Extra-Heavy Oil Aquathermolysis Using Nickel-Based Catalyst: Some Aspects of In-Situ Transformation of Catalyst Precursor
Abstract
:1. Introduction
2. Results and Discussions
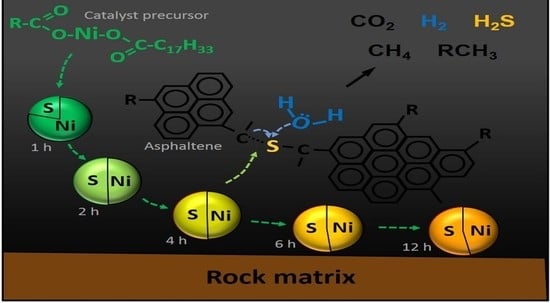
2.1. Activation of Catalyst Precursors
2.2. Gas Phase Products of Catalytic and Non-Catalytic Aquathermolysis
2.3. SARA Analysis of Core Extracts before and after Catalytic Aquathermolysis
2.4. GC-MS Analysis of Saturates and Aromatics Hydrocarbons
2.5. FT-IR Spectroscopy Results
2.6. Elemental Analysis Results
2.7. Matrix-Activated Laser Desorption/Ionization (MALDI) Analysis Results
3. Research Methods
3.1. Materials
3.2. Catalytic and Non-Catalytic Aquathermolysis Modeling in Batch Reactor Coupled with Gas Chromatography
3.3. Transformation of Catalyst Precursors
3.3.1. Isolation of Nickel-Based Catalyst
3.3.2. X-ray Diffraction Analysis
3.3.3. Scanning Electron Microscope (SEM) Analysis
3.4. Products of Catalytic and Non-Catalytic Aquathermolysis
3.4.1. SARA-Analysis
3.4.2. Gas Chromatography-Mass Spectroscopy (GC-MS)
3.4.3. Fourier Transform Infrared Spectral (FT-IR) Analysis
3.4.4. Elemental Analysis
3.4.5. Matrix-Activated Laser Desorption/Ionization (MALDI) Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, T.; Zhang, D. A critical review of comparative global historical energy consumption and future demand: The story told so far. Energy Rep. 2020, 6, 1973–1991. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X. A steam injection distribution optimization method for SAGD oil field using LSTM and dynamic programming. ISA Trans. 2020. In Press. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, X.; Liu, Y.; Zhong, L. In situ upgrading heavy oil by aquathermolytic treatment under steam injection conditions. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 2–4 February 2005; Society of Petroleum Engineers: Richardson, TX, USA, 2005. [Google Scholar]
- Giacchetta, G.; Leporini, M.; Marchetti, B. Economic and environmental analysis of a Steam Assisted Gravity Drainage (SAGD) facility for oil recovery from Canadian oil sands. Appl. Energy 2015, 142, 1–9. [Google Scholar] [CrossRef]
- Hyne, J.B.; Greidanus, J.W.; Tyrer, J.D.; Verona, D.; Rizek, C.; Clark, P.D.; Clarke, R.A.; Koo, J. Aquathermolysis of heavy oils. Rev. Tec. Intevep 1982, 2, 87–94. [Google Scholar]
- Vakhin, A.V.; Mukhamatdinov, I.I.; Aliev, F.A.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Sitnov, S.A.; Chemodanov, A.E.; Varfolomeev, M.A.; Nurgaliev, D.K. Aquathermolysis of heavy oil in reservoir conditions with the use of oil-soluble catalysts: Part II–changes in composition of aromatic hydrocarbons. Pet. Sci. Technol. 2018, 36, 1850–1856. [Google Scholar] [CrossRef]
- Ovalles, C.; Vallejos, C.; Vasquez, T.; Martinis, J.; Perez-Perez, A.; Cotte, E.; Castellanos, L.; Rodriguez, H. Extra-heavy crude oil downhole upgrading process using hydrogen donors under steam injection conditions. In Proceedings of the SPE International Thermal Operations and Heavy Oil Symposium, Porlamar, Venezuela, 12–14 March 2001. [Google Scholar]
- Alemán-Vázquez, L.O.; Torres-Mancera, P.; Ancheyta, J.; Ramírez-Salgado, J. Use of Hydrogen Donors for Partial Upgrading of Heavy Petroleum. Energy Fuels 2016, 30, 9050–9060. [Google Scholar] [CrossRef]
- Hart, A.; Lewis, C.; White, T.; Greaves, M.; Wood, J. Effect of cyclohexane as hydrogen-donor in ultradispersed catalytic upgrading of heavy oil. Fuel Process. Technol. 2015, 138, 724–733. [Google Scholar] [CrossRef] [Green Version]
- Fujimoto, K.; Ohno, A.; Kunugi, T. Liquid phase hydrogenolysis of thiophene by decaline as hydrogen donor with metal supported active carbon catalysts. Stud. Surf. Sci. Catal. 1983, 17, 241–249. [Google Scholar]
- Liu, Y.; Fan, H. The effect of hydrogen donor additive on the viscosity of heavy oil during steam stimulation. Energy Fuels 2002, 16, 842–846. [Google Scholar] [CrossRef]
- Feoktistov, D.A.; Kayukova, G.P.; Vakhin, A.V.; Sitnov, S.A. Catalytic aquathermolysis of high-viscosity oil using iron, cobalt, and copper tallates. Chem. Technol. Fuels Oils 2018, 53, 905–912. [Google Scholar] [CrossRef]
- Kayukova, G.P.; Mikhailova, A.N.; Kosachev, I.P.; Feoktistov, D.A.; Vakhin, A. V Conversion of heavy oil with different chemical compositions under catalytic aquathermolysis with an amphiphilic Fe-Co-Cu catalyst and kaolin. Energy Fuels 2018, 32, 6488–6497. [Google Scholar] [CrossRef]
- Zhao, F.J.; Liu, Y.J.; Wu, Y.; Si, Y.; Zhang, B.; Wu, H.R. Research on the Asphaltene Structure and Thermal Analysis in Catalytic Aquathermolysis of Heavy Oil. In Key Engineering Materials; Trans Tech Publications Ltd.: Stäfa, Switzerland, 2011; Volume 474, pp. 893–897. [Google Scholar]
- Vakhin, A.V.; Aliev, F.A.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Sitnov, S.A.; Mukhamatdinov, I.I.; Varfolomeev, M.A.; Nurgaliev, D.K. Aquathermolysis of heavy oil in reservoir conditions with the use of oil-soluble catalysts: Part I–changes in composition of saturated hydrocarbons. Pet. Sci. Technol. 2018, 36, 1829–1836. [Google Scholar] [CrossRef]
- Sitnov, S.A.; Mukhamatdinov, I.I.; Shmeleva, E.I.; Aliev, F.A.; Vakhin, A.V. Influence of nanosized iron oxides (II, III) on conversion of biodegradated oil. Pet. Sci. Technol. 2019, 37, 971–976. [Google Scholar] [CrossRef]
- Mukhamatdinov, I.I.; Khaidarova, A.R.; Zaripova, R.D.; Mukhamatdinova, R.E.; Sitnov, S.A.; Vakhin, A.V. The composition and structure of ultra-dispersed mixed oxide (Ii, iii) particles and their influence on in-situ conversion of heavy oil. Catalysts 2020, 10, 114. [Google Scholar] [CrossRef] [Green Version]
- Sitnov, S.A.; Petrovnina, M.S.; Feoktistov, D.A.; Isakov, D.R.; Nurgaliev, D.K.; Amerkhanov, M.I. Intensification of thermal steam methods of production of heavy oil using a catalyst based on cobalt. Neft. Khozyaystvo Oil Ind. 2016, 2016, 106–108. [Google Scholar]
- Sitnov, S.A.; Mukhamatdinov, I.I.; Vakhin, A.V.; Ivanova, A.G.; Voronina, E. V Composition of aquathermolysis catalysts forming in situ from oil-soluble catalyst precursor mixtures. J. Pet. Sci. Eng. 2018, 169, 44–50. [Google Scholar] [CrossRef]
- Maity, S.K.; Ancheyta, J.; Marroquín, G. Catalytic aquathermolysis used for viscosity reduction of heavy crude oils: A review. Energy Fuels 2010, 24, 2809–2816. [Google Scholar] [CrossRef]
- Fan, H.-F.; Liu, Y.-J.; Zhong, L.-G. Studies on the synergetic effects of mineral and steam on the composition changes of heavy oils. Energy Fuels 2001, 15, 1475–1479. [Google Scholar] [CrossRef]
- Tumanyan, B.P.; Petrukhina, N.N.; Kayukova, G.P.; Nurgaliev, D.K.; Foss, L.E.; Romanov, G.V. Aquathermolysis of crude oils and natural bitumen: Chemistry, catalysts and prospects for industrial implementation. Russ. Chem. Rev. 2015, 84, 1145. [Google Scholar] [CrossRef]
- Clark, P.D.; Dowling, N.I.; Lesage, K.L.; Hyne, J.B. Chemistry of organosulphur compound types occurring in heavy oil sands: 5. Reaction of thiophene and tetrahydrothiophene with aqueous Group VIIIB metal species at high temperature. Fuel 1987, 66, 1699–1702. [Google Scholar] [CrossRef]
- Clark, P.D.; Kirk, M.J. Studies on the upgrading of bituminous oils with water and transition metal catalysts. Energy Fuels 1994, 8, 380–387. [Google Scholar] [CrossRef]
- Clark, P.D.; Clarke, R.A.; Hyne, J.B.; Lesage, K.L. Studies on the effect of metal species on oil sands undergoing steam treatments. Aostra J. Res. 1990, 6, 53–64. [Google Scholar]
- Zhong, L.G.; Liu, Y.J.; Fan, H.F.; Jiang, S.J. Liaohe extra-heavy crude oil underground aquathermolytic treatments using catalyst and hydrogen donors under steam injection conditions. In Proceedings of the SPE International Improved Oil Recovery Conference in Asia Pacific, Kuala Lumpur, Malaysia, 20–21 October 2003. [Google Scholar]
- Zhang, F.L.; Zhao, H.Y. Steam based recovery technologies of heavy oil reservoirs in Liaohe oilfield. 2007. [Google Scholar]
- Fan, H.F.; Liu, Y.J.; Zhao, X.F.; Zhong, L.G. Studies on effect of metal ions on aquathermolysis reaction of Liaohe heavy oils under steam treatment. J. Fuel Chem. Technol. 2001, 25, 430–433. [Google Scholar]
- Cheraghian, G.; Rostami, S.; Afrand, M. Nanotechnology in enhanced oil recovery. Processes 2020, 8, 1073. [Google Scholar] [CrossRef]
- Pevneva, G.S.; Voronetskaya, N.G.; Sviridenko, N.N.; Golovko, A.K. Effect of WC/Ni–Cr additive on changes in the composition of an atmospheric residue in the course of cracking. Pet. Sci. 2020, 17, 499–508. [Google Scholar] [CrossRef] [Green Version]
- Karakhanov, E.A.; Glotov, A.P.; Nikiforova, A.G.; Vutolkina, A.V.; Ivanov, A.O.; Kardashev, S.V.; Maksimov, A.L.; Lysenko, S. V Catalytic cracking additives based on mesoporous MCM-41 for sulfur removal. Fuel Process. Technol. 2016, 153, 50–57. [Google Scholar] [CrossRef]
- Kadieva, M.K.; Khadzhiev, S.N.; Kadiev, K.M.; Gyul’Maliev, A.M.; Yakovenko, T.V. The formation of nanosized molybdenum oxide particles in a hydrocarbon medium. Pet. Chem. 2011, 51, 16–23. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, Y.; Fu, Z.; Zhao, X. Using hydrogen donor with oil-soluble catalysts for upgrading heavy oil. Russ. J. Appl. Chem. 2014, 87, 1498–1506. [Google Scholar] [CrossRef]
- Yusuf, A.; Al-Hajri, R.S.; Al-Waheibi, Y.M.; Jibril, B.Y. In-situ upgrading of Omani heavy oil with catalyst and hydrogen donor. J. Anal. Appl. Pyrolysis 2016, 121, 102–112. [Google Scholar] [CrossRef]
- Ren, R.; Liu, H.; Chen, Y.; Li, J.; Chen, Y. Improving the Aquathermolysis Efficiency of Aromatics in Extra-Heavy Oil by Introducing Hydrogen-Donating Ligands to Catalysts. Energy Fuels 2015, 29, 7793–7799. [Google Scholar] [CrossRef]
- Huibers, D.T.A. Tall oil. Kirk-Othmer Encycl. Chem. Technol. 2000. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Sitnov, S.A.; Mukhamatdinov, I.I.; Aliev, F.A.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Varfolomeev, M.A.; Nurgaliev, D.K. Aquathermolysis of heavy oil in reservoir conditions with the use of oil-soluble catalysts: Part III--changes in composition resins and asphaltenes. Pet. Sci. Technol. 2018, 36, 1857–1863. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Aliev, F.A.; Mukhamatdinov, I.I.; Sitnov, S.A.; Sharifullin, A.V.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Nurgaliev, D.K. Catalytic aquathermolysis of boca de jaruco heavy oil with nickel-based oil-soluble catalyst. Processes 2020, 8, 532. [Google Scholar] [CrossRef]
- Hyne, J.B.; Greidanus, J.W.; Tyrer, J.D.; Verona, D.; Rizek, C.; Clark, P.D.; Clarke, R.A.; Koo, J. The Second International Conference on heavy crude and tar sands. Caracas Venez. 1982, 1, 1–8. [Google Scholar]
- Al-Muntaser, A.A.; Varfolomeev, M.A.; Suwaid, M.A.; Feoktistov, D.A.; Yuan, C.; Klimovitskii, A.E.; Gareev, B.I.; Djimasbe, R.; Nurgaliev, D.K.; Kudryashov, S.I. Hydrogen donating capacity of water in catalytic and non-catalytic aquathermolysis of extra-heavy oil: Deuterium tracing study. Fuel 2021, 283, 118957. [Google Scholar] [CrossRef]
- Hosseinpour, M.; Fatemi, S.; Ahmadi, S.J. Deuterium tracing study of unsaturated aliphatics hydrogenation by supercritical water in upgrading heavy oil. Part II: Hydrogen donating capacity of water in the presence of iron(III) oxide nanocatalyst. J. Supercrit. Fluids 2016, 110, 75–82. [Google Scholar] [CrossRef]
- Foss, L.E.; Kayukova, G.P.; Tumanyan, B.P.; Petrukhina, N.N.; Nikolaev, V.F.; Romanov, G.V. Change in the Hydrocarbon and Component Compositions of Heavy Crude Ashalchinsk Oil Upon Catalytic Aquathermolysis. Chem. Technol. Fuels Oils 2017, 53, 173–180. [Google Scholar] [CrossRef]
- Ganeeva, Y.M.; Yusupova, T.N.; Romanov, G. V Asphaltene nano-aggregates: Structure, phase transitions and effect on petroleum systems. Russ. Chem. Rev. 2011, 80, 993. [Google Scholar] [CrossRef]
- Tang, X.D.; Chen, X.D.; Li, J.J.; Deng, L.Y.; Liang, G.J. Experimental Study on Homogeneous Catalytic Upgrading of Heavy Oil. Pet. Chem. 2017, 57, 1018–1023. [Google Scholar] [CrossRef]
- Galukhin, A.V.; Erokhin, A.A.; Gerasimov, A.V.; Eskin, A.A.; Nurgaliev, D.K. Influence of iron pentacarbonyl on catalytic aquathermolysis of heavy oil: Changes of oil’s parameters and formation of magnetic nanoparticles. In Proceedings of the Society of Petroleum Engineers—SPE Russian Petroleum Technology Conference, Moscow, Russia, 26–28 October 2015. [Google Scholar]
- Mukhamatdinov, I.I.; Salih, I.S.S.; Rakhmatullin, I.Z.; Sitnov, S.A.; Laikov, A.V.; Klochkov, V.V.; Vakhin, A.V. Influence of Co-based catalyst on subfractional composition of heavy oil asphaltenes during aquathermolysis. J. Pet. Sci. Eng. 2020, 186, 106721. [Google Scholar] [CrossRef]











| Aquathermolysis Duration, Hour(s) | Phases | Content, wt.% | Content of Ni and S, Rel.% | |
|---|---|---|---|---|
| Ni | S | |||
| 1 | NiS2 | 5 | 79.8 | 20.2 |
| Ni3S4 | 20 | |||
| Ni3S2 | 22 | |||
| Ni9S8 | 24 | |||
| CaSO4 | 28 | |||
| 2 | NiS2 | 6 | 49.4 | 50.6 |
| Ni3S2 | 8 | |||
| Ni3S4 | 9 | |||
| Ni9S8 | 20 | |||
| CaSO4 | 58 | |||
| 4 | K2Ca(CO3)2 | 4 | 48.2 | 51.8 |
| NiS2 | 8 | |||
| Ni3S2 | 0 | |||
| CaSO4 | 10 | |||
| Ni3S4 | 25 | |||
| Ni9S8 | 53 | |||
| 6 | K2Ca(CO3)2 | 4 | 48.4 | 51.6 |
| NiS2 | 6 | |||
| Ni3S4 | 21 | |||
| Ni3S2 | 0 | |||
| CaSO4 | 24 | |||
| Ni9S8 | 45 | |||
| 12 | NiS2 | 3 | 47.0 | 53.0 |
| K2Ca(CO3)2 | 4 | |||
| CaSO4 | 7 | |||
| Ni3S2 | 0 | |||
| Ni3S4 | 24 | |||
| Ni9S8 | 62 | |||
| Experimental Conditions | With Catalyst | Without Catalyst | ||||
|---|---|---|---|---|---|---|
| Duration, Hours | 48 | 72 | 96 | 96 | ||
| Composition of gaseous products, wt.% | H2 | 0.54 | 0.71 | - | 0.42 | |
| CO2 | 55.71 | 58.61 | 54.65 | 45.17 | ||
| H2S | 17.09 | 10.38 | 13.14 | 14.70 | ||
| Saturated hydrocarbons | CH4 | 13.26 | 14.38 | 15.82 | 16.54 | |
| C2H6 | 7.21 | 9.28 | 9.20 | 9.54 | ||
| C3H8 | - | - | - | 6.85 | ||
| normal (n)-C4H10 | 1.49 | 2.02 | 2.14 | 2.11 | ||
| iso (i)-C4H10 | 0.64 | 0.91 | 0.94 | 1.05 | ||
| n-C5H12 | 0.52 | 0.65 | 0.72 | 0.64 | ||
| neo-C5H12 | 0.21 | 0.22 | 0.24 | - | ||
| i-C5H12 | 0.35 | 0.50 | 0.54 | 0.57 | ||
| n-C6H14 | 0.27 | 0.25 | 0.26 | 0.24 | ||
| i-C6H14 | 0.18 | 0.20 | 0.21 | 0.22 | ||
| Isomers C7–C8 | 0.42 | 0.32 | 0.15 | 0.38 | ||
| Unsaturated hydrocarbons | C2H4 | 0.09 | - | - | - | |
| C3H6 | 0.40 | - | - | - | ||
| C4–C5 | 0.48 | 0.37 | 0.06 | 0.28 | ||
| C6–C7 | 0.17 | 0.17 | 0.23 | 0.15 | ||
| Sum. | 1.14 | 0.54 | 0.29 | 0.43 | ||
| Total amount gaseous products, g/100 g of core | 0.622 | 0.686 | 0.742 | 0.725 | ||
| Alkanes | Initial Core Extract | After catalytic Upgrading, % | ||
|---|---|---|---|---|
| 48 h | 72 h | 96 h | ||
| C11 | Not detected | 15.38 | 15.43 | 0.41 |
| C12 | 36.54 | 33.95 | 9.13 | |
| C13 | 17,63 | 29.63 | 18.26 | |
| C14 | 6.41 | 9.26 | 17.24 | |
| C15 | 9.62 | 6.17 | 20.28 | |
| C16 | 4.81 | 0.93 | 10.75 | |
| C17 | 4.81 | 2.78 | 11.36 | |
| C18 | 0.64 | 0.93 | 4.06 | |
| C19 | 0.64 | 0.31 | 3.45 | |
| C20 | 2.88 | 0.62 | 4.06 | |
| C21 | 0.32 | 0.00 | 0.61 | |
| C22 | 0.32 | 0.00 | 0.41 | |
| C11–C15 | 85.58 | 94.44 | 65.31 | |
| Experimental Conditions | Spectral Coefficients | |||||
|---|---|---|---|---|---|---|
| *C1 | *C2 | *C3 | *C4 | *C5 | ||
| Initial core extracts | 0.33 | 0.12 | 0.58 | 7.38 | 0.20 | |
| 48 h | Hydrothermal treatment in the presence of catalyst and [H]-donor | 0.39 | 0.11 | 0.58 | 7.00 | 0.15 |
| 72 h | 0.37 | 0.10 | 0.58 | 7.32 | 0.15 | |
| 96 h | 0.34 | 0.11 | 0.57 | 7.63 | 0.13 | |
| Without catalyst | 0.33 | 0.04 | 0.57 | 7.52 | 0.14 | |
| Experimental Conditions | Object | Elemental Composition, wt.% | H/Cat | |||||
|---|---|---|---|---|---|---|---|---|
| C | H | N | S | O | ||||
| Initial | Core extracts | 83.9 | 9.1 | 0.4 | 2.6 | 4.0 | 1.30 | |
| SARA-fractions | S | 84.5 | 12.8 | 0.1 | 1.0 | 1.6 | 1.82 | |
| A | 80.3 | 9.9 | 0.1 | 4.0 | 5.7 | 1.47 | ||
| R | 79.7 | 8.4 | 0.6 | 4.7 | 6.5 | 1.26 | ||
| A | 73.3 | 8.0 | 0.8 | 7.7 | 10.2 | 1.31 | ||
| 48 | Core extracts | 83.1 | 10.3 | 0.4 | 2.3 | 4.0 | 1.49 | |
| SARA-fractions | S | 84.5 | 12.8 | 0.1 | 1.1 | 1.6 | 1.82 | |
| A | 82.1 | 9.2 | 0.1 | 3.5 | 5.0 | 1.35 | ||
| R | 75.2 | 8.6 | 0.8 | 6.4 | 8.9 | 1.38 | ||
| A | 77.7 | 6.3 | 1.3 | 6.3 | 8.4 | 0.98 | ||
| 72 | Core extracts | 82.9 | 10.5 | 0.3 | 2.1 | 4.2 | 1.52 | |
| SARA-fractions | S | 85.3 | 13.0 | 0.1 | 0.6 | 1.0 | 1.83 | |
| A | 81.9 | 9.2 | 0.1 | 3.6 | 5.2 | 1.35 | ||
| R | 78.1 | 8.8 | 1.0 | 5.1 | 7.0 | 1.35 | ||
| A | 78.6 | 6.6 | 1.2 | 5.9 | 7.7 | 1.01 | ||
| 96 | Core extracts | 83.1 | 10.7 | 0.4 | 1.9 | 3.9 | 1.55 | |
| SARA-fractions | S | 84.5 | 12.8 | 0.1 | 1.0 | 1.6 | 1.82 | |
| A | 80.3 | 9.9 | 0.1 | 4.0 | 5.7 | 1.47 | ||
| R | 79.7 | 8.4 | 0.6 | 4.7 | 6.5 | 1.26 | ||
| A | 83.3 | 8.0 | 0.8 | 5.8 | 9.5 | 0.99 | ||
| 96 without catalyst | Core extracts | 82.4 | 10.1 | 0.3 | 2.9 | 4.3 | 1.47 | |
| SARA-fractions | S | 81.5 | 12.6 | 0.1 | 2.3 | 3.5 | 1.86 | |
| A | 81.4 | 8.8 | 0.1 | 4.0 | 5.7 | 1.30 | ||
| R | 79.9 | 8.2 | 1.3 | 4.5 | 6.2 | 1.24 | ||
| A | 80.6 | 6.8 | 1.2 | 4.9 | 6.5 | 1.01 | ||
| Sample | SARA Fractions, wt.% | |||||
|---|---|---|---|---|---|---|
| Core extracts | Saturates | Aromatics | Resins | Asphaltenes | ||
| 16.5 | 31.8 | 26.4 | 25.3 | |||
| Elemental analysis, wt.% | ||||||
| C | H | N | S | O | H/C | |
| 83.91 | 9.09 | 0.38 | 2.65 | 3.97 | 1.301 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vakhin, A.V.; Aliev, F.A.; Mukhamatdinov, I.I.; Sitnov, S.A.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Nurgaliev, D.K. Extra-Heavy Oil Aquathermolysis Using Nickel-Based Catalyst: Some Aspects of In-Situ Transformation of Catalyst Precursor. Catalysts 2021, 11, 189. https://doi.org/10.3390/catal11020189
Vakhin AV, Aliev FA, Mukhamatdinov II, Sitnov SA, Kudryashov SI, Afanasiev IS, Petrashov OV, Nurgaliev DK. Extra-Heavy Oil Aquathermolysis Using Nickel-Based Catalyst: Some Aspects of In-Situ Transformation of Catalyst Precursor. Catalysts. 2021; 11(2):189. https://doi.org/10.3390/catal11020189
Chicago/Turabian StyleVakhin, Alexey V., Firdavs A. Aliev, Irek I. Mukhamatdinov, Sergey A. Sitnov, Sergey I. Kudryashov, Igor S. Afanasiev, Oleg V. Petrashov, and Danis K. Nurgaliev. 2021. "Extra-Heavy Oil Aquathermolysis Using Nickel-Based Catalyst: Some Aspects of In-Situ Transformation of Catalyst Precursor" Catalysts 11, no. 2: 189. https://doi.org/10.3390/catal11020189