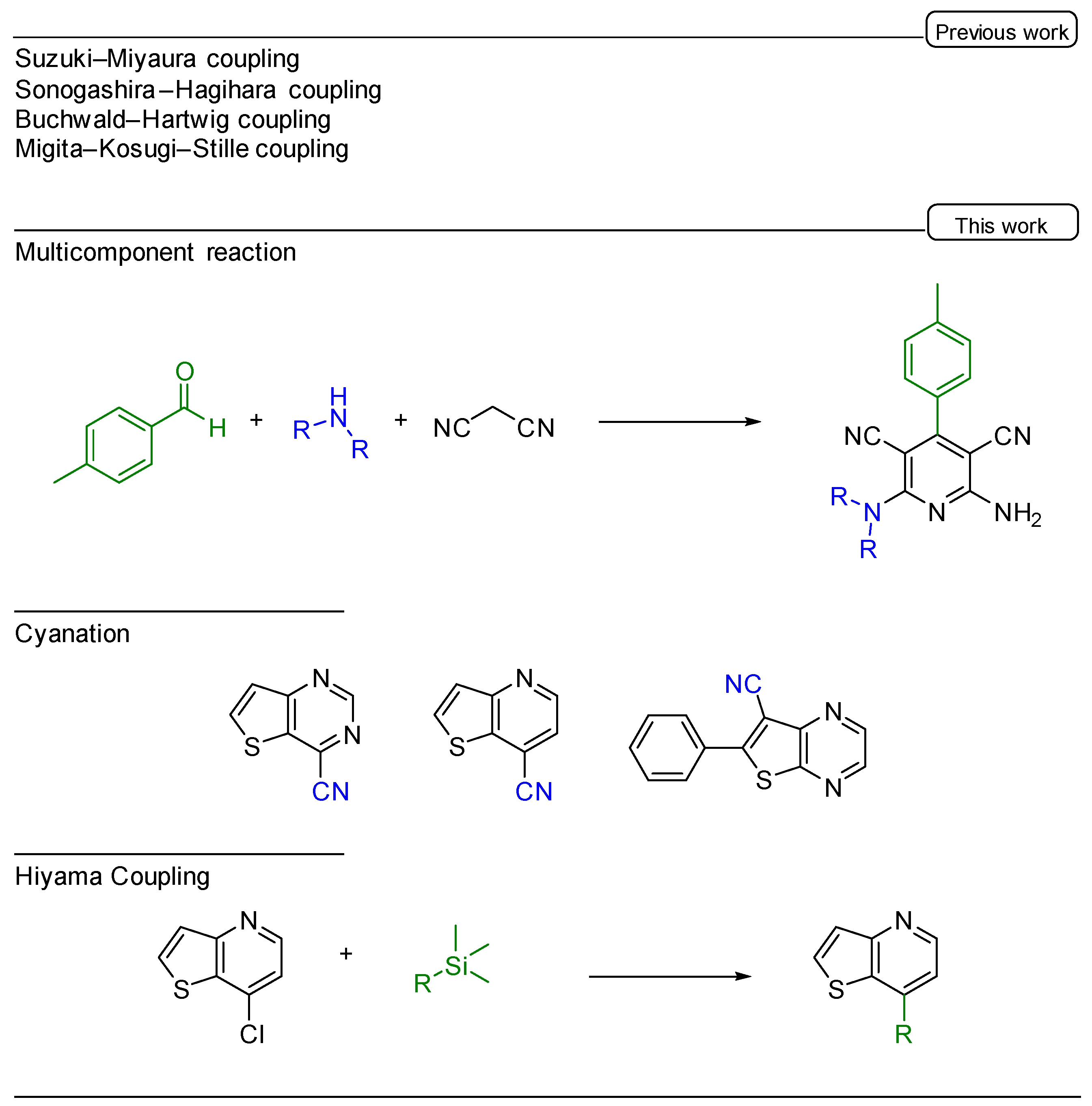
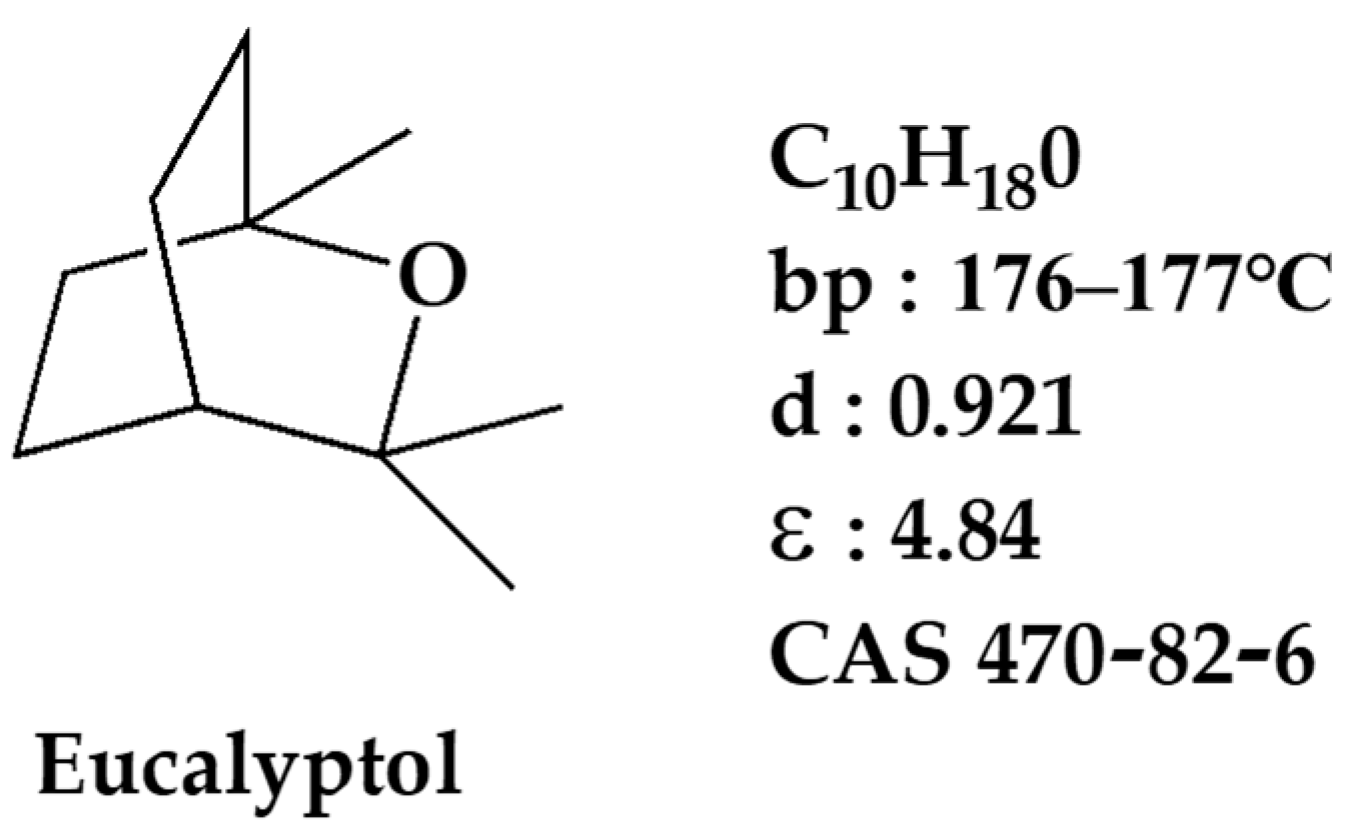
:1. Introduction
2. Results and Discussion
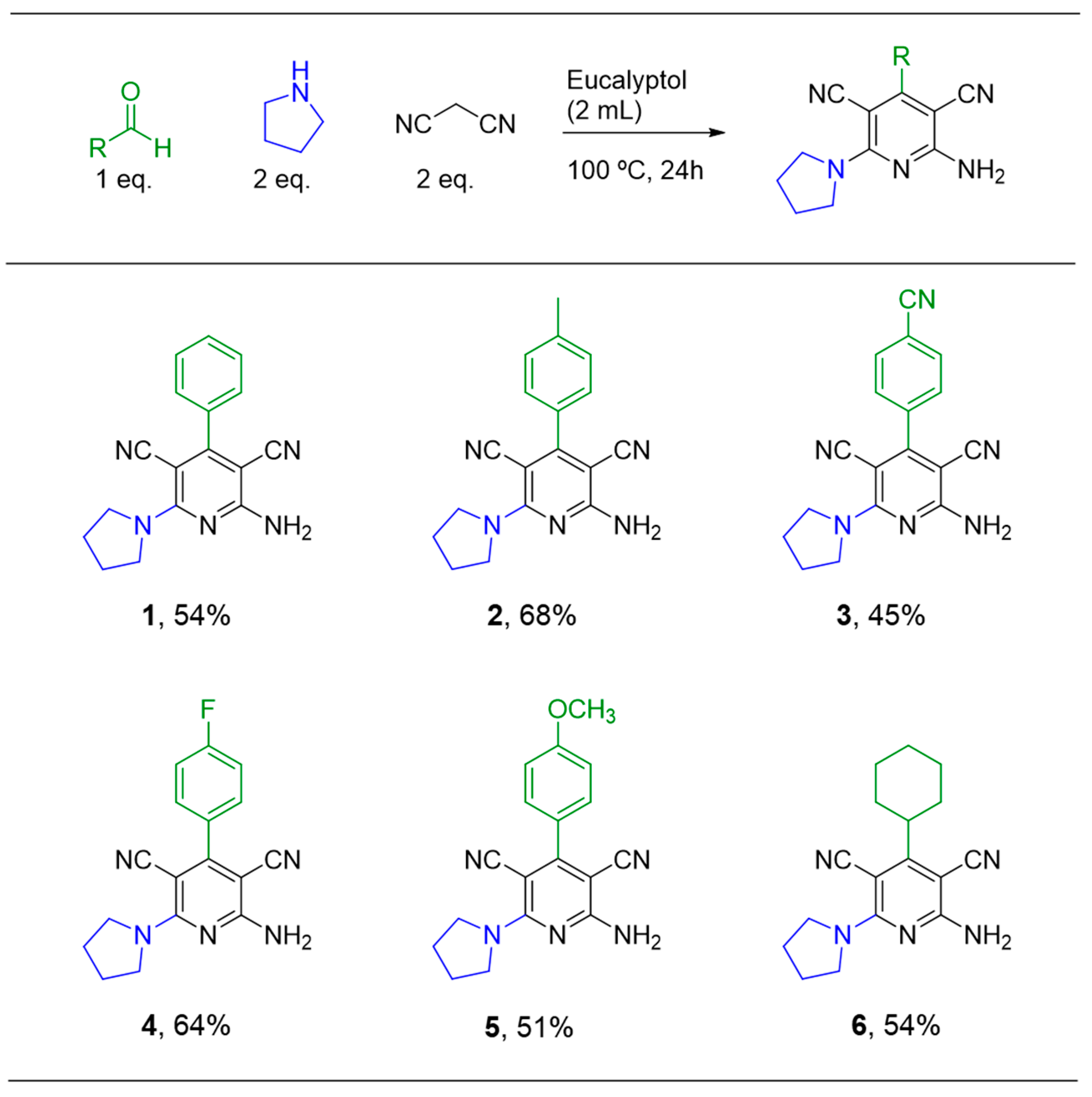
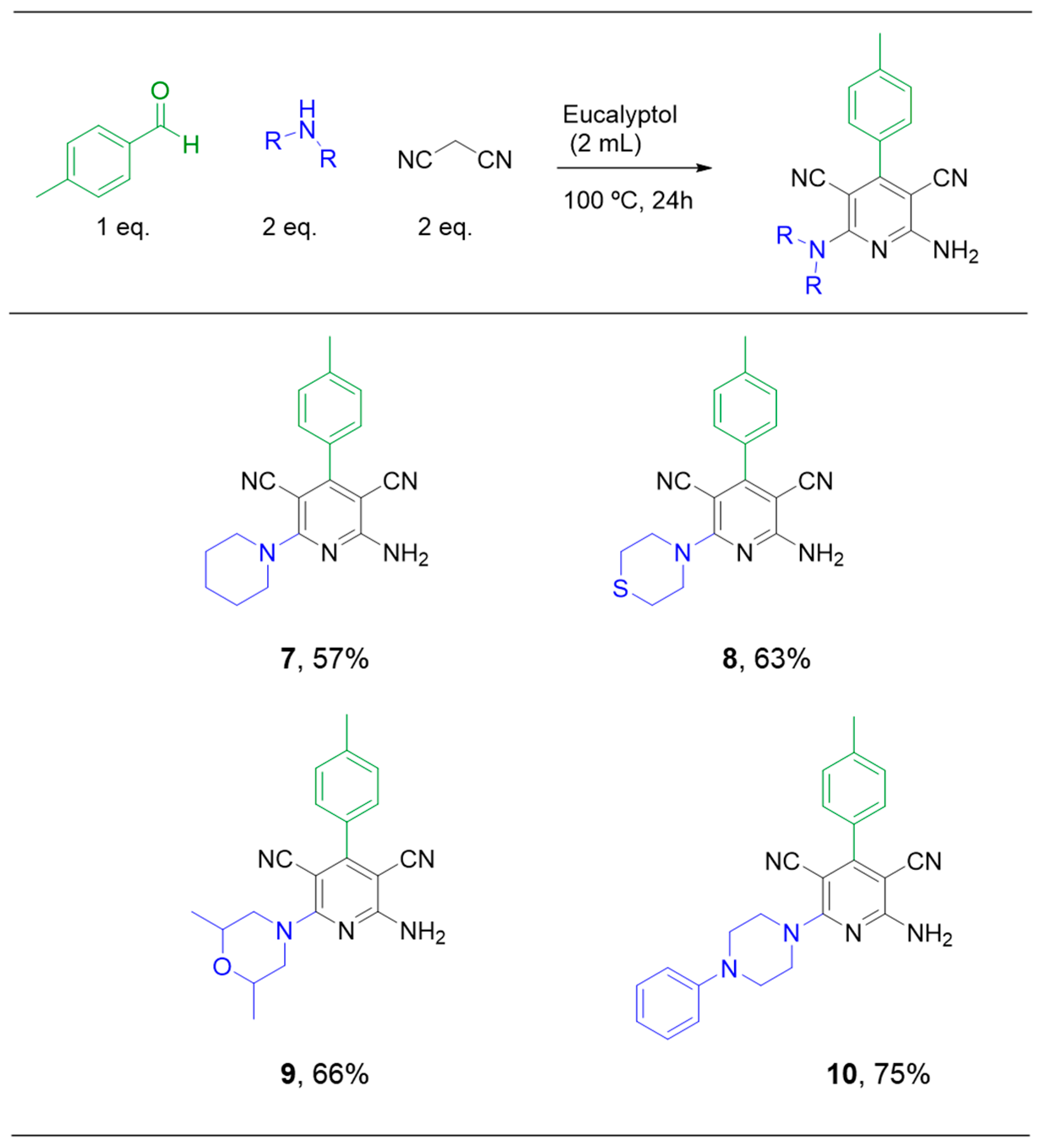
2.1. Multicomponent Reaction
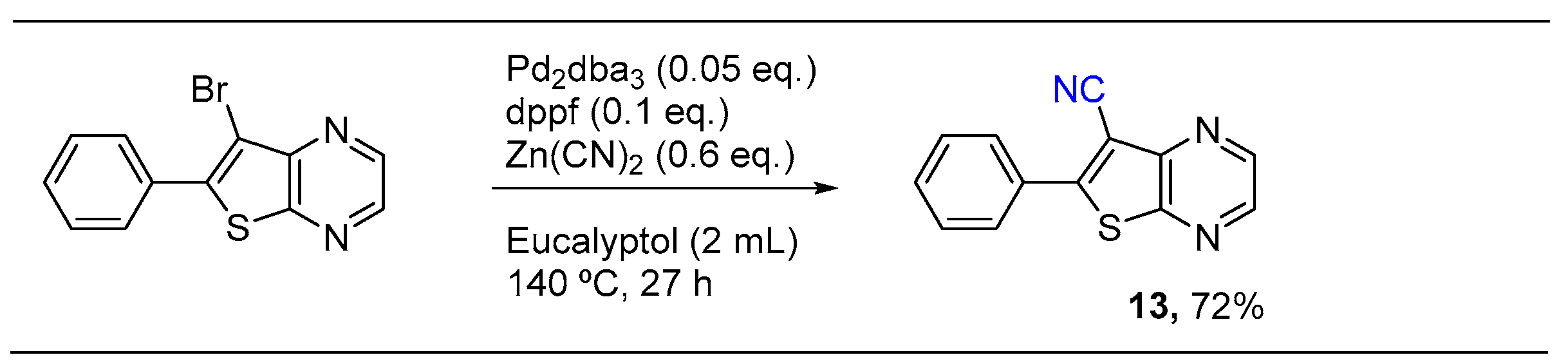
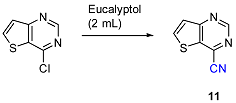
2.2. Palladium Catalyzed Cyanation
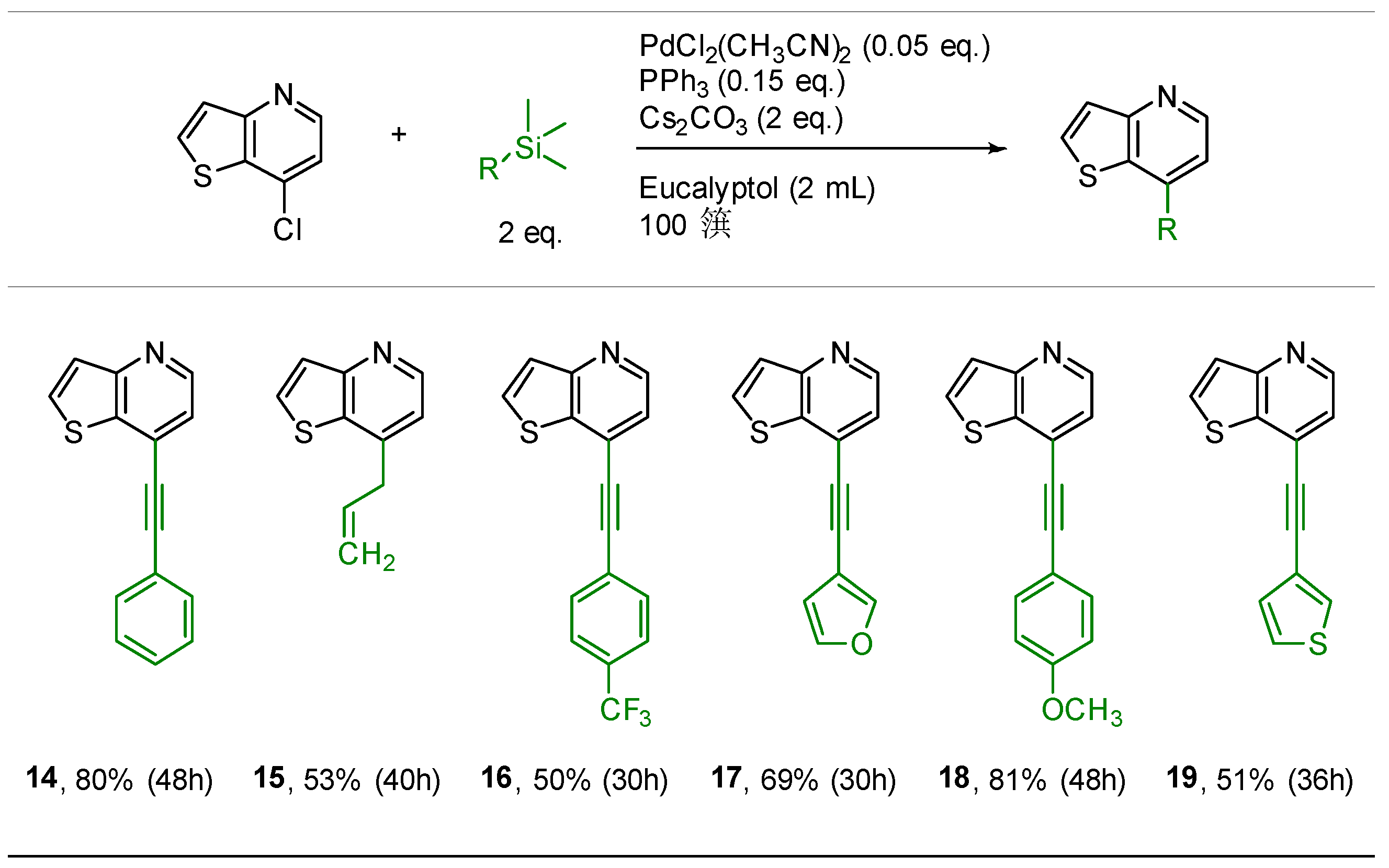
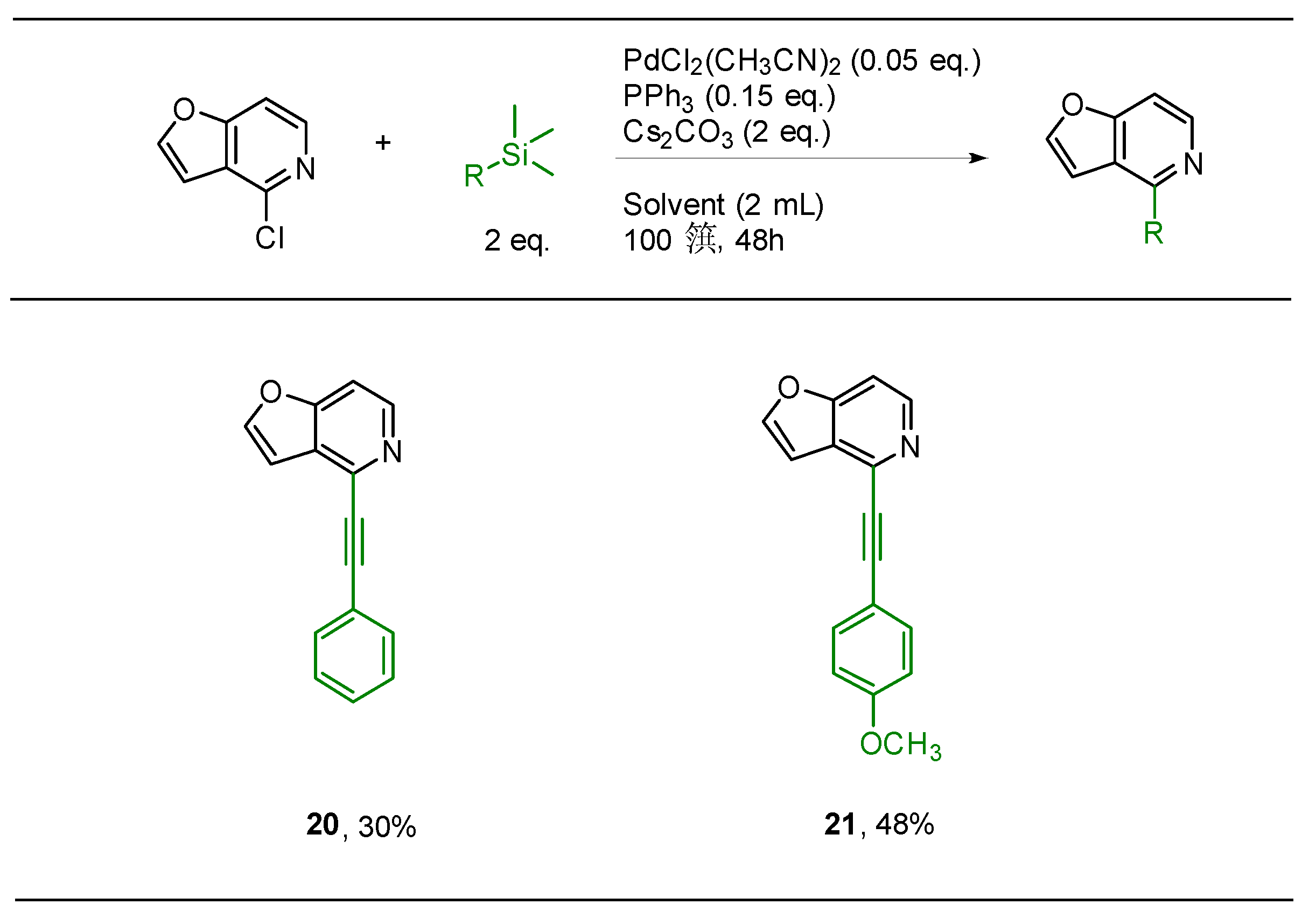
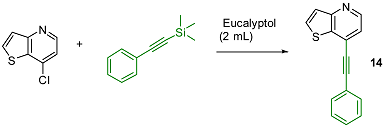
2.3. Hiyama Coupling
2.4. Recyclability of the Solvent
3. Materials and Methods
3.1. General Methods
3.2. Multicomponent Reaction: General Procedure for Synthesis of Compounds 1–10
3.3. Palladium Catalyzed Cyanation: General Procedure for Synthesis of Compounds 11–13
3.4. Hiyama Coupling: General Procedure for Synthesis of Compounds 14–21
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Henderson, R.K.; Jiménez-González, C.; Constable, D.J.C.; Alston, S.R.; Inglis, G.G.A.; Fisher, G.; Sherwood, J.; Binks, S.P.; Curzons, A.D. Expanding GSK’s solvent selection guide—Embedding sustainability into solvent selection starting at medicinal chemistry. Green Chem. 2011, 13, 854–862. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef]
- Capello, C.; Fischer, U.; Hungerbühler, K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007, 9, 927–934. [Google Scholar] [CrossRef]
- Alfonsi, K.; Colberg, J.; Dunn, P.J.; Fevig, T.; Jennings, S.; Johnson, T.A.; Kleine, H.P.; Knight, C.; Nagy, M.A.; Perry, D.A.; et al. Green chemistry tools to influence a medicinal chemistry and research chemistry based organization. Green Chem. 2008, 10, 31–36. [Google Scholar] [CrossRef]
- Prat, D.; Pardigon, O.; Flemming, H.W.; Letestu, S.; Ducandas, V.; Isnard, P.; Guntrum, E.; Senac, T.; Ruisseau, S.; Cruciani, P.; et al. Sanofi’s Solvent Selection Guide: A Step Toward More Sustainable Processes. Org. Process Res. Dev. 2013, 17, 1517–1525. [Google Scholar] [CrossRef]
- Diorazio, L.J.; Hose, D.R.J.; Adlington, N.K. Toward a More Holistic Framework for Solvent Selection. Org. Process Res. Dev. 2016, 20, 760–773. [Google Scholar] [CrossRef] [Green Version]
- Campos, J.F.; Berteina-Raboin, S. Greener Synthesis of Nitrogen-Containing Heterocycles in Water, PEG and Bio-Based Solvents. Catalysts 2020, 10, 429. [Google Scholar] [CrossRef]
- Campos, J.F.; Pacheco-Benichou, A.; Fruit, C.; Besson, T.; Berteina-Raboin, S. Synthesis of Benzo-Fused 11H-Pyrido[2,1-b]quinazolin-11-ones by a Buchwald–Hartwig Coupling/Pyridine Dearomatization Sequence in Eucalyptol. Synthesis 2020, 52, 3071–3076. [Google Scholar] [CrossRef]
- Fresneau, N.; Hiebel, M.-A.; Agrofoglio, L.A.; Berteina-Raboin, S. Efficient Synthesis of Unprotected C-5-Aryl/Heteroaryl-2′-deoxyuridine via a Suzuki-Miyaura Reaction in Aqueous Media. Molecules 2012, 17, 14409. [Google Scholar] [CrossRef] [Green Version]
- Fresneau, N.; Hiebel, M.-A.; Agrofoglio, L.A.; Berteina-Raboin, S. One-pot Sonogashira-cyclization protocol to obtain substituted furopyrimidine nucleosides in aqueous conditions. Tetrahedron Lett. 2012, 53, 1760–1763. [Google Scholar] [CrossRef]
- Hiebel, M.-A.; Fall, Y.; Scherrmann, M.-C.; Berteina-Raboin, S. Straightforward Synthesis of Various 2,3-Diarylimidazo[1,2-a]pyridines in PEG400 Medium through One-Pot Condensation and C–H Arylation. Eur. J. Org. Chem. 2014, 21, 4643–4650. [Google Scholar] [CrossRef]
- Hiebel, M.-A.; Berteina-Raboin, S. Iodine-catalyzed regioselective sulfenylation of imidazoheterocycles in PEG400. Green Chem. 2015, 17, 937–944. [Google Scholar] [CrossRef]
- Dumonteil, G.; Hiebel, M.-A.; Scherrmann, M.-C.; Berteina-Raboin, S. Iodine-catalyzed formation of substituted 2-aminobenzothiazole derivatives in PEG400. RSC Adv. 2016, 6, 73517–73521. [Google Scholar] [CrossRef]
- Campos, J.F.; Loubidi, M.; Scherrmann, M.-C.; Berteina-Raboin, S. A Greener and Efficient Method for Nucleophilic Aromatic Substitution of Nitrogen-Containing Fused Heterocycles. Molecules 2018, 23, 684. [Google Scholar] [CrossRef] [Green Version]
- Campos, J.F.; Scherrmann, M.-C.; Berteina-Raboin, S. Eucalyptol: A new solvent for the synthesis of heterocycles containing oxygen, sulfur and nitrogen. Green Chem. 2019, 21, 1531–1539. [Google Scholar] [CrossRef]
- Campos, J.F.; Berteina-Raboin, S. Eucalyptol as bio-based solvent for Migita-Kosugi-Stille coupling reaction on O,S,N-Heterocycles. Catal. Today 2020, 358, 138–142. [Google Scholar] [CrossRef]
- Campos, J.F.; Berteina-Raboin, S. Eucalyptol as a Bio-Based Solvent for Buchwald-Hartwig Reaction on O,S,N-Heterocycles. Catalysts 2019, 9, 840. [Google Scholar] [CrossRef] [Green Version]
- Lombardino, J.G.; Lowe, J.A., III. A Guide to drug discovery: The Role of Medicinal Chemist in Drug Discovery-Then and Now. Nat. Rev. Drug Discov. 2004, 3, 853–862. [Google Scholar] [CrossRef]
- Hayashi, Y. Pot economy and one-pot synthesis. Chem. Sci. 2016, 7, 866–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, B.; Rajale, T.; Wever, W.; Tu, S.-J.; Li, G. Multicomponent Reactions for the Synthesis of Heterocycles. Chem. Asian J. 2010, 5, 2318–2335. [Google Scholar] [CrossRef]
- Malinakova, H.C. Recent advances in the discovery and design of multicomponent reactions for the generation of small-molecule libraries. Rep. Org. Chem. 2015, 5, 75–90. [Google Scholar] [CrossRef] [Green Version]
- Altaf, A.A.; Shahzad, A.; Gul, Z.; Rasool, N.; Badshah, A.; Lal, B.; Khan, E. A Review on the Medicinal Importance of Pyridine Derivatives. JDDMC 2015, 1, 1–11. [Google Scholar] [CrossRef]
- Fuentes, L.; Vaquero, J.J.; Soto, J.L. Heterocycle synthesis. XVI. Reaction of malononitrile with benzylidenemalononitriles in presence of amines. AN QUIM C-ORG BIOQ 1980, 76, 68–69. [Google Scholar]
- Raghukumar, V.; Thirumalai, D.; Ramakrishnan, V.T.; Karunakara, V.; Ramamurthy, P. Synthesis of nicotinonitrile derivatives as a new class of Non-linear optical materials. Tetrahedron 2003, 59, 3761–3768. [Google Scholar] [CrossRef]
- Sarkar, S.; Das, D.K.; Khan, A.T. Synthesis of fully-substituted pyridines and dihydropyridines in a highly chemoselective manner utilizing a multicomponent reaction (MCR) strategy. RSC Adv. 2014, 4, 53752–53760. [Google Scholar] [CrossRef]
- Rosenmund, K.W.; Struck, E. Das am Ringkohlenstoff gebundene Halogen und sein Ersatz durch andere Substituenten. I. Mitteilung: Ersatz des Halogens durch die Carboxylgruppe. Chem. Ber. 1919, 52, 1749–1756. [Google Scholar] [CrossRef] [Green Version]
- Pongratz, A. Untersuchungen über Perylen und seine Derivate. Monatsh. Chem. 1927, 48, 585–591. [Google Scholar] [CrossRef]
- von Braun, J.; Manz, G. Fluoranthen und seine Derivate. III. Mitteilung. Liebigs Ann. Chem. 1931, 488, 111–126. [Google Scholar] [CrossRef]
- Connor, J.A.; Leeming, S.W.; Price, R. Influence of substrate structure on copper(I)-assisted cyanide substitution in aryl halides. J. Chem. Soc. Perkin Trans. 1990, 1, 1127–1132. [Google Scholar] [CrossRef]
- Ellis, G.P.; Romney-Alexander, T.M. Cyanation of aromatic halides. Chem. Rev. 1987, 87, 779–794. [Google Scholar] [CrossRef]
- Campos, J.F.; Queiroz, M.-J.R.P.; Berteina-Raboin, S. The first catalytic direct C-H arylation on C2 and C3 of thiophene ring applied to thieno-pyridines, -pyrimidines and -pyrazines. Catalysts 2018, 8, 137. [Google Scholar] [CrossRef] [Green Version]
- Campos, J.F.; Queiroz, M.-J.R.P.; Berteina-Raboin, S. Synthesis of new thieno[3,2-b]pyridines and thieno[3,2-b]pyrazines by palladium cross-coupling. ChemistrySelect 2017, 24, 6945–6948. [Google Scholar] [CrossRef]
- Schareina, T.; Zapf, A.; Beller, M. An environmentally benign procedure for the Cu-catalyzed cyanation of aryl bromides. Tetrahedron Lett. 2005, 15, 2585–2588. [Google Scholar] [CrossRef]
- Schareina, T.; Zapf, A.; Mägerlein, W.; Müller, N.; Beller, M. A State-of-the-Art Cyanation of Aryl Bromides: A Novel and Versatile Copper Catalyst System Inspired by Nature. Chem. Eur. J. 2007, 21, 6249–6254. [Google Scholar] [CrossRef]
- Schareina, T.; Zapf, A.; Cotté, A.; Müller, N.; Beller, M. A Bio-inspired Copper Catalyst System for Practical Catalytic Cyanation of Aryl Bromides. Synthesis 2008, 20, 3351–3355. [Google Scholar] [CrossRef]
- Schareina, T.; Zapf, A.; Mägerlein, W.; Müller, N.; Beller, M. Copper-Catalyzed Cyanation of Heteroaryl Bromides: A Novel and Versatile Catalyst System Inspired by Nature. Synlett 2007, 4, 555–558. [Google Scholar] [CrossRef]
- Anbarasan, P.; Schareina, T.; Beller, M. Recent developments and perspectives in palladium-catalyzed cyanation of aryl halides: Synthesis of benzonitriles. Chem. Soc. Rev. 2011, 40, 5049–5067. [Google Scholar] [CrossRef] [PubMed]
- Montel, F.; Lamberth, C.; Jung, P.M.J. First synthesis of 7-amido-[1,2,4]triazolo[1,5-a]pyrimidines using halogen–metal exchange. Tetrahedron 2008, 27, 6372–6376. [Google Scholar] [CrossRef]
- Miyashita, A.; Suzuki, Y.; Ohta, K.; Higashino, T. Preparation of Heteroarenecarbonitriles by Reaction of Haloheteroarenes with Potassium Cyanide Catalyzed by Sodium p-Toluenesulfinate. Heterocycles 1994, 39, 345–356. [Google Scholar] [CrossRef]
- Ballini, R.; Belderrain, T.R.; Bruneau, C.; Cokoja, M.; Dong, D. Science of Synthesis: C-1 Building Blocks in Organic Synthesis Vol. 2: Alkenations, Cross Couplings, Insertions, Substitutions, and Halomethylations (English Edition); Thieme Publishers: New York, NY, USA; Georg Thieme Verlag KG: Stuttgart, Germany, 2014; ISBN 9783131751317. [Google Scholar]
- Schareina, T.; Zapf, A.; Beller, M. Potassium hexacyanoferrate(ii)—A new cyanating agent for the palladium-catalyzed cyanation of aryl halides. Chem. Commun. 2004, 1388–1389. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J. Hiyama Cross-Coupling Reaction in: Name Reactions; Springer: Berlin/Heidelberg, Germany, 2003; pp. 187–188. [Google Scholar] [CrossRef]
- Li, J.-H.; Deng, C.-L.; Liu, W.-J.; Xie, Y.-X. Pd(OAc)2/DABCO as an Inexpensive and Efficient Catalytic System for Hiyama- Cross-Coupling Reactions of Aryl Halides with Aryltrimethoxysilanes. Synthesis 2005, 18, 3039–3044. [Google Scholar] [CrossRef]
- Molander, G.A.; Iannazzo, L. Palladium-Catalyzed Hiyama Cross-Coupling of Aryltrifluorosilanes with Aryl and Heteroaryl Chlorides. J. Org. Chem. 2011, 76, 9102–9108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monguchi, Y.; Yanase, T.; Mori, S.; Sajiki, H. A Practical Protocol for the Hiyama Cross-Coupling Reaction Catalyzed by Palladium on Carbon. Synthesis 2013, 45, 40–44. [Google Scholar] [CrossRef] [Green Version]
- Raders, S.M.; Kingston, J.V.; Verkade, J.G. Advantageous Use of tBu2P-N=P(iBuNCH2CH2)3N in the Hiyama Coupling of Aryl Bromides and Chlorides. J. Org. Chem. 2010, 75, 1744–1747. [Google Scholar] [CrossRef] [PubMed]
- Srimani, D.; Bej, A.; Sarkar, A. Palladium Nanoparticle Catalyzed Hiyama Coupling Reaction of Benzyl Halides. J. Org. Chem. 2010, 75, 4296–4299. [Google Scholar] [CrossRef]
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | A (equiv.) | B (equiv.) | C (equiv.) | Cat (equiv.) | T (°C) | t (h) | Yield a (%) |
| 1 | 1 | 1 | 2 | - | 100 | 12 | 39 |
| 2 | 1 | 2 | 2 | - | 100 | 24 | 54 |
| 3 | 1 | 1 | 2 | - | 80 | 24 | 46 |
| 4 | 1 | 2 | 2 | - | 80 | 24 | 49 |
| 5 | 1 | 1 | 2 | - | r.t. | 24 | 50 |
| 6 | 1 | 2 | 2 | - | r.t. | 24 | 38 |
| 7 | 1 | 1 | 2 | DMAP | r.t. | 24 | 28 |
| 8 | 1 | 2 | 2 | DIPEA | 100 | 24 | 47 |
| 9 | 1 | 2 | 2 | Cs2CO3 | 100 | 24 | 42 |
| 10 | 1 | 1 | 2 | - | 100 | 24 | 46 b |
| 11 | 1 | 2 | 2 | - | 100 | 24 | 43 b |
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Pd (eq.) | Lig (eq.) | CN (eq.) | T (°C) | t (h) | Yield a (%) |
| 1 | Pd(PPh3)4 (0.07) | - | Zn(CN)2 (0.6) | 100 | 96 | 7 |
| 2 c | Pd2(dba)3 (0.05) | dppf (0.05) | Zn(CN)2 (0.6) | 100 | 96 | 11 |
| 3 c | Pd2(dba)3 (0.05) | dppf (0.05) | Zn(CN)2 (0.6) | 140 | 44 | 55 |
| 4 | Pd(PPh3)4 (0.05) | - | KCN (1.5) | 140 | 61 | 0 |
| 5 | PdCl2(PPh3)2 (0.05) | - | KCN (2) | 140 | 61 | 0 |
| 6 d | Pd(OAc)2 (0.05) | dppe (0.1) | KCN (1) | 140 | 61 | 0 |
| 7 d | Pd(OAc)2 (0.05) | dppe (0.1) | KCN (1) | 140 | 61 | 0 |
| 8 d | Pd(OAc)2 (0.05) | dppe (0.1) | KCN (1) | 140 | 61 | 0 |
| 9 d | Pd(OAc)2 (0.05) | dppe (0.1) | KCN (1) | 140 | 61 | 0 |
| 10 | Pd2(dba)3 (0.1) | dppf (0.4) | KCN (2) | 140 | 44 | 24 |
| 11 e | Pd(OAc)2 (0.03) | cataCXium (0.09) | K4[Fe(CN)6] b (0.2) | 140 | 41 | traces |
| 12 | Pd2(dba)3 (0.03) | cataCXium (0.09) | K4[Fe(CN)6] b (0.2) | 140 | 41 | traces |
| 13 e | Pd(TFA)2 (0.03) | TTBP·HBF4 (0.09) | K4[Fe(CN)6] b (0.2) | 140 | 41 | traces |
| 14 e | PdCl2 (0.03) | TTBP·HBF4 (0.09) | K4[Fe(CN)6] b (0.2) | 140 | 41 | traces |
| 15 c | Pd2(dba)3 (0.05) | dppf (0.05) | Zn(CN)2 (0.6) | 140 | 96 | 43 |
| 16 e | Pd(OAc)2 (0.05) | X-Phos (0.1) | K4[Fe(CN)6] b (0.25) | 140 | 60 | 56 |
| 17 c | Pd2(dba)3 (0.05) | PCy3 (0.05) | Zn(CN)2 (0.6) | 140 | 48 | 48 |
| 18 e | Pd(OAc)2 (0.05) | dppf (0.1) | K4[Fe(CN)6] b (0.2) | 140 | 60 | 43 |
| 19 c | Pd2(dba)3 (0.05) | dppf (0.1) | Zn(CN)2 (0.6) | 170 | 26 | 39 |
| 20 | - | - | NaCN (5) | rt | 26 | 0 |
| 21 | - | - | NaCN (5) | 170 | 24 | 0 |
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Pd (eq.) | Lig (eq.) | CN (eq.) | T (°C) | t (h) | Yield a (%) |
| 1 c | Pd2(dba)3 (0.05) | PCy3 (0.1) | Zn(CN)2 (0.06) | 170 | 48 | 9 |
| 2 c,d | Pd(OAc)2 (0.05) | X-Phos (0.1) | K4[Fe(CN)6] b (0.2) | 170 | 48 | 0 |
| 3 c | Pd2(dba)3 (0.05) | dppf (0.1) | Zn(CN)2 (0.6) | 170 | 26 | 61 |
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Pd (eq.) | Lig (eq.) | CN (eq.) | T (°C) | t (h) | Yield a (%) |
| 1 | Pd(OAc)2 (0.025) | X-Phos (0.05) | TBAF·3H2O (2.5) | 100 | 72 | 67 |
| 2 | PdCl2(PPh3)2 (0.1) | Ph3As (0.4) | - | 100 | 48 | 0 |
| 3 | Pd(OAc)2 (0.1) | P(Cy3) (0.4) | CsF (1.5) | 100 | 30 | 42 |
| 4 | Pd(OAc)2 (0.1) | DABCO (0.2) | TBAF·3H2O (2.5) | 100 | 48 | 0 |
| 5 | Pd(PPh3)4 (0.1) | - | CsF (4) | 100 | 30 | 30 |
| 6 | [PdCl(allyl)]2 (0.05) | P(Cy3) (0.1) | TBAF in THF (3) | 100 | 96 | 0 |
| 7 | [PdCl(allyl)]2 (0.05) | X-Phos (0.2) | TBAF·3H2O (5) | 100 | 48 | 51 |
| 8 | PdCl2(PPh3)2 (0.1) | - | KF (5) | 100 | 96 | 43 |
| 9 | Pd2(dba)3 (0.05) | X-Phos (0.1) | TBAF·3H2O (5) | 100 | 48 | 56 |
| 10 | Pd(CH3CN)2Cl2 (0.05) | X-Phos (0.1) | TBAF·3H2O (5) | 100 | 48 | 65 |
| 11 | Pd(CH3CN)2Cl2 (0.05) | PPh3 (0.15) | Cs2CO3 (2) | 100 | 48 | 80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, J.F.; Ferreira, V.; Berteina-Raboin, S. Eucalyptol: A Bio-Based Solvent for the Synthesis of O,S,N-Heterocycles. Application to Hiyama Coupling, Cyanation, and Multicomponent Reactions. Catalysts 2021, 11, 222. https://doi.org/10.3390/catal11020222
Campos JF, Ferreira V, Berteina-Raboin S. Eucalyptol: A Bio-Based Solvent for the Synthesis of O,S,N-Heterocycles. Application to Hiyama Coupling, Cyanation, and Multicomponent Reactions. Catalysts. 2021; 11(2):222. https://doi.org/10.3390/catal11020222
Chicago/Turabian StyleCampos, Joana F., Véronique Ferreira, and Sabine Berteina-Raboin. 2021. "Eucalyptol: A Bio-Based Solvent for the Synthesis of O,S,N-Heterocycles. Application to Hiyama Coupling, Cyanation, and Multicomponent Reactions" Catalysts 11, no. 2: 222. https://doi.org/10.3390/catal11020222







