Pd/Alumina Catalysts for Beneficial Transformation of Harmful Freon R-22
Abstract
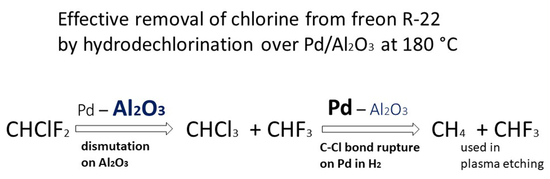
:1. Introduction
2. Results and Discussion
3. Methods
3.1. Catalysts
3.2. Catalyst Characterization
3.3. Catalytic Screening
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chlorodifluoromethane. Available online: https://en.wikipedia.org/wiki/Chlorodifluoromethane (accessed on 30 August 2021).
- Montzka, S.A.; McFarland, M.; Andersen, S.O.; Miller, B.R.; Fahey, D.W.; Hall, B.D.; Hu, L.; Siso, C.; Elkins, J.W. Recent trends in global emissions of hydrochlorofluorocarbons and hydrofluorocarbons: Reflecting on the 2007 Adjustments to the Montreal Protocol. J. Phys. Chem. A 2015, 119, 4439–4449. [Google Scholar] [CrossRef] [PubMed]
- Montzka, S.A.; Dutton, G.S.; Yu, P.; Ray, E.; Portmann, R.W.; Daniel, J.S.; Kuijpers, L.; Hall, B.D.; Mondeel, D.; Siso, C.; et al. An unexpected and persistent increase in global emissions of ozone-depleting CFC-11. Nature 2018, 557, 413–417. [Google Scholar] [CrossRef]
- Prignon, M.; Chabrillat, S.; Minganti, D.; O’Doherty, S.; Servais, C.; Stiller, G.; Toon, G.C.; Vollmer, M.K.; Mahieu, E. Improved FTIR retrieval strategy for HCFC-22 (CHClF2), comparisons with in situ and satellite datasets with the support of models, and determination of its long-term trend above Jungfraujoch. Atmos. Chem. Phys. 2019, 19, 12309–12324. [Google Scholar] [CrossRef] [Green Version]
- Oram, D.E.; Ashfold, M.J.; Laube, J.C.; Gooch, L.J.; Humphrey, S.; Sturges, W.T.; Leedham-Elvidge, E.; Forster, G.L.; Harris, N.R.P.; Iqbal Mead, M.; et al. A growing threat to the ozone layer from short-lived anthropogenic chlorocarbons. Atmos. Chem. Phys. 2017, 17, 11929–11941. [Google Scholar] [CrossRef] [Green Version]
- Eltanany, G.; Rüdiger, S.; Kemnitz, E. Supported high surface AlF3: A very strong solid Lewis acid for catalytic applications. J. Mater. Chem. 2008, 18, 2268–2275. [Google Scholar] [CrossRef]
- Patil, P.T.; Dimitrov, A.; Kirmse, H.; Neumann, W.; Kemnitz, E. Non-aqueous sol–gel synthesis, characterization and catalytic properties of metal fluoride supported palladium nanoparticles. Appl. Catal. B Environ. 2008, 78, 80–91. [Google Scholar] [CrossRef]
- Radlik, M.; Juszczyk, W.; Matus, K.; Raróg-Pilecka, W.; Karpiński, Z. Hydrodechlorination of CHClF2 (HCFC-22) over Pd–Pt catalysts supported on thermally modified activated carbon catalysts. Catalysts 2020, 10, 1291. [Google Scholar] [CrossRef]
- Martin-Martinez, M.; Gómez-Sainero, L.M. Progress in Catalytic Hydrodechlorination. Catalysts 2021, 11, 272. [Google Scholar] [CrossRef]
- Han, W.; Li, X.; Liu, B.; Li, L.; Tang, H.; Li, Y.; Lu, C.H.; Li, X. Microwave assisted combustion of phytic acid for the preparation of Ni2P@C as a robust catalyst for hydrodechlorination. Chem. Commun. 2019, 55, 9279–9282. [Google Scholar] [CrossRef]
- Flid, M.R.; Kartashov, L.M.; Treger, Y.A. Theoretical and Applied Aspects of Hydrodechlorination Processes-Catalysts and Technologies. Catalysts 2020, 10, 216. [Google Scholar] [CrossRef] [Green Version]
- Sarıbıyık, O.Y.; Weilach, C.; Serin, S.; Rupprechter, G. The Effect of Shape-Controlled Pt and Pd Nanoparticles on Selective Catalytic Hydrodechlorination of Trichloroethylene. Catalysts 2020, 10, 1314. [Google Scholar] [CrossRef]
- Ha, J.-M.; Kim, D.; Kim, J.; Ahn, B.S.; Kim, Y.; Kang, J.W. High-temperature hydrodechlorination of ozone-depleting chlorodifluoromethane (HCFC-22) on supported Pd and Ni catalysts. J. Environ. Sci. Health Part A 2011, 46, 989–996. [Google Scholar] [CrossRef]
- van de Sandt, E.J.A.X.; Wiersma, A.; Makkee, M.; van Bekkum, H.; Moulijn, J.A. Mechanistic study of the selective hydrogenolysis of CCl2F2 (CFC-12) into CH2F2 (HFC-32) over palladium on activated carbon. Recl. Trav. Pays-Bas 1996, 115, 505–510. [Google Scholar] [CrossRef]
- Rolland, L.; Peignon, M.C.; Cardinaud, C.; Turban, G. SiO2/Si Selectivity in High Density CHF3/CH4 Plasmas: Role of the Fluorocarbon Layer. Microelectron. Eng. 2000, 53, 375–379. [Google Scholar] [CrossRef]
- Gaboriau, F.; Cartry, G.; Peignon, M.-C.; Cardinaud, C. Selective and deep plasma etching of SiO2: Comparison between different fluorocarbon gases (CF4, C2F6, CHF3) mixed with CH4 or H2 and influence of the residence time. J. Vac. Sci. Technol. B 2002, 20, 1514–1521. [Google Scholar] [CrossRef]
- Raghuram, U. Reactive Ion Etching of Si/SiO2 by CHF3/CH4/O2 Gas Mixture. Master’s Thesis, San Jose State University, San Jose, CA, USA, 1993. [Google Scholar]
- Kanazawa, T.; Motoyama, S.; Wakayama, T.; Akinaga, H. Reactive Ion Etching of NiFe Film with Organic Resist Mask and Metal Mask by Inductively Coupled Plasma. J. Magnetics 2007, 12, 81–83. [Google Scholar] [CrossRef]
- Radlik, M.; Śrębowata, A.; Juszczyk, W.; Matus, K.; Małolepszy, A.; Karpiński, Z. n-Hexane conversion on γ-alumina supported palladium–platinum catalysts. Adsorption 2019, 25, 843–853. [Google Scholar] [CrossRef] [Green Version]
- Rousset, J.L.; Bertolini, J.C.; Miegge, P. Theory of segregation using the equivalent-medium approximation and bond-strength modifications at surfaces: Application to fcc Pd-X alloys. Phys. Rev. B 1996, 53, 4947–4957. [Google Scholar] [CrossRef]
- Ichikawa, S.; Poppa, H.; Boudart, M. Disproportionation of CO on small particles of silica-supported palladium. J. Catal. 1985, 91, 1–10. [Google Scholar] [CrossRef]
- Rachmady, V.; Vannice, M.A. Acetic acid hydrogenation over supported platinum catalysts. J. Catal. 2000, 192, 322–334. [Google Scholar] [CrossRef]
- Xu, L.; Bhandari, S.; Chen, J.; Glasgow, J.; Mavrikakis, M. Chloroform Hydrodechlorination on Palladium Surfaces: A Comparative DFT Study on Pd(111), Pd(100), and Pd(211). Top. Catal. 2020, 63, 762–776. [Google Scholar] [CrossRef]
- Chen, N.; Rioux, R.M.; Barbosa, L.A.M.M.; Ribeiro, F.H. Kinetic and theoretical study of the hydrodechlorination of CH4-xClx (x = 1–4) compounds on palladium. Langmuir 2010, 26, 16615–16624. [Google Scholar] [CrossRef]
- Deeth, R.J.; Jenkins, H.D.B. A density functional and thermochemical study of M-X bond lengths and energies in [MX6]2− complexes: LDA versus Becke88/Perdew86 gradient-corrected functionals. J. Phys. Chem. A 1997, 101, 4793–4798. [Google Scholar] [CrossRef]
- Erley, W. Chlorine adsorption on the (111) faces of Pd and Pt. Surf. Sci. 1980, 94, 281–292. [Google Scholar] [CrossRef]
- Erley, W. Chlorine adsorption on the (110) faces of Ni, Pd and Pt. Surf. Sci. 1982, 114, 47–64. [Google Scholar] [CrossRef]
- Bonarowska, M.; Kaszkur, Z.; Kępiński, L.; Karpiński, Z. Hydrodechlorination of tetrachloromethane on alumina- and silica-supported platinum catalysts. App. Catal. B Environ. 2010, 99, 248–256. [Google Scholar] [CrossRef]
- Kwak, J.H.; Hu, J.; Mei, D.; Yi, C.-W.; Kim, D.H.; Peden, C.H.F.; Allard, L.F.; Sanyi, J. Coordinatively Unsaturated Al3+ Centers as Binding Sites for Active Catalyst Phases of Platinum on γ-Al2O3. Science 2009, 325, 1670–1673. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Montero, M.A.; Gomez-Sainero, L.M.; Martin-Martinez, M.; Heras, F.; Rodriguez, J.J. Hydrodechlorination of chloromethanes with Pd on activated carbon catalysts for the treatment of residual gas streams. Appl. Catal. B Environ. 2010, 96, 148–156. [Google Scholar] [CrossRef]
- Alvarez-Montero, M.A.; Gomez-Sainero, L.M.; Mayoral, A.; Diaz, I.; Baker, R.T.; Rodriguez, J.J. Hydrodechlorination of chloromethanes with a highly stable Pt on activated carbon catalyst. J. Catal. 2011, 279, 389–396. [Google Scholar] [CrossRef]





| Catalyst a | Metal Dispersion b (D), % | Metal Particle Size, nm | ||
|---|---|---|---|---|
| dTEM c | dXRD d | dchem e | ||
| Pd/Al2O3 | 28 | 2.5 | 3.3 | 4.0 |
| Pd80Pt20/Al2O3 | 23.4 | 2.6 | n.m. f | 4.8 |
| Pd50Pt50/Al2O3 | 33.4 | 2.4 | n.m. f | 3.4 |
| Pt/Al2O3 | 44.5 | 1.3 | <2 g | 2.5 |
| Product Selectivity, % | Al2O3 | Pd/Al2O3 | Pd80Pt20/Al2O3 | Pd50Pt50/Al2O3 | Pt/Al2O3 |
|---|---|---|---|---|---|
| CH4 | - | 37.7 | 36.4 | 32.0 | 3.7 |
| CHF3 | 60.4 | 59.6 | 59.9 | 62.2 | 61.0 |
| C2H6 | - | 0.9 | 0.5 | 0.7 | 0.3 |
| CHFCl2 | 6.3 | 1.8 | 3.2 | 5.0 | 3.0 |
| CCl3F | 0.1 | - | - | - | - |
| CH2Cl2 | - | - | - | - | 2.6 |
| CHCl3 | 33.1 | - | - | - | 29.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radlik, M.; Juszczyk, W.; Kemnitz, E.; Karpiński, Z. Pd/Alumina Catalysts for Beneficial Transformation of Harmful Freon R-22. Catalysts 2021, 11, 1178. https://doi.org/10.3390/catal11101178
Radlik M, Juszczyk W, Kemnitz E, Karpiński Z. Pd/Alumina Catalysts for Beneficial Transformation of Harmful Freon R-22. Catalysts. 2021; 11(10):1178. https://doi.org/10.3390/catal11101178
Chicago/Turabian StyleRadlik, Monika, Wojciech Juszczyk, Erhard Kemnitz, and Zbigniew Karpiński. 2021. "Pd/Alumina Catalysts for Beneficial Transformation of Harmful Freon R-22" Catalysts 11, no. 10: 1178. https://doi.org/10.3390/catal11101178