Production of Levulinic Acid from Cellulose and Cellulosic Biomass in Different Catalytic Systems
Abstract
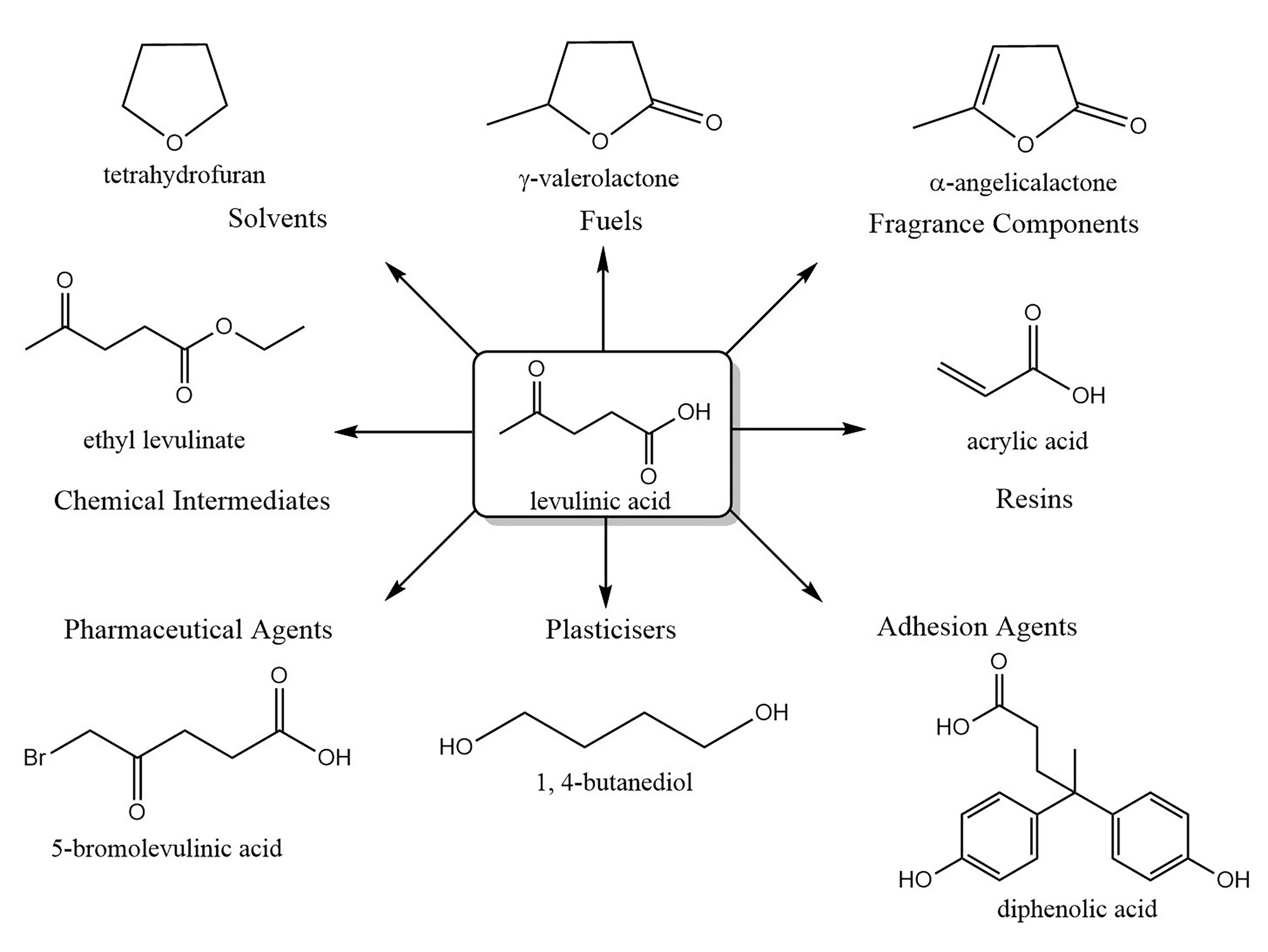
:1. Introduction
2. Mechanism Studies on the Production of LA
3. Pretreatment
4. Catalytic Systems for the Preparation of LA
4.1. Homogeneous Catalysts
4.1.1. Conventional Mineral Acids
4.1.2. Ionic Liquids
4.2. Heterogeneous Catalysts
4.2.1. Metal Salts
4.2.2. Solid Acids
5. Conclusions and Outlook
- (1)
- Future research should mainly focus on reducing energy input and waste generation, and developing environmentally friendly processes to increase the yield of LA. Researchers should also conduct techno-economic analyses to facilitate the commercial production of LA.
- (2)
- The formation of by-products such as humins is a bottleneck in the industrial production of LA. This problem is even more prominent when lignocellulosic biomass is used as a feedstock. A suitable solvent system can decrease the formation of humins to promote the selectivity of LA. Biphasic solvents work well on a laboratory scale and can be tried for use on a plant scale. Low temperatures and high-concentration acid could possibly prevent the formation of humins.
- (3)
- Catalysts containing special structures should be developed to reduce carbon deposition. Additionally, another strategy to avoid carbon deposition is exploring a specific catalytic route to synthesize LA under mild conditions. The calcination and air oxidation in the temperature range of 400–500 °C are the preferred methods to remove humins [152]. It is recommended to wash the catalysts with H2O2, HCl, NaOH, ethanol, or acetone when working with temperature limitations. When the SO3H-functionalized catalyst is washed with methanol, methyl sulfonate can be constituted on the solid surface and the catalytic activity will reduce after consecutive washing [153]. The sulfonation method should be optimized to avoid the loss of acidic sites, thereby improving the stability.
- (4)
- Water is safe and eco-friendly, with a high thermal conductivity as well as a low viscosity for the LA production from biomass. On the other hand, water is not recognized as a suitable solvent since the feedstock is insoluble, especially since mass transfer is limited by a heterogeneous catalyst [116]. Therefore, using a suitable organic solvent is a favorable alternative. However, the separation and purification of LA from organic solvent is still a challenge. Generating a higher concentration of LA in the product stream may reduce the amount of waste liquid and energy cost.
- (5)
- Brønsted/Lewis acid molar ratio is a key factor in catalyst activity. Therefore, designing novel catalysts with adjustable acid sites is instructive for seeking an effective way to obtain LA. Developing green heterogeneous catalysts should focus on significant factors—e.g., proper shape selectivity, acid site accessibility, recyclability, and long-term stability.
- (6)
- It is necessary to find a more efficient and green catalytic system and further optimize the separation and purification technology to achieve industrial production. Unavoidable by-products can be converted into valuable new carbon materials. An in-depth study of the preparation of LA from cellulose is significant for future development, and future research in this area will remain the focus of the high-value utilization of biomass resources.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abbasi, T.; Abbasi, S.A. Decarbonization of fossil fuels as a strategy to control global warming. Renew. Sustain. Energy Rev. 2011, 15, 1828–1834. [Google Scholar] [CrossRef]
- Yu, Z.; Lu, X.; Xiong, J.; Ji, N. Transformation of Levulinic Acid to Valeric Biofuels: A Review on Heterogeneous Bifunctional Catalytic Systems. Chemsuschem 2019, 12, 3915–3930. [Google Scholar] [CrossRef]
- Mansoor, M.; Mariun, N.; Ismail, N.; Wahab, N.I.A. A guidance chart for most probable solution directions in sustainable energy developments. Renew. Sustain. Energy Rev. 2013, 24, 306–313. [Google Scholar] [CrossRef]
- Yu, Z.; Lu, X.; Liu, C.; Han, Y.; Ji, N. Synthesis of gamma-valerolactone from different biomass-derived feedstocks: Recent advances on reaction mechanisms and catalytic systems. Renew. Sustain. Energy Rev. 2019, 112, 140–157. [Google Scholar] [CrossRef]
- Kim, S.; Dale, B.E. Global potential bioethanol production from wasted crops and crop residues. Biomass Bioenergy 2004, 26, 361–375. [Google Scholar] [CrossRef]
- Li, X.; Lu, X.; Liang, M.; Xu, R.; Yu, Z.; Duan, B.; Lu, L.; Si, C. Conversion of waste lignocellulose to furfural using sulfonated carbon microspheres as catalyst. Waste Manag. 2020, 108, 119–126. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; West, R.M.; Durnesic, J.A. Catalytic Conversion of Renewable Biomass Resources to Fuels and Chemicals. In Annual Review of Chemical and Biomolecular Engineering; Prausnitz, J.M., Doherty, M.F., Segalman, R.A., Eds.; Annual Reviews: Palo Alto, CA, USA, 2010; Volume 1, pp. 79–100. [Google Scholar]
- Dhillon, R.S.; von Wuehlisch, G. Mitigation of global warming through renewable biomass. Biomass Bioenergy 2013, 48, 75–89. [Google Scholar] [CrossRef]
- Wettstein, S.G.; Alonso, D.M.; Guerbuez, E.I.; Dumesic, J.A. A roadmap for conversion of lignocellulosic biomass to chemicals and fuels. Curr. Opin. Chem. Eng. 2012, 1, 218–224. [Google Scholar] [CrossRef]
- Liu, G.; Bao, J. Evaluation of electricity generation from lignin residue and biogas in cellulosic ethanol production. Bioresour. Technol. 2017, 243, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Brewer, C.E. Producing jet fuel from biomass lignin: Potential pathways to alkyl benzenes and cycloalkanes. Renew. Sustain. Energy Rev. 2017, 72, 673–722. [Google Scholar] [CrossRef]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindstrom, T.; Nietzsche, S.; et al. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Yao, Q.; Liu, J.; Sun, J.; Zhu, Q.; Chen, H. Processing nanocellulose to bulk materials: A review. Cellulose 2019, 26, 7585–7617. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.R.; Sharma, S.K.; Lindstrom, T.; Hsiao, B.S. Nanocellulose-Enabled Membranes for Water Purification: Perspectives. Adv. Sustain. Syst. 2020, 4. [Google Scholar] [CrossRef]
- Salimi, S.; Sotudeh-Gharebagh, R.; Zarghami, R.; Chan, S.Y.; Yuen, K.H. Production of Nanocellulose and Its Applications in Drug Delivery: A Critical Review. ACS Sustain. Chem. Eng. 2019, 7, 15800–15827. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.; Lee, S.Y.; Wei, T.; Li, J.; Fan, Z. Nanocellulose: A promising nanomaterial for advanced electrochemical energy storage. Chem. Soc. Rev. 2018, 47, 2837–2872. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Hsiao, B.S. Efficient Removal of UO22+ from Water Using Carboxycellulose Nanofibers Prepared by the Nitro-Oxidation Method. Ind. Eng. Chem. Res. 2017, 56, 13885–13893. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Geng, L.; Amiralian, N.; Martin, D.; Hsiao, B.S. Nanocellulose from Spinifex as an Effective Adsorbent to Remove Cadmium(II) from Water. ACS Sustain. Chem. Eng. 2018, 6, 3279–3290. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Athira, K.B.; Rubiyah, M.H.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef]
- Sharma, P.R.; Joshi, R.; Sharma, S.K.; Hsiao, B.S. A Simple Approach to Prepare Carboxycellulose Nanofibers from Untreated Biomass. Biomacromolecules 2017, 18, 2333–2342. [Google Scholar] [CrossRef]
- Alvira, P.; Tomas-Pejo, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Nolte, M.W.; Shanks, B.H. Catalytic dehydration of C6 carbohydrates for the production of hydroxymethylfurfural (HMF) as a versatile platform chemical. Green Chem. 2014, 16, 548–572. [Google Scholar] [CrossRef]
- Saha, B.; Abu-Omar, M.M. Advances in 5-hydroxymethylfurfural production from biomass in biphasic solvents. Green Chem. 2014, 16, 24–38. [Google Scholar] [CrossRef]
- Morales, M.; Quintero, J.; Conejeros, R.; Aroca, G. Life cycle assessment of lignocellulosic bioethanol: Environmental impacts and energy balance. Renew. Sustain. Energy Rev. 2015, 42, 1349–1361. [Google Scholar] [CrossRef]
- Bohre, A.; Saha, B.; Abu-Omar, M.M. Catalytic Upgrading of 5-Hydroxymethylfurfural to Drop-in Biofuels by Solid Base and Bifunctional Metal-Acid Catalysts. Chemsuschem 2015, 8, 4022–4029. [Google Scholar] [CrossRef] [PubMed]
- Bozell, J.J. CHEMISTRY Connecting Biomass and Petroleum Processing with a Chemical Bridge. Science 2010, 329, 522–523. [Google Scholar] [CrossRef]
- Li, X.; Lu, X.; Nie, S.; Liang, M.; Yu, Z.; Duan, B.; Yang, J.; Xu, R.; Lu, L.; Si, C. Efficient catalytic production of biomass-derived levulinic acid over phosphotungstic acid in deep eutectic solvent. Ind. Crop. Prod. 2020, 145. [Google Scholar] [CrossRef]
- Yu, Z.; Lu, X.; Bai, H.; Xiong, J.; Feng, W.; Ji, N. Effects of Solid Acid Supports on the Bifunctional Catalysis of Levulinic Acid to gamma-Valerolactone: Catalytic Activity and Stability. Chem. Asian J. 2020, 15, 1182–1201. [Google Scholar] [CrossRef]
- Zheng, X.; Gu, X.; Ren, Y.; Zhi, Z.; Lu, X. Production of 5-hydroxymethyl furfural and levulinic acid from lignocellulose in aqueous solution and different solvents. Biofuels Bioprod. Biorefin. 2016, 10, 917–931. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Yan, K.; Lafleur, T.; Wu, X.; Chai, J.; Wu, G.; Xie, X. Cascade upgrading of gamma-valerolactone to biofuels. Chem. Commun. 2015, 51, 6984–6987. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Sasaki, T. Focus on materials science of topological insulators and superconductors Foreword. Sci. Technol. Adv. Mater. 2015, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maity, S.K. Opportunities, recent trends and challenges of integrated biorefinery: Part I. Renew. Sustain. Energy Rev. 2015, 43, 1427–1445. [Google Scholar] [CrossRef] [Green Version]
- Bozell, J.J.; Moens, L.; Elliott, D.C.; Wang, Y.; Neuenscwander, G.G.; Fitzpatrick, S.W.; Bilski, R.J.; Jarnefeld, J.L. Production of levulinic acid and use as a platform chemical for derived products. Resour. Conserv. Recycl. 2000, 28, 227–239. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Bhanage, B.M. The green metric evaluation and synthesis of diesel-blend compounds from biomass derived levulinic acid in supercritical carbon dioxide. Biomass Bioenergy 2016, 84, 12–21. [Google Scholar] [CrossRef]
- Yan, K.; Jarvis, C.; Gu, J.; Yan, Y. Production and catalytic transformation of levulinic acid: A platform for speciality chemicals and fuels. Renew. Sustain. Energy Rev. 2015, 51, 986–997. [Google Scholar] [CrossRef]
- Hayes, G.C.; Becer, C.R. Levulinic acid: A sustainable platform chemical for novel polymer architectures. Polym. Chem. 2020, 11, 4068–4077. [Google Scholar] [CrossRef]
- Demolis, A.; Essayem, N.; Rataboul, F. Synthesis and Applications of Alkyl Levulinates. ACS Sustain. Chem. Eng. 2014, 2, 1338–1352. [Google Scholar] [CrossRef]
- Harmsen, P.F.H.; Hackmann, M.M.; Bos, H.L. Green building blocks for bio-based plastics. Biofuels Bioprod. Biorefin. 2014, 8, 306–324. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Wang, D.; Dumesic, J.A. Catalytic upgrading of levulinic acid to 5-nonanone. Green Chem. 2010, 12, 574–577. [Google Scholar] [CrossRef]
- Bond, J.Q.; Alonso, D.M.; Wang, D.; West, R.M.; Dumesic, J.A. Integrated Catalytic Conversion of gamma-Valerolactone to Liquid Alkenes for Transportation Fuels. Science 2010, 327, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.P.; Price, R.; Ayoub, P.M.; Louis, J.; Petrus, L.; Clarke, L.; Gosselink, H. Valeric Biofuels: A Platform of Cellulosic Transportation Fuels. Angew. Chem. Int. Ed. 2010, 49, 4479–4483. [Google Scholar] [CrossRef] [PubMed]
- Chan-Thaw, C.E.; Marelli, M.; Psaro, R.; Ravasio, N.; Zaccheria, F. New generation biofuels: Gamma-valerolactone into valeric esters in one pot. RSC Adv. 2013, 3, 1302–1306. [Google Scholar] [CrossRef]
- Pan, T.; Deng, J.; Xu, Q.; Xu, Y.; Guo, Q.X.; Fu, Y. Catalytic conversion of biomass-derived levulinic acid to valerate esters as oxygenated fuels using supported ruthenium catalysts. Green Chem. 2013, 15, 2967–2974. [Google Scholar] [CrossRef]
- Deng, W.; Zhang, Q.; Wang, Y. Catalytic transformations of cellulose and its derived carbohydrates into 5-hydroxymethylfurfural, levulinic acid, and lactic acid. Sci. China Chem. 2015, 58, 29–46. [Google Scholar] [CrossRef]
- Mukherjee, A.; Dumont, M.J.; Raghauan, V. Review: Sustainable production of hydroxymethylfurfural and levulinic acid: Challenges and opportunities. Biomass Bioenergy 2015, 72, 143–183. [Google Scholar] [CrossRef]
- Rinaldi, R.; Schueth, F. Acid Hydrolysis of Cellulose as the Entry Point into Biorefinery Schemes. Chemsuschem 2009, 2, 1096–1107. [Google Scholar] [CrossRef]
- Yang, G.; Pidko, E.A.; Hensen, E.J.M. Mechanism of Brønsted acid-catalyzed conversion of carbohydrates. J. Catal. 2012, 295, 122–132. [Google Scholar] [CrossRef]
- Assary, R.S.; Redfern, P.C.; Hammond, J.R.; Greeley, J.; Curtiss, L.A. Computational Studies of the Thermochemistry for Conversion of Glucose to Levulinic Acid. J. Phys. Chem. B 2010, 114, 9002–9009. [Google Scholar] [CrossRef]
- Garces, D.; Diaz, E.; Ordonez, S. Aqueous Phase Conversion of Hexoses into 5-Hydroxymethylfurfural and Levulinic Acid in the Presence of Hydrochloric Acid: Mechanism and Kinetics. Ind. Eng. Chem. Res. 2017, 56, 5221–5230. [Google Scholar] [CrossRef]
- Hu, L.; Zhao, G.; Hao, W.; Tang, X.; Sun, Y.; Lin, L.; Liu, S. Catalytic conversion of biomass-derived carbohydrates into fuels and chemicals via furanic aldehydes. RSC Adv. 2012, 2, 11184–11206. [Google Scholar] [CrossRef]
- Daorattanachai, P.; Khemthong, P.; Viriya-empikul, N.; Laosiripojana, N.; Faungnawakij, K. Conversion of fructose, glucose, and cellulose to 5-hydroxymethylfurfural by alkaline earth phosphate catalysts in hot compressed water. Carbohydr. Res. 2012, 363, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Aizawa, Y.; Iida, T.; Nishimura, R.; Inomata, H. Catalytic glucose and fructose conversions with TiO2 and ZrO2 in water at 473 K: Relationship between reactivity and acid-base property determined by TPD measurement. Appl. Catal. A Gen. 2005, 295, 150–156. [Google Scholar] [CrossRef]
- Qi, X.; Watanabe, M.; Aida, T.M.; Smith, R.L., Jr. Catalytical conversion of fructose and glucose into 5-hydroxymethylfurfural in hot compressed water by microwave heating. Catal. Commun. 2008, 9, 2244–2249. [Google Scholar] [CrossRef]
- Antal, M.J., Jr.; Mok, W.S.; Richards, G.N. Mechanism of formation of 5-(hydroxymethyl)-2-furaldehyde from D-fructose an sucrose. Carbohydr. Res. 1990, 199, 91–109. [Google Scholar] [CrossRef]
- Moreau, C.; Durand, R.; Razigade, S.; Duhamet, J.; Faugeras, P.; Rivalier, P.; Ros, P.; Avignon, G. Dehydration of fructose to 5-hydroxymethylfurfural over H-mordenites. Appl. Catal. A Gen. 1996, 145, 211–224. [Google Scholar] [CrossRef]
- Li, K.; Bai, L.; Amaniampong, P.N.; Jia, X.; Lee, J.M.; Yang, Y. One-Pot Transformation of Cellobiose to Formic Acid and Levulinic Acid over Ionic-Liquid-based Polyoxometalate Hybrids. Chemsuschem 2014, 7, 2670–2677. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Wilson, L.D.; Bhanage, B.M. Recent advances for sustainable production of levulinic acid in ionic liquids from biomass: Current scenario, opportunities and challenges. Renew. Sustain. Energy Rev. 2019, 102, 266–284. [Google Scholar] [CrossRef]
- Ahlkvist, J.; Warna, J.; Salmi, T.; Mikkola, J.P. Heterogeneously catalyzed conversion of nordic pulp to levulinic and formic acids. React. Kinet. Mech. Catal. 2016, 119, 415–427. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Yu, J. An intensified reaction technology for high levulinic acid concentration from lignocellulosic biomass. Biomass Bioenergy 2016, 95, 214–220. [Google Scholar] [CrossRef]
- Himmel, M.E.; Ding, S.Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Vyver, S.; Thomas, J.; Geboers, J.; Keyzer, S.; Smet, M.; Dehaen, W.; Jacobs, P.A.; Sels, B.F. Catalytic production of levulinic acid from cellulose and other biomass-derived carbohydrates with sulfonated hyperbranched poly(arylene oxindole)s. Energy Environ. Sci. 2011, 4, 3601–3610. [Google Scholar] [CrossRef]
- Morone, A.; Pandey, R.A. Lignocellulosic biobutanol production: Gridlocks and potential remedies. Renew. Sustain. Energy Rev. 2014, 37, 21–35. [Google Scholar] [CrossRef]
- Bevilaqua, D.B.; Rambo, M.K.D.; Rizzetti, T.M.; Cardoso, A.L.; Martins, A.F. Cleaner production: Levulinic acid from rice husks. J. Clean. Prod. 2013, 47, 96–101. [Google Scholar] [CrossRef]
- Kang, S.; Fu, J.; Zhang, G. From lignocellulosic biomass to levulinic acid: A review on acid-catalyzed hydrolysis. Renew. Sustain. Energy Rev. 2018, 94, 340–362. [Google Scholar] [CrossRef]
- Cao, S.; Pu, Y.; Studer, M.; Wyman, C.; Ragauskas, A.J. Chemical transformations of Populus trichocarpa during dilute acid pretreatment. RSC Adv. 2012, 2, 10925–10936. [Google Scholar] [CrossRef]
- Jeong, H.; Jang, S.K.; Hong, C.Y.; Kim, S.H.; Lee, S.Y.; Lee, S.M.; Choi, J.W.; Choi, I.G. Levulinic acid production by two-step acid-catalyzed treatment of Quercus mongolica using dilute sulfuric acid. Bioresour. Technol. 2017, 225, 183–190. [Google Scholar] [CrossRef]
- Cha, J.S.; Um, B.H. Levulinic acid production through two-step acidic and thermal treatment of food waste using dilute hydrochloric acid. Korean J. Chem. Eng. 2020, 37, 1149–1156. [Google Scholar] [CrossRef]
- Yang, Z.; Kang, H.; Guo, Y.; Zhuang, G.; Bai, Z.; Zhang, H.; Feng, C.; Dong, Y. Dilute-acid conversion of cotton straw to sugars and levulinic acid via 2-stage hydrolysis. Ind. Crop. Prod. 2013, 46, 205–209. [Google Scholar] [CrossRef]
- Li, J.; Ding, D.J.; Xu, L.J.; Guo, Q.X.; Fu, Y. The breakdown of reticent biomass to soluble components and their conversion to levulinic acid as a fuel precursor. RSC Adv. 2014, 4, 14985–14992. [Google Scholar] [CrossRef]
- Muranaka, Y.; Suzuki, T.; Sawanishi, H.; Hasegawa, I.; Mae, K. Effective Production of Levulinic Acid from Biomass through Pretreatment Using Phosphoric Acid, Hydrochloric Acid, or Ionic Liquid. Ind. Eng. Chem. Res. 2014, 53, 11611–11621. [Google Scholar] [CrossRef]
- Heeres, H.; Handana, R.; Chunai, D.; Rasrendra, C.B.; Girisuta, B.; Heeres, H.J. Combined dehydration/(transfer)-hydrogenation of C6-sugars (D-glucose and D-fructose) to gamma-valerolactone using ruthenium catalysts. Green Chem. 2009, 11, 1247–1255. [Google Scholar] [CrossRef] [Green Version]
- Morone, A.; Apte, M.; Pandey, R.A. Levulinic acid production from renewable waste resources: Bottlenecks, potential remedies, advancements and applications. Renew. Sustain. Energy Rev. 2015, 51, 548–565. [Google Scholar] [CrossRef]
- Pileidis, F.D.; Titirici, M.M. Levulinic Acid Biorefineries: New Challenges for Efficient Utilization of Biomass. Chemsuschem 2016, 9, 562–582. [Google Scholar] [CrossRef]
- Meramo-Hurtado, S.I.; Ojeda, K.A.; Sanchez-Tuiran, E. Environmental and Safety Assessments of Industrial Production of Levulinic Acid via Acid-Catalyzed Dehydration. ACS Omega 2019, 4, 22302–22312. [Google Scholar] [CrossRef] [Green Version]
- Signoretto, M.; Taghavi, S.; Ghedini, E.; Menegazzo, F. Catalytic Production of Levulinic Acid (LA) from Actual Biomass. Molecules 2019, 24, 2760. [Google Scholar] [CrossRef] [Green Version]
- Galletti, A.M.R.; Antonetti, C.; De Luise, V.; Licursi, D.; Di Nasso, N.N.O. Levulinic acid production from waste biomass. Bioresources 2012, 7, 1824–1835. [Google Scholar]
- Fachri, B.A.; Abdilla, R.M.; van de Bovenkamp, H.H.; Rasrendra, C.B.; Heeres, H.J. Experimental and Kinetic Modeling Studies on the Sulfuric Acid Catalyzed Conversion of D-Fructose to 5-Hydroxymethylfurfural and Levulinic Acid in Water. ACS Sustain. Chem. Eng. 2015, 3, 3024–3034. [Google Scholar] [CrossRef]
- Wettstein, S.G.; Alonso, D.M.; Chong, Y.; Dumesic, J.A. Production of levulinic acid and gamma-valerolactone (GVL) from cellulose using GVL as a solvent in biphasic systems. Energy Environ. Sci. 2012, 5, 8199–8203. [Google Scholar] [CrossRef]
- Fang, Q.; Hanna, M.A. Experimental studies for levulinic acid production from whole kernel grain sorghum. Bioresour. Technol. 2002, 81, 187–192. [Google Scholar] [CrossRef]
- Dussan, K.; Girisuta, B.; Haverty, D.; Leahy, J.J.; Hayes, M.H.B. Kinetics of levulinic acid and furfural production from Miscanthus × giganteus. Bioresour. Technol. 2013, 149, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Takahashi, Y.; Yamada, Y.; Sato, S. Efficient formation of angelica lactones in a vapor-phase conversion of levulinic acid. Appl. Catal. A Gen. 2016, 526, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Putrakumar, B.; Nagaraju, N.; Kumar, V.P.; Chary, K.V.R. Hydrogenation of levulinic acid to gamma-valerolactone over copper catalysts supported on gamma-Al2O3. Catal. Today 2015, 250, 209–217. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, F.; Yin, H.; Du, Y. Conversion of saccharides into levulinic acid and 5-hydroxymethylfurfural over WO3-Ta2O5 catalysts. RSC Adv. 2016, 6, 49760–49763. [Google Scholar] [CrossRef]
- Yan, L.; Greenwood, A.A.; Hossain, A.; Yang, B. A comprehensive mechanistic kinetic model for dilute acid hydrolysis of switchgrass cellulose to glucose, 5-HMF and levulinic acid. RSC Adv. 2014, 4, 23492–23504. [Google Scholar] [CrossRef]
- Antonetti, C.; Licursi, D.; Fulignati, S.; Valentini, G.; Galletti, A.M.R. New Frontiers in the Catalytic Synthesis of Levulinic Acid: From Sugars to Raw and Waste Biomass as Starting Feedstock. Catalysts 2016, 6, 196. [Google Scholar] [CrossRef]
- De la Hoz, A.; Diaz-Ortiz, A.; Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef]
- Rivas, S.; Raspolli-Galletti, A.M.; Antonetti, C.; Santos, V.; Carlos Parajo, J. Sustainable conversion of Pinus pinaster wood into biofuel precursors: A biorefinery approach. Fuel 2016, 164, 51–58. [Google Scholar] [CrossRef]
- Jeong, G.-T.; Kim, S.-K. Valorization of thermochemical conversion of lipid-extracted microalgae to levulinic acid. Bioresour. Technol. 2020, 313. [Google Scholar] [CrossRef]
- Rackemann, D.W.; Doherty, W.O.S. The conversion of lignocellulosics to levulinic acid. Biofuels Bioprod. Biorefin.-Biofpr 2011, 5, 198–214. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.; Girisuta, B.; Zhou, Y.; Liu, L. Selective and recyclable depolymerization of cellulose to levulinic acid catalyzed by acidic ionic liquid. Carbohydr. Polym. 2015, 117, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Sun, J.-K.; Yi, Y.-X.; Wang, B.; Xu, F.; Sun, R.-C. One-pot synthesis of levulinic acid from cellulose in ionic liquids. Bioresour. Technol. 2015, 192, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Ramli, N.A.S.; Amin, N.A.S. A new functionalized ionic liquid for efficient glucose conversion to 5-hydroxymethyl furfural and levulinic acid. J. Mol. Catal. A Chem. 2015, 407, 113–121. [Google Scholar] [CrossRef]
- Ramli, N.A.S.; Amin, N.A.S. Optimization of Biomass Conversion to Levulinic Acid in Acidic Ionic Liquid and Upgrading of Levulinic Acid to Ethyl Levulinate. Bioenergy Res. 2017, 10, 50–63. [Google Scholar] [CrossRef]
- Sun, Z.; Cheng, M.; Li, H.; Shi, T.; Yuan, M.; Wang, X.; Jiang, Z. One-pot depolymerization of cellulose into glucose and levulinic acid by heteropolyacid ionic liquid catalysis. RSC Adv. 2012, 2, 9058–9065. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Wiredu, B. Acidic Ionic Liquid Catalyzed One-Pot Conversion of Cellulose to Ethyl Levulinate and Levulinic Acid in Ethanol-Water Solvent System. Bioenergy Res. 2014, 7, 1237–1243. [Google Scholar] [CrossRef]
- Ren, H.; Zhou, Y.; Liu, L. Selective conversion of cellulose to levulinic acid via microwave-assisted synthesis in ionic liquids. Bioresour. Technol. 2013, 129, 616–619. [Google Scholar] [CrossRef]
- Ribeiro, M.C.C. High Viscosity of Imidazolium Ionic Liquids with the Hydrogen Sulfate Anion: A Raman Spectroscopy Study. J. Phys. Chem. B 2012, 116, 7281–7290. [Google Scholar] [CrossRef]
- Khan, A.S.; Man, Z.; Bustam, M.A.; Nasrullah, A.; Ullah, Z.; Sarwono, A.; Shah, F.U.; Muhammad, N. Efficient conversion of lignocellulosic biomass to levulinic acid using acidic ionic liquids. Carbohydr. Polym. 2018, 181, 208–214. [Google Scholar] [CrossRef]
- Khan, A.S.; Man, Z.; Bustam, M.A.; Kait, C.F.; Nasrullah, A.; Ullah, Z.; Sarwono, A.; Ahamd, P.; Muhammad, N. Dicationic ionic liquids as sustainable approach for direct conversion of cellulose to levulinic acid. J. Clean. Prod. 2018, 170, 591–600. [Google Scholar] [CrossRef]
- Liu, J.; Tang, Y.; Wu, K.; Bi, C.; Cui, Q. Conversion of fructose into 5-hydroxymethylfurfural (HMF) and its derivatives promoted by inorganic salt in alcohol. Carbohydr. Res. 2012, 350, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Pidko, E.A.; Hensen, E.J.M. The Mechanism of Glucose Isomerization to Fructose over Sn-BEA Zeolite: A Periodic Density Functional Theory Study. Chemsuschem 2013, 6, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Lin, L.; Zhang, J.; Zhuang, J.; Zhang, B.; Gong, Y. Catalytic Conversion of Cellulose to Levulinic Acid by Metal Chlorides. Molecules 2010, 15, 5258–5272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhary, V.; Mushrif, S.H.; Ho, C.; Anderko, A.; Nikolakis, V.; Marinkovic, N.S.; Frenkel, A.I.; Sandler, S.I.; Vlachos, D.G. Insights into the Interplay of Lewis and Brønsted Acid Catalysts in Glucose and Fructose Conversion to 5-(Hydroxymethyl)furfural and Levulinic Acid in Aqueous Media. J. Am. Chem. Soc. 2013, 135, 3997–4006. [Google Scholar] [CrossRef]
- Yang, F.; Fu, J.; Mo, J.; Lu, X. Synergy of Lewis and Bronsted Acids on Catalytic Hydrothermal Decomposition of Hexose to Levulinic Acid. Energy Fuels 2013, 27, 6973–6978. [Google Scholar] [CrossRef]
- Lappalainen, K.; Vogeler, N.; Karkkainen, J.; Dong, Y.; Niemela, M.; Rusanen, A.; Ruotsalainen, A.L.; Wali, P.; Markkola, A.; Lassi, U. Microwave-assisted conversion of novel biomass materials into levulinic acid. Biomass Convers. Biorefin. 2018, 8, 965–970. [Google Scholar] [CrossRef] [Green Version]
- Efremov, A.A.; Pervyshina, G.G.; Kuznetsov, B.N. Production of levulinic acid from wood raw material in the presence of sulfuric acid and its salts. Chem. Nat. Compd. 1998, 34, 182–185. [Google Scholar] [CrossRef]
- Zhi, Z.; Li, N.; Qiao, Y.; Zheng, X.; Wang, H.; Lu, X. Kinetic study of levulinic acid production from corn stalk at relatively high temperature using FeCl3 as catalyst: A simplified model evaluated. Ind. Crop. Prod. 2015, 76, 672–680. [Google Scholar] [CrossRef]
- Cao, X.; Peng, X.; Sun, S.; Zhong, L.; Chen, W.; Wang, S.; Sun, R.-C. Hydrothermal conversion of xylose, glucose, and cellulose under the catalysis of transition metal sulfates. Carbohydr. Polym. 2015, 118, 44–51. [Google Scholar] [CrossRef]
- Potvin, J.; Sorlien, E.; Hegner, J.; DeBoef, B.; Lucht, B.L. Effect of NaCl on the conversion of cellulose to glucose and levulinic acid via solid supported acid catalysis. Tetrahedron Lett. 2011, 52, 5891–5893. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Z.; Hu, L.; Hu, C. Selective Conversion of Cellulose in Corncob Residue to Levulinic Acid in an Aluminum Trichloride-Sodium Chloride System. Chemsuschem 2014, 7, 2482–2488. [Google Scholar] [CrossRef] [PubMed]
- Moeller, M.; Harnisch, F.; Schroeder, U. Microwave-assisted hydrothermal degradation of fructose and glucose in subcritical water. Biomass Bioenergy 2012, 39, 389–398. [Google Scholar] [CrossRef]
- Jow, J.; Rorrer, G.L.; Hawley, M.C.; Lamport, D.T. Dehydration of D-fructose to levulinic acid over LZY zeolite catalyst. Biomass 1987, 14, 185–194. [Google Scholar] [CrossRef]
- Chen, S.S.; Maneerung, T.; Tsang, D.C.W.; Ok, Y.S.; Wang, C.-H. Valorization of biomass to hydroxymethylfurfural, levulinic acid, and fatty acid methyl ester by heterogeneous catalysts. Chem. Eng. J. 2017, 328, 246–273. [Google Scholar] [CrossRef]
- Ennaert, T.; Geboers, J.; Gobechiya, E.; Courtin, C.M.; Kurttepeli, M.; Houthoofd, K.; Kirschhock, C.E.A.; Magusin, P.C.M.M.; Bals, S.; Jacobs, P.A.; et al. Conceptual Frame Rationalizing the Self-Stabilization of H-USY Zeolites in Hot Liquid Water. ACS Catal. 2015, 5, 754–768. [Google Scholar] [CrossRef]
- Ya’aini, N.; Amin, N.A.S.; Asmadi, M. Optimization of levulinic acid from lignocellulosic biomass using a new hybrid catalyst. Bioresour. Technol. 2012, 116, 58–65. [Google Scholar] [CrossRef]
- Ramli, N.A.S.; Amin, N.A.S. Catalytic hydrolysis of cellulose and oil palm biomass in ionic liquid to reducing sugar for levulinic acid production. Fuel Process. Technol. 2014, 128, 490–498. [Google Scholar] [CrossRef]
- Xiang, M.; Liu, J.; Fu, W.; Tang, T.; Wu, D. Improved Activity for Cellulose Conversion to Levulinic Acid through Hierarchization of ETS-10 Zeolite. ACS Sustain. Chem. Eng. 2017, 5, 5800–5809. [Google Scholar] [CrossRef]
- Joshi, S.S.; Zodge, A.D.; Pandare, K.V.; Kulkarni, B.D. Efficient Conversion of Cellulose to Levulinic Acid by Hydrothermal Treatment Using Zirconium Dioxide as a Recyclable Solid Acid Catalyst. Ind. Eng. Chem. Res. 2014, 53, 18796–18805. [Google Scholar] [CrossRef]
- Weingarten, R.; Conner, W.C., Jr.; Huber, G.W. Production of levulinic acid from cellulose by hydrothermal decomposition combined with aqueous phase dehydration with a solid acid catalyst. Energy Environ. Sci. 2012, 5, 7559–7574. [Google Scholar] [CrossRef]
- Ding, D.; Wang, J.; Xi, J.; Liu, X.; Lu, G.; Wang, Y. High-yield production of levulinic acid from cellulose and its upgrading to gamma-valerolactone. Green Chem. 2014, 16, 3846–3853. [Google Scholar] [CrossRef]
- Hegner, J.; Pereira, K.C.; DeBoef, B.; Lucht, B.L. Conversion of cellulose to glucose and levulinic acid via solid-supported acid catalysis. Tetrahedron Lett. 2010, 51, 2356–2358. [Google Scholar] [CrossRef]
- Alonso, D.M.; Gallo, J.M.R.; Mellmer, M.A.; Wettstein, S.G.; Dumesic, J.A. Direct conversion of cellulose to levulinic acid and gamma-valerolactone using solid acid catalysts. Catal. Sci. Technol. 2013, 3, 927–931. [Google Scholar] [CrossRef]
- Yang, H.; Wang, L.; Jia, L.; Qiu, C.; Pang, Q.; Pan, X. Selective Decomposition of Cellulose into Glucose and Levulinic Acid over Fe-Resin Catalyst in NaCl Solution under Hydrothermal Conditions. Ind. Eng. Chem. Res. 2014, 53, 6562–6568. [Google Scholar] [CrossRef]
- Siril, P.R.; Cross, H.E.; Brown, D.R. New polystyrene sulfonic acid resin catalysts with enhanced acidic and catalytic properties. J. Mol. Catal. A Chem. 2008, 279, 63–68. [Google Scholar] [CrossRef]
- Chen, L.; Ji, T.; Yuan, R.; Mu, L.; Brisbin, L.; Zhu, J. Unveiling Mesopore Evolution in Carbonized Wood: Interfacial Separation, Migration, and Degradation of Lignin Phase. ACS Sustain. Chem. Eng. 2015, 3, 2489–2495. [Google Scholar] [CrossRef]
- Foo, G.S.; Van Pelt, A.H.; Kroetschel, D.; Sauk, B.F.; Rogers, A.K.; Jolly, C.R.; Yung, M.M.; Sievers, C. Hydrolysis of Cellobiose over Selective and Stable Sulfonated Activated Carbon Catalysts. ACS Sustain. Chem. Eng. 2015, 3, 1934–1942. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, J.; Liang, X.; Wu, H.; Xu, J. Direct Conversion of Cellulose to Levulinic Acid over Multifunctional Sulfonated Humins in Sulfolane-Water Solution. ACS Sustain. Chem. Eng. 2018, 6, 15092–15099. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Lin, Q.; Cheng, B.; Kong, F.; Li, H.; Ren, J. Solid acid-induced hydrothermal treatment of bagasse for production of furfural and levulinic acid by a two-step process. Ind. Crop. Prod. 2018, 123, 118–127. [Google Scholar] [CrossRef]
- Han, Y.; Ye, L.; Gu, X.; Zhu, P.; Lu, X. Lignin-based solid acid catalyst for the conversion of cellulose to levulinic acid using gamma-valerolactone as solvent. Ind. Crop. Prod. 2019, 127, 88–93. [Google Scholar] [CrossRef]
- Chatterjee, S.; Saito, T. Lignin-Derived Advanced Carbon Materials. Chemsuschem 2015, 8, 3941–3958. [Google Scholar] [CrossRef] [PubMed]
- Boonyakarn, T.; Wataniyakul, P.; Boonnoun, P.; Quitain, A.T.; Kida, T.; Sasaki, M.; Laosiripojana, N.; Jongsomjit, B.; Shotipruk, A. Enhanced Levulinic Acid Production from Cellulose by Combined Bronsted Hydrothermal Carbon and Lewis Acid Catalysts. Ind. Eng. Chem. Res. 2019, 58, 2697–2703. [Google Scholar] [CrossRef]
- Pang, Q.; Wang, L.; Yang, H.; Jia, L.; Pan, X.; Qiu, C. Cellulose-derived carbon bearing -Cl and -SO3H groups as a highly selective catalyst for the hydrolysis of cellulose to glucose. RSC Adv. 2014, 4, 41212–41218. [Google Scholar] [CrossRef]
- Shen, S.; Cai, B.; Wang, C.; Li, H.; Dai, G.; Qin, H. Preparation of a novel carbon-based solid acid from cocarbonized starch and polyvinyl chloride for cellulose hydrolysis. Appl. Catal. A-Gen. 2014, 473, 70–74. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhang, Y.; Fu, Y. Catalytic Conversion of Cellulose into Levulinic Acid by a Sulfonated Chloromethyl Polystyrene Solid Acid Catalyst. Chemcatchem 2014, 6, 753–757. [Google Scholar] [CrossRef]
- Shen, F.; Smith, R.L., Jr.; Li, L.; Yan, L.; Qi, X. Eco-friendly Method for Efficient Conversion of Cellulose into Levulinic Acid in Pure Water with Cellulase-Mimetic Solid Acid Catalyst. ACS Sustain. Chem. Eng. 2017, 5, 2421–2427. [Google Scholar] [CrossRef]
- Lai, D.-M.; Deng, L.; Guo, Q.-X.; Fu, Y. Hydrolysis of biomass by magnetic solid acid. Energy Environ. Sci. 2011, 4, 3552–3557. [Google Scholar] [CrossRef]
- Li, X.; Lei, T.; Wang, Z.; Li, X.; Wen, M.; Yang, M.; Chen, G.; He, X.; Xu, H.; Guan, Q.; et al. Catalytic pyrolysis of corn straw with magnetic solid acid catalyst to prepare levulinic acid by response surface methodology. Ind. Crop. Prod. 2018, 116, 73–80. [Google Scholar] [CrossRef]
- Wang, P.; Zhan, S.; Yu, H. Production of Levulinic Acid from Cellulose Catalyzed by Environmental-Friendly Catalyst. In Advance in Ecological Environment Functional Materials and Ion Industry; Liang, J., Wang, L., Eds.; Trans Tech Publications Ltd.: Dürnten, Switzerland, 2010; Volume 96, pp. 183–187. [Google Scholar]
- Chen, H.; Yu, B.; Jin, S. Production of levulinic acid from steam exploded rice straw via solid superacid, S2O82−/ZrO2-SiO2-Sm2O3. Bioresour. Technol. 2011, 102, 3568–3570. [Google Scholar] [CrossRef]
- Yan, L.; Yang, N.; Pang, H.; Liao, B. Production of levulinic acid from bagasse and paddy straw by liquefaction in the presence of hydrochloride acid. Clean-Soil Air Water 2008, 36, 158–163. [Google Scholar] [CrossRef]
- Victor, A.; Pulidindi, I.N.; Gedanken, A. Levulinic acid production from Cicer arietinum, cotton, Pinus radiata and sugarcane bagasse. RSC Adv. 2014, 4, 44706–44711. [Google Scholar] [CrossRef]
- Szabolcs, A.; Molnar, M.; Dibo, G.; Mika, L.T. Microwave-assisted conversion of carbohydrates to levulinic acid: An essential step in biomass conversion. Green Chem. 2013, 15, 439–445. [Google Scholar] [CrossRef]
- Girisuta, B.; Janssen, L.P.B.M.; Heeres, H.J. Kinetic study on the acid-catalyzed hydrolysis of cellulose to levulinic acid. Ind. Eng. Chem. Res. 2007, 46, 1696–1708. [Google Scholar] [CrossRef] [Green Version]
- Girisuta, B.; Danon, B.; Manurung, R.; Janssen, L.P.B.M.; Heeres, H.J. Experimental and kinetic modelling studies on the acid–catalysed hydrolysis of the water hyacinth plant to levulinic acid. Bioresour. Technol. 2008, 99, 8367–8375. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Ma, X.; Cen, P. Kinetic Studies on Wheat Straw Hydrolysis to Levulinic Acid. Chin. J. Chem. Eng. 2009, 17, 835–839. [Google Scholar] [CrossRef]
- Girisuta, B.; Dussan, K.; Haverty, D.; Leahy, J.J.; Hayes, M.H.B. A kinetic study of acid catalysed hydrolysis of sugar cane bagasse to levulinic acid. Chem. Eng. J. 2013, 217, 61–70. [Google Scholar] [CrossRef]
- Zhou, C.; Yu, X.; Ma, H.; He, R.; Vittayapadung, S. Optimization on the Conversion of Bamboo Shoot Shell to Levulinic Acid with Environmentally Benign Acidic Ionic Liquid and Response Surface Analysis. Chin. J. Chem. Eng. 2013, 21, 544–550. [Google Scholar] [CrossRef]
- Zheng, X.; Zhi, Z.; Gu, X.; Li, X.; Zhang, R.; Lu, X. Kinetic study of levulinic acid production from corn stalk at mild temperature using FeCl3 as catalyst. Fuel 2017, 187, 261–267. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kaiki, H.; Shrotri, A.; Techikawara, K.; Fukuoka, A. Hydrolysis of woody biomass by a biomass-derived reusable heterogeneous catalyst. Chem. Sci. 2016, 7, 692–696. [Google Scholar] [CrossRef] [Green Version]
- Hara, M. Biomass conversion by a solid acid catalyst. Energy Environ. Sci. 2010, 3, 601–607. [Google Scholar] [CrossRef]


| Physical Properties Items | Values |
|---|---|
| Molecular weight | 116.12 |
| Color | White |
| Density | 1.13 |
| Solubility | Soluble |
| Melting point | 37 °C |
| Boiling point | 245–246 °C |
| Substrate | Catalyst | T (°C) | t | YLA (%) | Ref. |
|---|---|---|---|---|---|
| 1 g Pretreated rice husks | 4.5% HCl | 170 | 1 h | 59.4 (based on the cellulose amount) | [65] |
| 1 g Pretreated rice husks | 4% H2SO4 | 170 | 1 h | 45.7 (based on the cellulose amount) | [65] |
| 1.75 g Olive tree pruning | 37% HCl | 200 | 1 h | 20.1 | [78] |
| 1.75 g Poplar sawdust | 37% HCl | 200 | 1 h | 29.3 | [78] |
| 1.75 g Paper sludge | 37% HCl | 200 | 1 h | 31.4 | [78] |
| 1.75 g Paper sludge | 98% H2SO4 | 200 | 1 h | 15.4 | [78] |
| 1.98 wt% Cellulose | 1.25 M HCl + 35 wt% NaCl | 155 | 1.5 h | 72 | [80] |
| Sorghum flour (10% flour loading) | 8% H2SO4 | 200 | 30 min | 32.6 | [81] |
| 0.5 g Pennisetum alopecuroides | 1% H2SO4 | 190 | 1 h | 50.5 (based on the glucan amount) | [86] |
| 1 g Bagasse | 4.45 wt% HCl | 220 | 45 min | 22.8 | [143] |
| 1 g Paddy Straw | 4.45 wt% HCl | 220 | 45 min | 23.7 | [143] |
| 1 g Cotton | 1 M HCl | 150 | 2 h | 44 | [144] |
| 0.4 g Cellulose | 2 M H2SO4 | 170 | 50 min | 34.2 | [145] |
| 1.7 wt% Cellulose | 1 M H2SO4 | 150 | 2 h | 60 | [146] |
| 5 wt% Water hyacinth | 1 M H2SO4 | 175 | 30 min | 53 (based on the C6 sugars amount) | [147] |
| Wheat straw | 3% H2SO4 | 210 | 42 min | 41 | [148] |
| Bagasse | 0.55 M H2SO4 | 150 | 8 h | 63 (based on the glucan amount) | [149] |
| 0.02 g Cellulose | 16.7 wt% [C3SO3Hmim]HSO4 | 170 | 5 h | 86.1 | [92] |
| 0.05 g Cellulose | 1.5 g [BSMim]HSO4 | 120 | 2 h | 39.4 | [93] |
| 0.1 g Glucose | 10 g [BMIM]FeCl4 | 150 | 4 h | 22.4 | [94] |
| 0.1 g Glucose | 10 g [SMIM]Cl | 150 | 4 h | 25.8 | [94] |
| 0.1 g Glucose | 10 g [SMIM]FeCl4 | 150 | 4 h | 67.8 | [94] |
| 0.18 g Oil palm fronds | 7.27 g [SMIM][FeCl4] | 154.5 | 3.7 h | 24.8 | [95] |
| 0.1 g Cellulose | 0.07 mmol heteropolyacid IL | 140 | 12 h | 63.1 | [96] |
| 0.15 g Cellulose | 50 mg Brønsted acidic IL | 150 | 48 h | 23.7 | [97] |
| 0.4 g Cellulose | 1 g [C3SO3Hmim]HSO4 | 160 | 30 min | 55 | [98] |
| 0.025 g Bamboo | 0.75 mL [C4(Mim)2][(2HSO4)(H2SO4)4] | 110 | 1 h | 47.5 (based on the glucose amount) | [100] |
| 0.025 g Cellulose | 1 mL [C4(Mim)2][(2HSO4)(H2SO4)2] | 100 | 3 h | 55 (based on the glucose amount) | [101] |
| 2 wt% Bamboo shoot shell | 0.9 M [C4mim]HSO4 | 145 | 104 min | 71 ± 0.4 (based on the C6 sugars amount) | [150] |
| 50 wt% Cellulose | 0.02 M CrCl3 | 200 | 3 h | 67 | [105] |
| 0.5 g Potato peel waste | 0.0075 M CrCl3 + 0.5 M H2SO4 | 180 | 15 min | 49 | [108] |
| Corn stalk | 0.5 M FeCl3 | 230 | 10 min | 48.7 (based on the glucan amount) | [110] |
| 4 g Corncob residue | 10 g/L AlCl3 + 40 wt% NaCl | 180 | 2 h | 46.8 (based on the cellulose amount) | [113] |
| 1 g Cellulose | 0.5 g Ni-HMETS-10 | 200 | 6 h | 91 | [120] |
| 2 g Cellulose | 2 wt% ZrO2 | 180 | 3 h | 53.9 | [121] |
| 0.5 g Cellulose | 0.4 g Al-NbOPO4 | 180 | 24 h | 52.9 | [123] |
| 2 wt% Cellulose | 6 wt% Amberlyst 70 | 160 | 16 h | 69 | [125] |
| 0.5 g Cellulose | 0.3 g Fe-resin + 5 wt% NaCl | 200 | 5 h | 33.3 | [126] |
| 10 g Cellulose | 1.67 g MSH | 180 | 2 h | 65.9 | [130] |
| 10 g Bamboo meal | 1.67 g MSH | 180 | 2 h | 45.6 | [130] |
| 0.15 g Cellulose | 0.15 g Lignin-based solid acid | 185 | 2 h | 35.6 | [132] |
| Cellulose | 5 wt% HTCG-SO3H + 0.015 M CrCl3 | 200 | 5 min | 40 | [134] |
| 0.1 g Cellulose | 0.3 g CP-SO3H-1.69 | 170 | 10 h | 65.5 | [137] |
| 0.05 g Cellulose | 0.2 g SA-SO3H | 180 | 12 h | 51.5 | [138] |
| 1.5 g Cellulose | 1.5 g Fe3O4-SBA-SO3H | 150 | 12 h | 45 | [139] |
| 1 g Cellulose | 0.7 g Sulfated TiO2 | 240 | 15 min | 27.2 | [141] |
| Rice straw | 13.3 wt% S2O82−/ZrO2-SiO2-Sm2O3 | 220 | 10 min | 22.8 | [142] |
| Corn stalk | 0.5 M FeCl3 | 180 | 40 min | 48.9 (based on the glucan amount) | [151] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Lu, X.; Yu, Z.; Xiong, J.; Bai, H.; Zhang, R. Production of Levulinic Acid from Cellulose and Cellulosic Biomass in Different Catalytic Systems. Catalysts 2020, 10, 1006. https://doi.org/10.3390/catal10091006
Liu C, Lu X, Yu Z, Xiong J, Bai H, Zhang R. Production of Levulinic Acid from Cellulose and Cellulosic Biomass in Different Catalytic Systems. Catalysts. 2020; 10(9):1006. https://doi.org/10.3390/catal10091006
Chicago/Turabian StyleLiu, Chen, Xuebin Lu, Zhihao Yu, Jian Xiong, Hui Bai, and Rui Zhang. 2020. "Production of Levulinic Acid from Cellulose and Cellulosic Biomass in Different Catalytic Systems" Catalysts 10, no. 9: 1006. https://doi.org/10.3390/catal10091006






