Selective Synthesis of 2-(1,2,3-Triazoyl) Quinazolinones through Copper-Catalyzed Multicomponent Reaction
Abstract
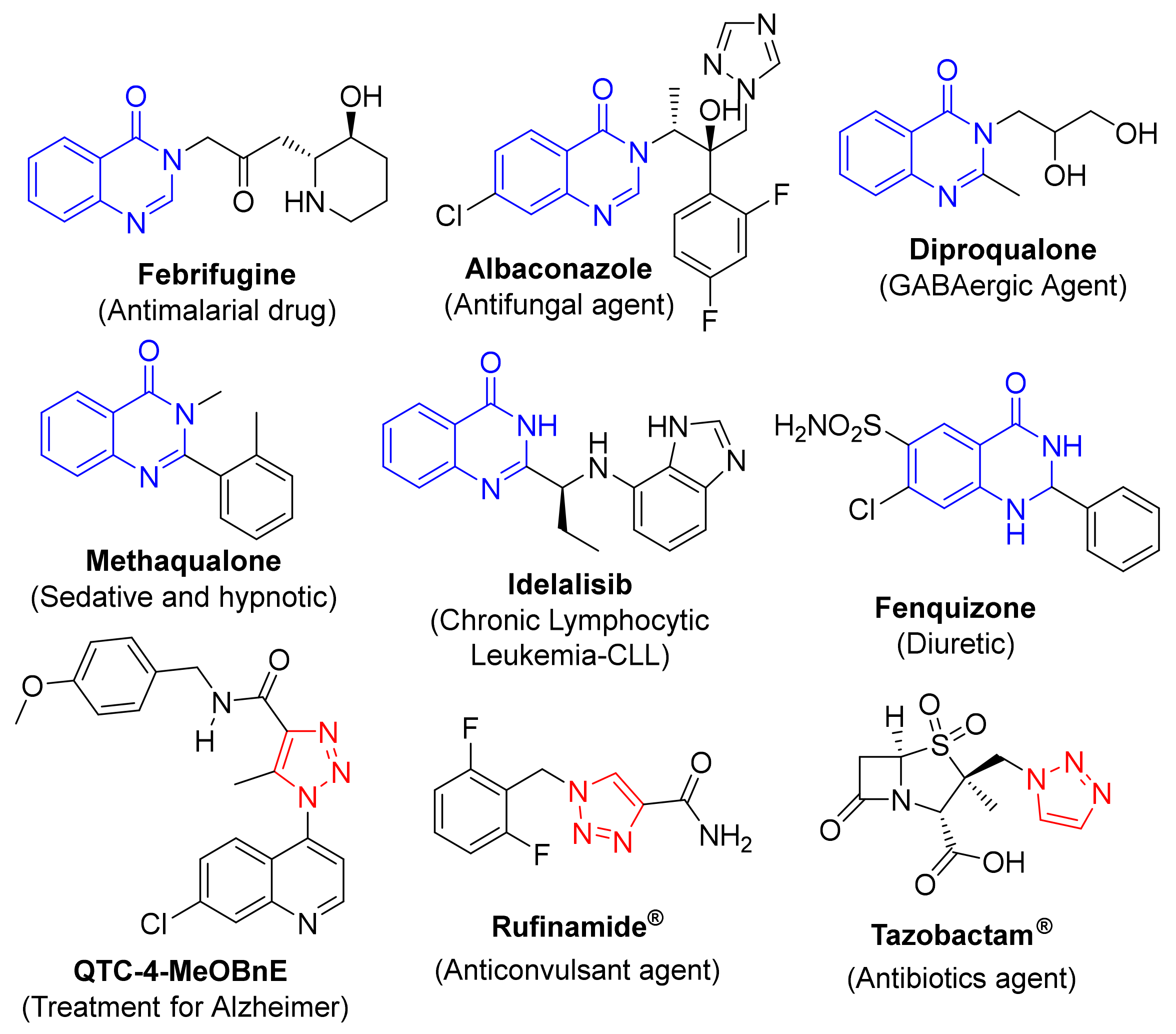
:1. Introduction
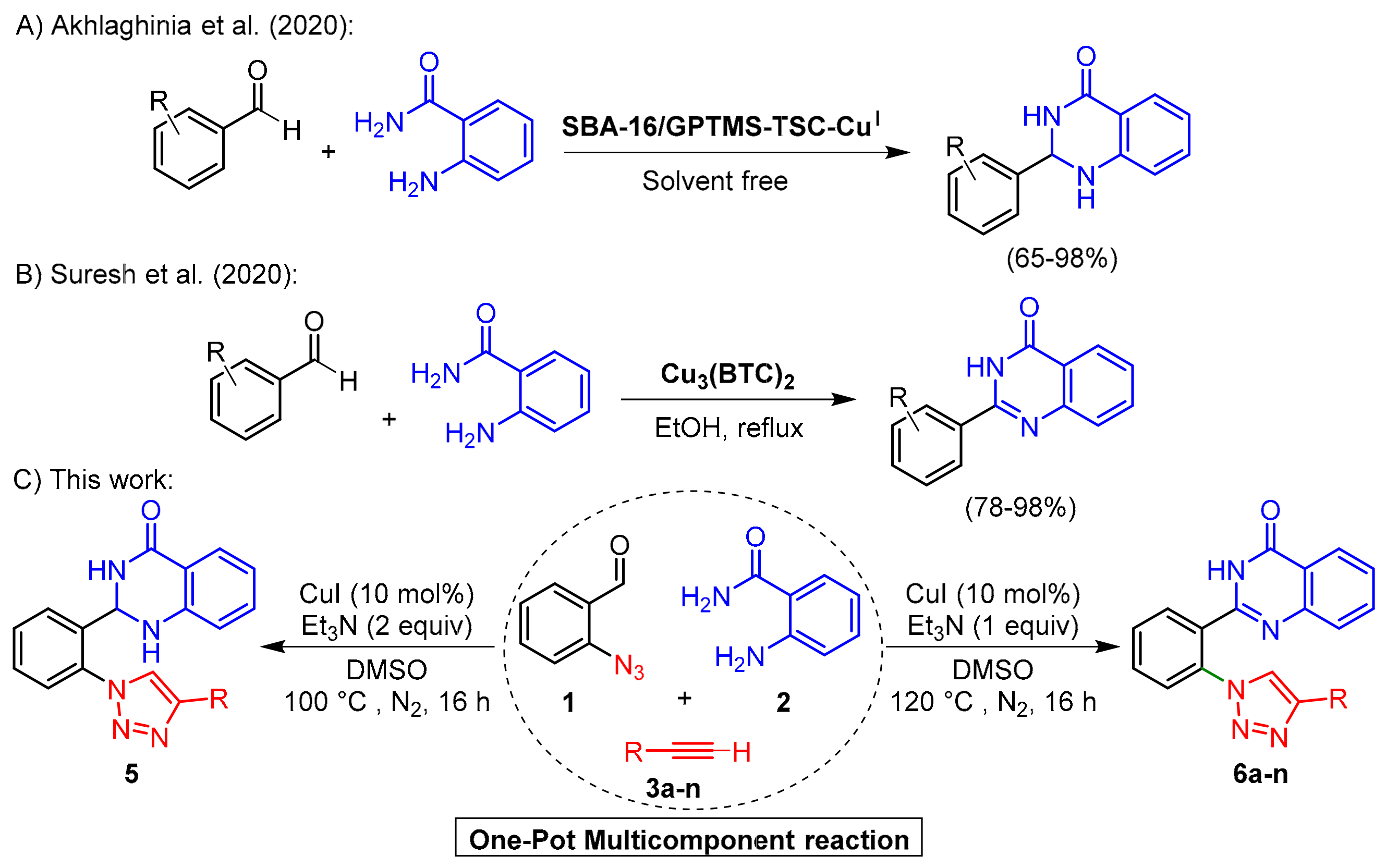
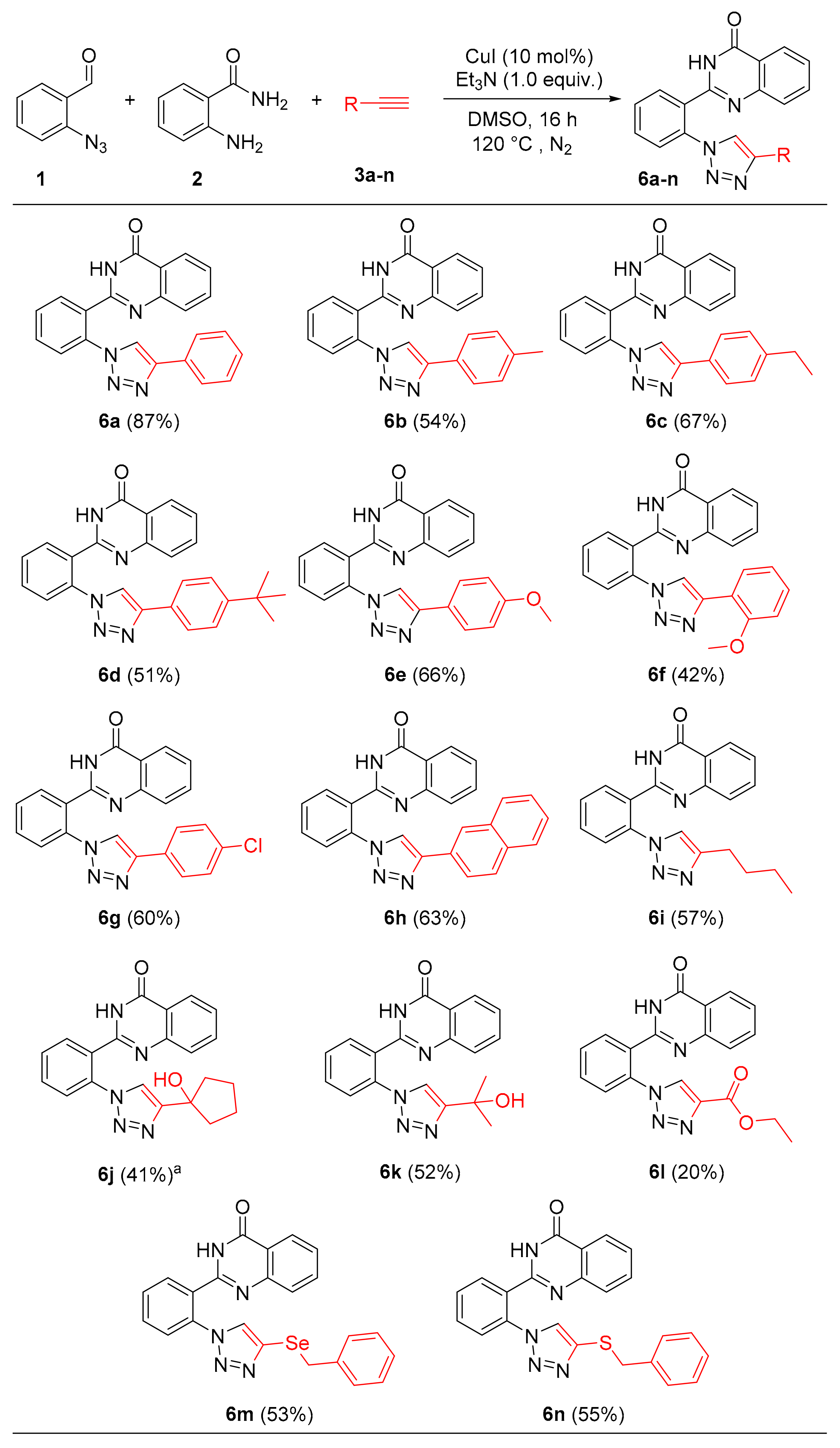
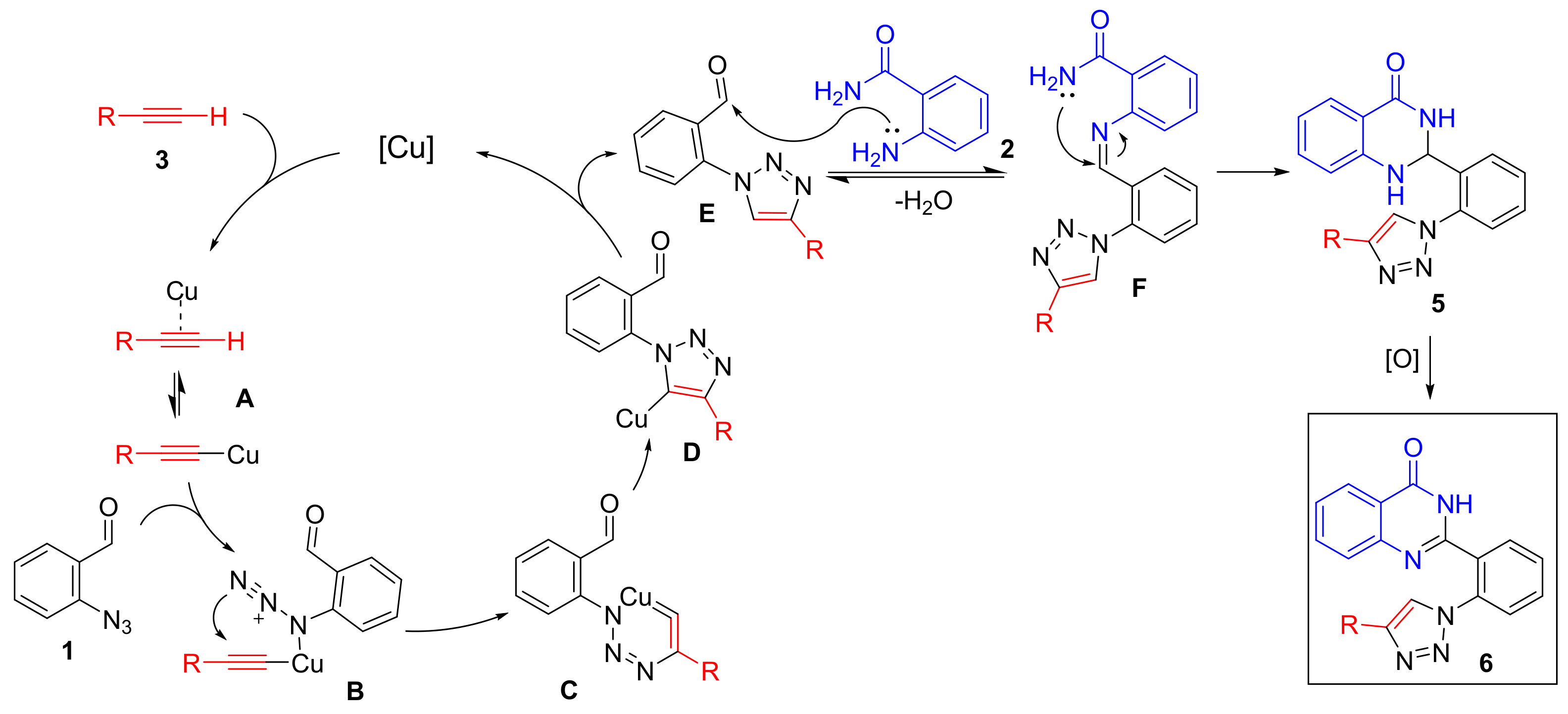
2. Results
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Synthesis of Triazoylquinazolin-4(3H)-ones 6a–n
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Auti, P.S.; George, G.; Paul, A.T. Recent advances in the pharmacological diversification of quinazoline/quinazolinone hybrids. RSC Adv. 2020, 10, 41353–41392. [Google Scholar] [CrossRef]
- Pandey, S.K.; Yadava, U.; Upadhyay, A.; Sharma, M.L. Synthesis, biological evaluation and molecular docking studies of novel quinazolinones as antitubercular and antimicrobial agents. Bioorg. Chem. 2021, 108, 104611. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.A.-M.; Abou-Zeid, L.A.; ElTahir, K.E.H.; Ayyad, R.R.; El-Sayed, M.A.-A.; El-Azab, A.S. Synthesis, anti-inflammatory, analgesic, COX-1/2 inhibitory activities and molecular docking studies of substituted 2-mercapto-4(3H)-quinazolinones. Eur. J. Med. Chem. 2016, 121, 410–421. [Google Scholar] [CrossRef]
- Brown, E.C.; Kong, T.; McNulty, J.; D’Aiuto, L.; Williamson, K.; McClain, L.; Piazza, P.; Nimgaonkar, L.V. Discovery of potent antiviral (HSV-1) quinazolinones and initial structure-activity relationship studies. Bioorg. Med. Chem. Lett. 2017, 27, 4601–4605. [Google Scholar] [CrossRef]
- Gatadi, S.; Pulivendala, G.; Gour, J.; Malasala, S.; Bujji, S.; Parupalli, R.; Shaikh, M.; Godugu, C.; Nanduri, S. Synthesis and evaluation of new 4(3H)-quinazolinone derivatives as potential anticancer agentes. J. Mol. Struct. 2020, 1200, 127097. [Google Scholar] [CrossRef]
- Decker, M. Novel inhibitors of acetyl- and butyrylcholinesterase derived from the alkaloids dehydroevodiamine and rutaecarpine. Eur. J. Med. Chem. 2005, 40, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Chandrashekar, G.; Meng, L.; Robinson, K.; Chatterji, D. Febrifugine analogue compounds: Synthesis and antimalarial evaluation. Bioorg. Med. Chem. 2012, 20, 927–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alagarsamy, V.; Chitra, K.; Saravanan, G.; Solomon, V.R.; Sulthana, M.T.; Narendhar, B. An overview of quinazolines: Pharmacological significance and recent developments. Eur. J. Med. Chem. 2018, 151, 628–685. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Wang, M.; Zhao, S.; Shu, Y.; Zeng, H.; Xiao, C.; Lu, C.; Liu, Y. Pharmaceutical prospects of naturally occurring quinazolinone and its derivatives. Fitoterapia 2017, 119, 136–149. [Google Scholar] [CrossRef]
- Gupta, R.; Arora, G.; Yadav, Y.; Dixit, R.; Srivastavab, A.; Sharma, R.K. A magnetically retrievable copper ionic liquid nanocatalyst for cyclooxidative synthesis of 2-phenylquinazolin-4(3H)-ones. Dalton Trans. 2021, 50, 890–898. [Google Scholar] [CrossRef]
- To, T.A.; Vo, Y.H.; Nguyen, H.T.T.; Ha, P.T.M.; Doan, S.H.; Doan, T.L.H.; Li, S.; Le, H.V.; Tu, T.N.; Phan, N.T.S. Iron-catalyzed one-pot sequential transformations: Synthesis of quinazolinones via oxidative Csp3-H bond activation using a new metal-organic framework as catalyst. J. Catal. 2019, 370, 11–20. [Google Scholar] [CrossRef]
- Kerdphon, S.; Sanghong, P.; Chatwichien, J.; Choommongkol, V.; Rithchumpon, P.; Singh, T.; Meepowpan, P. Commercial copper-catalyzed aerobic oxidative synthesis of quinazolinones from 2-aminobenzamide and methanol. Eur. J. Org. Chem. 2020, 2730–2734. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, X.; Chen, G.; Zhao, P. Direct synthesis of quinazolinones by heterogeneous Cu(OH)X/OMS-2 catalyst under oxygen. Catal. Commun. 2018, 104, 106–111. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, C.; Chen, X.; Ma, W.; Li, B.; Lin, Z.; Chen, X.; Li, Y.; Xie, F. Direct synthesis of quinazolinones via the carbon-supported acid-catalyzed cascade reaction of isatoic anhydrides with amides and aldehydes. Tetrahedron Lett. 2021, 66, 152835. [Google Scholar] [CrossRef]
- Wang, Q.; Lv, M.; Liu, J.; Li, Y.; Xu, Q.; Xu, Z.; Cao, H. Efficient synthesis of quinazolinones by transition-metal-free direct aerobic oxidative cascade annulation of alcohols with o-aminoarylnitriles. ChemSusChem 2019, 12, 3043–3048. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Tan, Z.; Jiang, H.; Zhu, Z.; Zhang, M. Copper-catalyzed oxidative multicomponent annulation reaction for direct synthesis of quinazolinones via an imine-protection strategy. Org. Lett. 2019, 21, 4725–4728. [Google Scholar] [CrossRef]
- Erfan, M.A.; Akhlaghinia, B.; Ghodsinia, S.S.E. An efficient green protocol for synthesis of 2,3-dihydroquinazolin-4(1H)-ones using SBA-16/GPTMS-TSC-CuI under solvent-free conditions. ChemistrySelect 2020, 5, 2306–2316. [Google Scholar] [CrossRef]
- Latha, G.; Devarajan, N.; Suresh, P. Framework copper catalyzed oxidative synthesis of quinazolinones: A benign approach using Cu3(BTC)2 MOF as an efficient and reusable catalyst. ChemistrySelect 2020, 5, 10041–10047. [Google Scholar] [CrossRef]
- Rani, A.; Singh, G.; Singh, A.; Maqbool, U.; Kaur, G.; Singh, J. CuAAC-ensembled 1,2,3-triazole-linked isosteres as pharmacophores in drug discovery: Review. RSC Adv. 2020, 10, 5610–5635. [Google Scholar] [CrossRef]
- Jain, A.; Piplani, P. Exploring the chemistry and therapeutic potential of triazoles: A comprehensive literature review. Mini Rev. Med. Chem. 2019, 19, 1298–1368. [Google Scholar] [CrossRef]
- Mantoani, S.P.; Andrade, P.; Chierrito, T.P.C.; Figueredo, A.S.; Carvalho, I. Potential triazole-based molecules for the treatment of neglected diseases. Curr. Med. Chem. 2019, 26, 4403–4434. [Google Scholar] [CrossRef]
- Asif, M. Pharmacological activities of triazole analogues as antibacterial, antifungal, antiviral agents. Pharm. Sci. Asia 2017, 44, 59–74. [Google Scholar] [CrossRef]
- Bonandi, E.; Christodoulou, M.S.; Fumagalli, G.; Perdicchia, D.; Rastelli, G.; Passarella, D. The 1,2,3-triazole ring as a bioisostere in medicinal chemistry. Drug Discov. Today 2017, 22, 1572–1581. [Google Scholar] [CrossRef]
- Massarotti, A.; Aprile, S.; Mercalli, V.; Del Grosso, E.; Grosa, G.; Sorba, G.; Tron, G.C. Are 1,4- and 1,5-disubstituted 1,2,3-triazoles good pharmacophoric groups? ChemMedChem 2014, 9, 2497–2508. [Google Scholar] [CrossRef]
- Fronza, M.G.; Baldinotti, R.; Fetter, J.; Sacramento, M.; Sousa, F.S.S.; Seixas, F.K.; Collares, T.; Alves, D.; Pratico, D.; Savegnago, L. QTC-4-MeOBnE Rescues Scopolamine-Induced Memory Deficits in Mice by Targeting Oxidative Stress, Neuronal Plasticity, and Apoptosis. ACS Chem. Neurosci. 2020, 11, 1259–1269. [Google Scholar] [CrossRef]
- Fronza, M.G.; Baldinotti, R.; Sacramento, M.; Gutierres, J.; Carvalho, F.B.; Fernandes, M.C.; Sousa, F.S.S.; Seixas, F.K.; Collares, T.; Alves, D.; et al. Effect of QTC-4-MeOBnE Treatment on Memory, Neurodegeneration, and Neurogenesis in a Streptozotocin-Induced Mouse Model of Alzheimer’s Disease. ACS Chem. Neurosci. 2021, 12, 109–122. [Google Scholar] [CrossRef]
- Cheng-Hakimian, A.; Anderson, G.D.; Miller, J.W. Rufinamide: Pharmacology, clinical trials, and role in clinical practice. Int. J. Clin. Pract. 2006, 60, 1497–1501. [Google Scholar] [CrossRef] [PubMed]
- Sanders, W.E., Jr.; Sanders, C.C. Piperacillin/Tazobactam: A critical review of the evolving clinical literature. Clin. Infect. Dis. 1996, 22, 107–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aufort, M.; Herscovici, J.; Bouhours, P.; Moreau, N.; Girard, C. Synthesis and antibiotic activity of a small molecules library of 1,2,3-triazole derivatives. Bioorg. Med. Chem. Lett. 2008, 18, 1195–1198. [Google Scholar] [CrossRef] [PubMed]
- Leaver, D.J.; Dawson, R.M.; White, J.M.; Polyzos, A.; Hughes, A.B. Synthesis of 1,2,3-triazole linked galactopyranosides and evaluation of cholera toxin inhibition. Org. Biomol. Chem. 2011, 9, 8465–8474. [Google Scholar] [CrossRef]
- Zhang, B. Comprehensive review on the anti-bacterial activity of 1,2,3-triazole hybrids. Eur. J. Med. Chem. 2019, 168, 357–372. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, S.-J.; Liu, Y. 1,2,3-Triazole-containing hybrids as potential anticancer agents: Current developments, action mechanisms and structure-activity relationships. Eur. J. Med. Chem. 2019, 183, 111700. [Google Scholar] [CrossRef]
- Lima-Neto, R.G.; Cavalcante, N.N.M.; Srivastava, R.M.; Junior, F.J.B.M.; Wanderley, A.G.; Neves, R.P.; Anjos, A.P. Synthesis of 1,2,3-triazole derivatives and in vitro antifungal evaluation on Candida strains. Molecules 2012, 17, 5882–5892. [Google Scholar] [CrossRef] [Green Version]
- Shalini, K.; Kumar, N.; Drabu, S. Advances in synthetic approach to and antifungal activity of triazoles. Beilstein J. Org. Chem. 2011, 7, 668–677. [Google Scholar] [CrossRef]
- Silva, F.C.; Souza, M.C.B.V.; Frugulhetti, I.I.P.; Castro, H.C.; Souza, S.L.O.; Souza, T.M.L.; Rodrigues, D.Q.; Souza, A.M.T.; Abreu, P.A.; Passamani, F.; et al. Synthesis, HIV-RT inhibitory activity and SAR of 1-benzyl-1H-1,2,3-triazole derivatives of carbohydrates. Eur. J. Med. Chem. 2009, 44, 373–383. [Google Scholar] [CrossRef]
- Raj, R.; Singh, P.; Singh, P.; Gut, J.; Rosenthal, P.J.; Kumar, V. Azide-alkyne cycloaddition en route to 1H-1,2,3-triazole-tethered 7-chloroquinoline-isatin chimeras: Synthesis and antimalarial evaluation. Eur. J. Med. Chem. 2013, 62, 590–596. [Google Scholar] [CrossRef] [Green Version]
- Im, C.; Maiti, S.N.; Micetich, R.G.; Daneshtalab, M.; Atchison, K.; Phillips, O.A.; Kunugita, C.J. Synthesis and β-lactamase inhibitory activity of 6-[(1-heteroarylthioethyl-1, 2, 3-triazol-4-yl)-methylene]penam sulfones. J. Antibiot. 1994, 47, 1030–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Ramachary, D.B.; Ramakumar, K.; Narayana, V.V. Amino Acid-Catalyzed Cascade [3+2]-Cycloaddition/Hydrolysis Reactions Based on the Push–Pull Dienamine Platform: Synthesis of Highly Functionalized NH-1,2,3-Triazoles. Chem. Eur. J. 2008, 14, 9143–9147. [Google Scholar] [CrossRef]
- Belkheira, M.; El Abed, D.; Pons, J.M.; Bressy, C. Organocatalytic Synthesis of 1,2,3-Triazoles from Unactivated Ketones and Arylazides. Chem. Eur. J. 2011, 17, 12917–12921. [Google Scholar] [CrossRef]
- Danence, L.J.T.; Gao, Y.; Li, M.; Huang, Y.; Wang, J. Organocatalytic Enamide–Azide Cycloaddition Reactions: Regiospecific Synthesis of 1,4,5-Trisubstituted-1,2,3-Triazoles. Chem. Eur. J. 2011, 17, 3584–3587. [Google Scholar] [CrossRef] [PubMed]
- Alagarsamy, V.; Solomon, V.R.; Murugan, M. Synthesis and pharmacological investigation of novel 4-benzyl-1-substituted-4H-[1,2,4]triazolo[4,3-a]quinazolin-5-ones as new class of H1-antihistaminic agents. Bioorg. Med. Chem. 2007, 15, 4009–4015. [Google Scholar] [CrossRef] [PubMed]
- Driowya, M.; Leclercq, J.; Verones, V.; Barczyk, A.; Lecoeur, M.; Renault, N.; Flouquet, N.; Ghinet, A.; Berthelot, P.; Lebegue, N. Synthesis of triazoloquinazolinone based compounds as tubulin polymerization inhibitors and vascular disrupting agents. Eur. J. Med. Chem. 2016, 115, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Ram, V.J.; Srimal, R.C.; Kushwaha, D.S.; Mishra, L. Chemotherapeutic agents. XIX. Synthesis of [1,2,4]-Triazoloquinazolinones and related compounds as antihypertensive agents. J. Prakt. Chem. 1990, 332, 629–639. [Google Scholar] [CrossRef]
- Bertelli, L.; Biagi, G.; Giorgi, I.; Livi, O.; Manera, C.; Scartoni, V.; Lucacchini, A.; Giannaccini, G.; Barili, P.L. Substituted 1,2,3-triazolo[1,5-a]quinazolines: Synthesis and binding to benzodiazepine and adenosine receptors. Eur. J. Med. Chem. 2000, 35, 333–341. [Google Scholar] [CrossRef]
- Peringer, F.; Nascimento, J.E.R.; Abib, P.B.; Barcellos, T.; Eycken, E.V.V.; Perin, G.; Jacob, R.G.; Alves, D. Copper-catalyzed multicomponent reactions: Synthesis of fused 1,2,3-triazolo-1,3,6-triazonines. Eur. J. Org. Chem. 2017, 2017, 2579. [Google Scholar] [CrossRef]
- Hein, J.E.; Fokin, V.V. Copper-catalyzed azide–alkynecycloaddition (CuAAC) and beyond: New reactivity of copper(i) acetylides. Chem. Soc. Rev. 2010, 39, 1302–1315. [Google Scholar] [CrossRef]
- Liang, L.; Astruc, D. The copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications. An overview. Coord. Chem. Rev. 2011, 255, 2933–2945. [Google Scholar] [CrossRef]
- Li, L.; Zang, Z. Development and Applications of the Copper-Catalyzed Azide-Alkyne Cycloaddition (CuAAC) as a Bioorthogonal Reaction. Molecules 2016, 21, 1393. [Google Scholar] [CrossRef]
- Rammah, M.M.; Gati, W.; Mtiraoui, H.; Rammah, M.E.B.; Ciamala, K.; Knorr, M.; Rousselin, Y.; Kubicki, M.M. Synthesis of Isoxazole and 1,2,3-Triazole Isoindole Derivatives via Silver- and Copper-Catalyzed 1,3-Dipolar Cycloaddition Reaction. Molecules 2016, 21, 307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; Li, Y.; Tao, L.; Zhang, W.; Fan, X. Rapid assembly of quinazolinone scaffold via copper-catalyzed tandem reaction of 2-bromobenzamides with aldehydes and aqueous ammonia: Application to the synthesis of the alkaloid tryptanthrin. RSC Adv. 2014, 4, 59289–59296. [Google Scholar] [CrossRef]
- Hu, B.-Q.; Wang, L.-X.; Yang, L.; Xiang, J.-F.; Tang, Y.-L. Copper-Catalyzed Intramolecular C–C Bond Cleavage To Construct 2-Substituted Quinazolinones. Eur. J. Org. Chem. 2015, 4504–4509. [Google Scholar] [CrossRef]
- Upadhyaya, K.; Thakur, R.K.; Shukla, S.K.; Tripathi, R.P. One-Pot Copper(I)-Catalyzed Ligand/Base-Free Tandem Cyclooxidative Synthesis of Quinazolinones. J. Org. Chem. 2016, 81, 5046–5055. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Brandenburg, K.; Putz, H. Diamond—Crystal and Molecular Structure Visualization; Crystal Impact: Bonn, Germany, 2014. [Google Scholar]
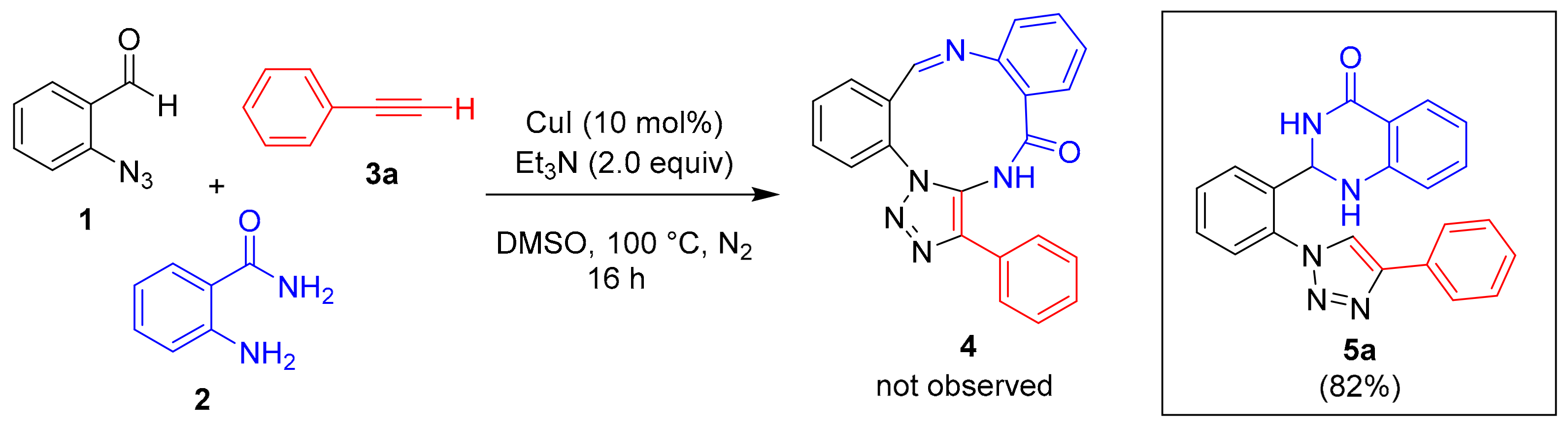
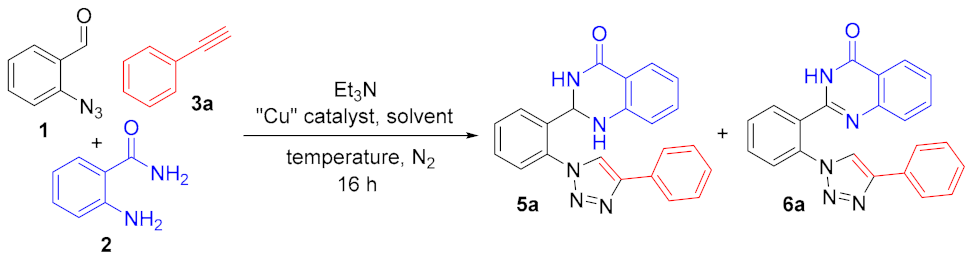
| Entry | Et3N (mmol) | Catalyst (10 mol%) | Solvent | Temp. (°C) | Yield (%) b | Ratio c 5a:6a |
|---|---|---|---|---|---|---|
| 1 | 1 | CuI | DMSO | 100 | 82 | 1:0 |
| 2 | 1 | CuBr | DMSO | 100 | 21 | 1:0 |
| 3 | 1 | CuCl | DMSO | 100 | 27 | 1:0 |
| 4 | 1 | CuBr2 | DMSO | 100 | n.f. | -- |
| 5 | 1 | CuCl2 | DMSO | 100 | n.f. | -- |
| 6 | 1 | Cu(OAc)2·H2O | DMSO | 100 | 38 | 1:0 |
| 7 d | 1 | CuI | DMSO | 100 | 81 | 1:0 |
| 8 e | 1 | CuI | DMSO | 100 | 31 | 1:0 |
| 9 | 1 | CuI | 1,4-dioxane | 100 | 36 | 1:0 |
| 10 | 1 | CuI | DMF | 100 | NF | 1:0 |
| 11 | 1 | CuI | PEG-400 | 100 | 58 | 1:0 |
| 12 | 1 | CuI | Toluene | 100 | 61 | 1:0 |
| 13 f | 1 | CuI | DMSO | 100 | 65 | 1:0 |
| 14 | 1 | CuI | DMSO | 80 | 60 | 1:0 |
| 15 | 1 | CuI | DMSO | 120 | 88 | 1:0.5 |
| 16 | 1 | CuI | DMSO | 140 | 90 | 1:0.7 |
| 17 | 0.5 | CuI | DMSO | 120 | 87 | 0:1 |
| 18 | 0.5 | CuI | DMSO | 100 | 66 | 0.2:1 |
| 19 | 0.5 | -- | DMSO | 120 | NF | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacramento, M.; Piúma, L.P.A.; Nascimento, J.E.R.; Cargnelutti, R.; Jacob, R.G.; Lenardão, E.J.; Alves, D. Selective Synthesis of 2-(1,2,3-Triazoyl) Quinazolinones through Copper-Catalyzed Multicomponent Reaction. Catalysts 2021, 11, 1170. https://doi.org/10.3390/catal11101170
Sacramento M, Piúma LPA, Nascimento JER, Cargnelutti R, Jacob RG, Lenardão EJ, Alves D. Selective Synthesis of 2-(1,2,3-Triazoyl) Quinazolinones through Copper-Catalyzed Multicomponent Reaction. Catalysts. 2021; 11(10):1170. https://doi.org/10.3390/catal11101170
Chicago/Turabian StyleSacramento, Manoela, Luís Pedro A. Piúma, José Edmilson R. Nascimento, Roberta Cargnelutti, Raquel G. Jacob, Eder João Lenardão, and Diego Alves. 2021. "Selective Synthesis of 2-(1,2,3-Triazoyl) Quinazolinones through Copper-Catalyzed Multicomponent Reaction" Catalysts 11, no. 10: 1170. https://doi.org/10.3390/catal11101170








