Recent Developments of Advanced Ti3+-Self-Doped TiO2 for Efficient Visible-Light-Driven Photocatalysis
Abstract
1. Introduction
2. Preparation of Ti3+–TiO2
2.1. Hydrogenation
2.2. Hydrothermal Reaction
2.3. Alkaline Metal Reduction
2.4. Sol-Gelation
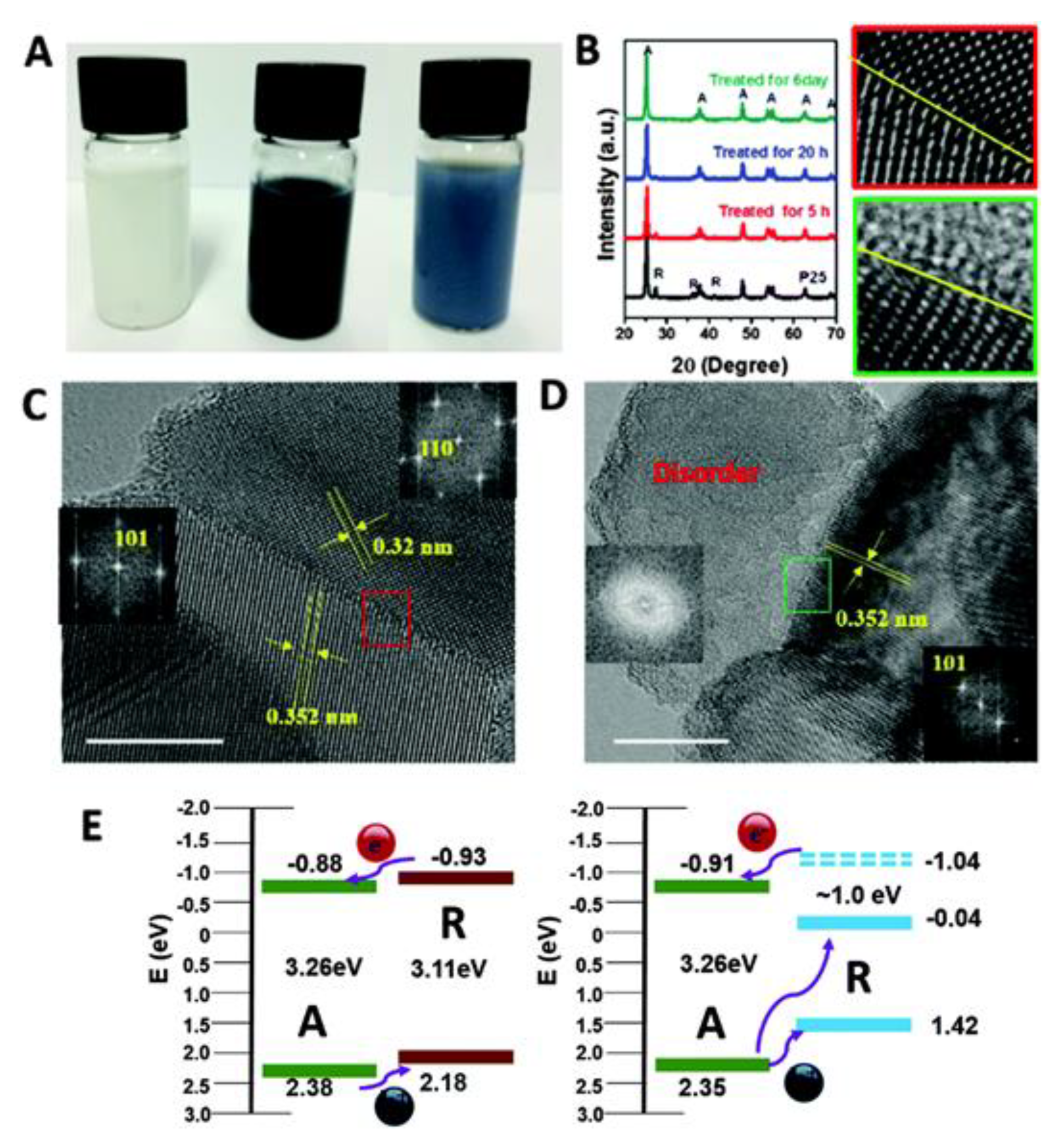
2.5. Phase-Selective Reduction
3. Enhancement of Photogenerated Charge Separation and Photocatalytic Activity in Advanced Ti3+–TiO2
3.1. Metal-Doping
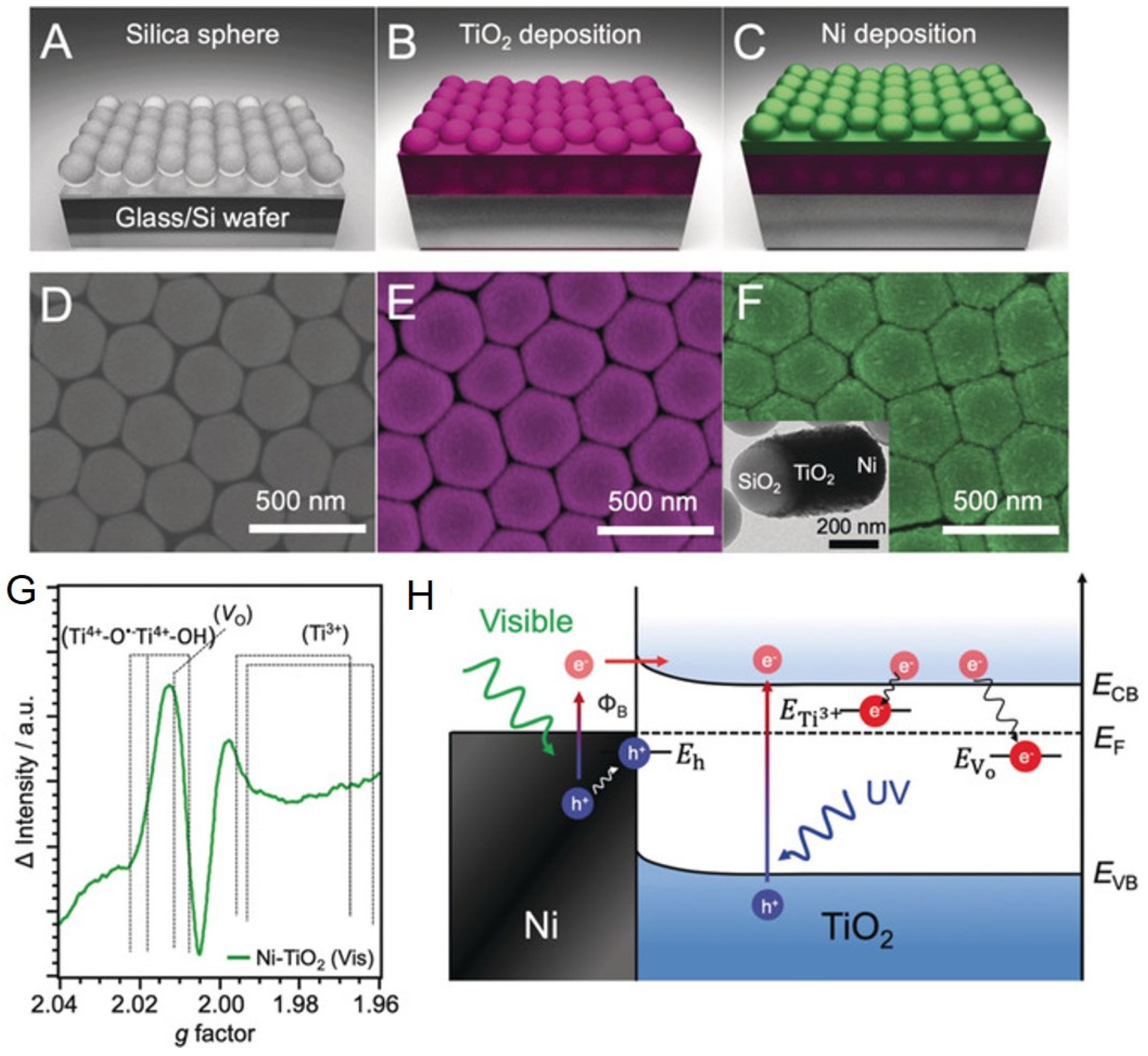
3.1.1. Surface Plasmon Effects on Ti3+–TiO2
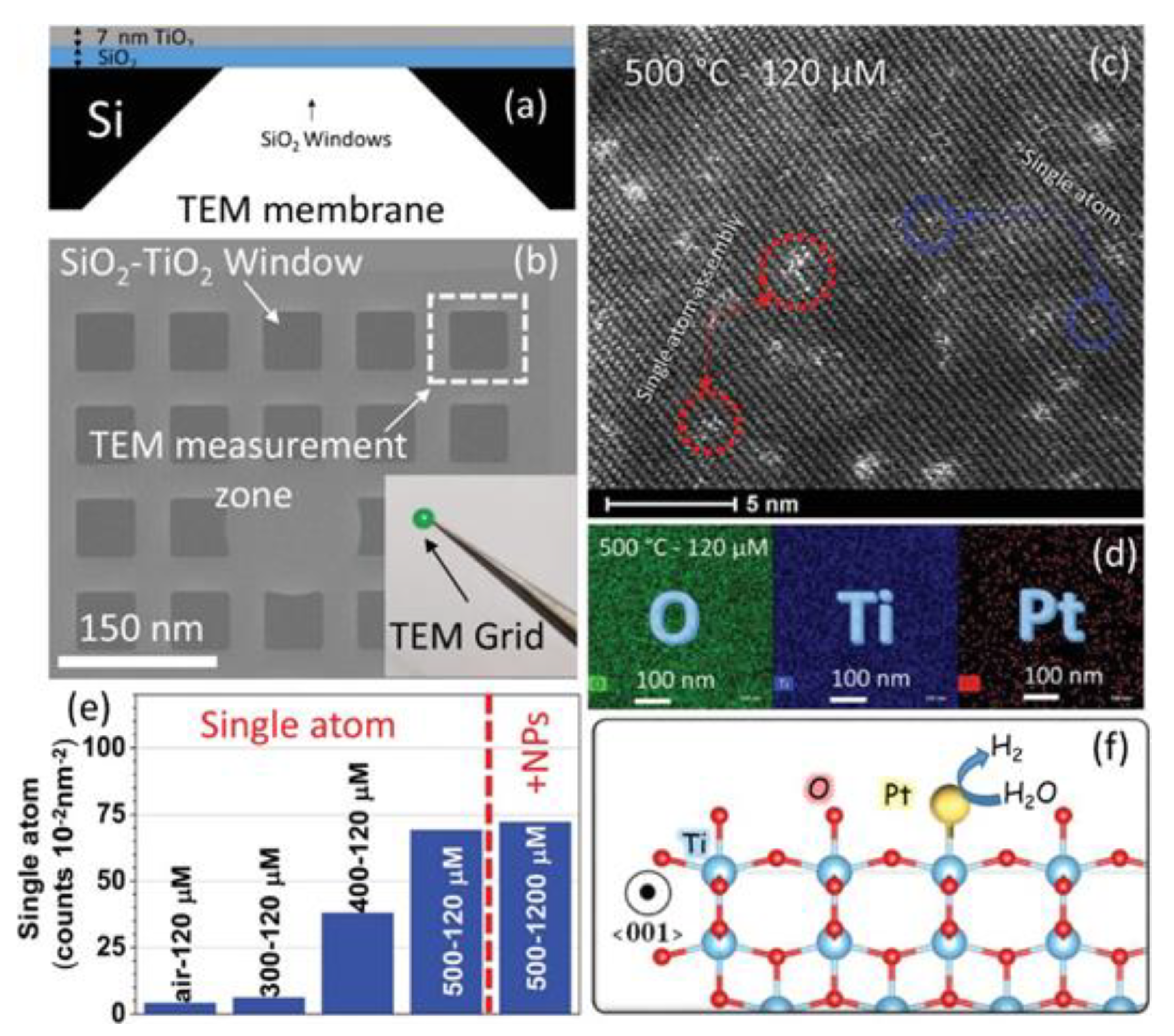
3.1.2. Single Atom Site Doping Effects on Ti3+–TiO2
3.1.3. Transition or Rare Metal Grafting on Ti3+–TiO2
3.2. Nonmetal Doping
3.2.1. N-Doped Ti3+–TiO2
3.2.2. Sulfur-Doped Ti3+–TiO2
3.2.3. Multi-Doped Ti3+–TiO2
3.3. Semiconducting Coupling
3.3.1. Transition Metal Dichalcogenide or Quantum Dot/Ti3+–TiO2
3.3.2. Carbon-Based Nonmetallic Semiconductors/Ti3+–TiO2
3.3.3. Metal Oxide/Ti3+–TiO2
3.4. Stoichiometry Modification
4. Conclusion and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ran, J.; Zhang, J.; Yu, J.; Jaroniec, M.; Qiao, S.Z. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 2014, 43, 7787–7812. [Google Scholar] [CrossRef]
- Dickinson, R.E. Land Surface Processes and Climate—Surface Albedos and Energy Balance. In Theory of Climate, Proceedings of a Symposium Commemorating the Two-Hundredth Anniversary of the Academy of Sciences of Lisbon; Saltzman, B., Ed.; Elsevier: Amsterdam, The Netherlands, 1983; Volume 25, pp. 305–353. [Google Scholar]
- Dines, W.H. The heat balance of the atmosphere. Q. J. R. Meteorol. Soc. 1917, 43, 151–158. [Google Scholar] [CrossRef]
- Stephens, G.L.; Li, J.; Wild, M.; Clayson, C.A.; Loeb, N.; Kato, S.; L’Ecuyer, T.; Stackhouse, P.W.; Lebsock, M.; Andrews, T. An update on Earth’s energy balance in light of the latest global observations. Nat. Geosci. 2012, 5, 691–696. [Google Scholar] [CrossRef]
- Kumar, A. A Review on the Factors Affecting the Photocatalytic Degradation of Hazardous Materials. Mater. Sci. Eng. C 2017, 1, 106–114. [Google Scholar] [CrossRef]
- Santhosh, C.; Malathi, A.; Daneshvar, E.; Kollu, P.; Bhatnagar, A. Photocatalytic degradation of toxic aquatic pollutants by novel magnetic 3D–TiO2@HPGA nanocomposite. Sci. Rep. 2018, 8, 15531. [Google Scholar] [CrossRef] [PubMed]
- Panthi, G.; Park, M.; Kim, H.-Y.; Lee, S.-Y.; Park, S.-J. Electrospun ZnO hybrid nanofibers for photodegradation of wastewater containing organic dyes: A review. J. Ind. Eng. Chem. 2015, 21, 26–35. [Google Scholar] [CrossRef]
- Gupta, V.K.; Jain, R.; Nayak, A.; Agarwal, S.; Shrivastava, M. Removal of the hazardous dye—Tartrazine by photodegradation on titanium dioxide surface. Mater. Sci. Eng. C 2011, 31, 1062–1067. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, W. Metal-organic frameworks for artificial photosynthesis and photocatalysis. Chem. Soc. Rev. 2014, 43, 5982–5993. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.R.; Moss, J.A.; Baum, M.M. Artificial photosynthesis: Semiconductor photocatalytic fixation of CO2 to afford higher organic compounds. Dalton Trans. 2011, 40, 5151–5158. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, J.H.; Park, C.B. Coupling Photocatalysis and Redox Biocatalysis toward Biocatalyzed Artificial Photosynthesis. Chem. Eur. 2013, 19, 4392–4406. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g–C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Osterloh, F.E. Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem. Soc. Rev. 2013, 42, 2294–2320. [Google Scholar] [CrossRef] [PubMed]
- Takanabe, K. Photocatalytic Water Splitting: Quantitative Approaches toward Photocatalyst by Design. ACS Catal. 2017, 7, 8006–8022. [Google Scholar] [CrossRef]
- She, X.; Wu, J.; Xu, H.; Zhong, J.; Wang, Y.; Song, Y.; Nie, K.; Liu, Y.; Yang, Y.; Rodrigues, M.-T.F.; et al. High Efficiency Photocatalytic Water Splitting Using 2D α-Fe2O3/g–C3N4 Z-Scheme Catalysts. Adv. Energy Mater. 2017, 7, 1700025. [Google Scholar] [CrossRef]
- Lin, L.; Yu, Z.; Wang, X. Crystalline Carbon Nitride Semiconductors for Photocatalytic Water Splitting. Angew. Chem. Int. Ed. 2019, 58, 6164–6175. [Google Scholar] [CrossRef]
- Zhu, M.; Zhu, C.; Wu, D.; Wang, X.; Wang, H.; Gao, J.; Huang, H.; Shi, C.; Liu, Y.; Kang, Z. Efficient photocatalytic water splitting through titanium silicalite stabilized CoO nanodots. Nanoscale 2019, 11, 15984–15990. [Google Scholar] [CrossRef]
- Collins, G.; Armstrong, E.; McNulty, D.; O’Hanlon, S.; Geaney, H.; O’Dwyer, C. 2D and 3D photonic crystal materials for photocatalysis and electrochemical energy storage and conversion. Sci. Technol. Adv. Mater. 2016, 17, 563–582. [Google Scholar] [CrossRef]
- Rangan, K.; Arachchige, S.M.; Brown, J.R.; Brewer, K.J. Solar energy conversion using photochemical molecular devices: Photocatalytic hydrogen production from water using mixed-metal supramolecular complexes. Energy Environ. Sci. 2009, 2, 410–419. [Google Scholar] [CrossRef]
- Bard, A.J. Inner-Sphere Heterogeneous Electrode Reactions. Electrocatalysis and Photocatalysis: The Challenge. J. Am. Chem. Soc. 2010, 132, 7559–7567. [Google Scholar] [CrossRef]
- Zhang, P.; Lou, X.W. Design of Heterostructured Hollow Photocatalysts for Solar-to-Chemical Energy Conversion. Adv. Mater. 2019, 31, 1900281. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Toi, S.; Ichikawa, S.; Hirai, T. Photocatalytic NH3 Splitting on TiO2 Particles Decorated with Pt–Au Bimetallic Alloy Nanoparticles. ACS Appl. Nano Mater. 2020, 3, 1612–1620. [Google Scholar] [CrossRef]
- Misra, M.; Chowdhury, S.R.; Singh, N. TiO2@Au@CoMn2O4 core–shell nanorods for photo‒electrochemical and photocatalytic activity for decomposition of toxic organic compounds and photo reduction of Cr6+ ion. J. Alloys Compd. 2020, 824, 153861. [Google Scholar] [CrossRef]
- Xu, H.; Ouyang, S.; Liu, L.; Reunchan, P.; Umezawa, N.; Ye, J. Recent advances in TiO2-based photocatalysis. J. Mater. Chem. A 2014, 2, 12642–12661. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Ramakrishna Matte, H.S.S.; Maitra, U. Graphene Analogues of Inorganic Layered Materials. Angew. Chem. Int. Ed. 2013, 52, 13162–13185. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Talreja, N.; Tao, H.; Texter, J.; Muhler, M.; Strunk, J.; Chen, J. Catalysis of Carbon Dioxide Photoreduction on Nanosheets: Fundamentals and Challenges. Angew. Chem. Int. Ed. 2018, 57, 7610–7627. [Google Scholar] [CrossRef]
- Sprick, R.S.; Bonillo, B.; Clowes, R.; Guiglion, P.; Brownbill, N.J.; Slater, B.J.; Blanc, F.; Zwijnenburg, M.A.; Adams, D.J.; Cooper, A.I. Visible-Light-Driven Hydrogen Evolution Using Planarized Conjugated Polymer Photocatalysts. Angew. Chem. Int. Ed. 2016, 55, 1792–1796. [Google Scholar] [CrossRef]
- Wang, S.; Hai, X.; Ding, X.; Jin, S.; Xiang, Y.; Wang, P.; Jiang, B.; Ichihara, F.; Oshikiri, M.; Meng, X.; et al. Intermolecular cascaded π-conjugation channels for electron delivery powering CO2 photoreduction. Nat. Commun. 2020, 11, 1149. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, L.; Wu, Y.; Yang, T.; Ding, Y.; Yang, H.G.; Hu, A. Hyperbranched Conjugated Polymer Dots: The Enhanced Photocatalytic Activity for Visible Light-Driven Hydrogen Production. Macromolecules 2019, 52, 4376–4384. [Google Scholar] [CrossRef]
- Lan, Z.-A.; Ren, W.; Chen, X.; Zhang, Y.; Wang, X. Conjugated donor-acceptor polymer photocatalysts with electron-output “tentacles” for efficient hydrogen evolution. Appl. Catal. B 2019, 245, 596–603. [Google Scholar] [CrossRef]
- Hasani, A.; Tekalgne, M.; Le, Q.V.; Jang, H.W.; Kim, S.Y. Two-dimensional materials as catalysts for solar fuels: Hydrogen evolution reaction and CO2 reduction. J. Mater. Chem. A 2019, 7, 430–454. [Google Scholar] [CrossRef]
- Di, J.; Yan, C.; Handoko, A.D.; Seh, Z.W.; Li, H.; Liu, Z. Ultrathin two-dimensional materials for photo- and electrocatalytic hydrogen evolution. Mater. Today 2018, 21, 749–770. [Google Scholar] [CrossRef]
- Li, J.; Zhan, G.; Yu, Y.; Zhang, L. Superior visible light hydrogen evolution of Janus bilayer junctions via atomic-level charge flow steering. Nat. Commun. 2016, 7, 11480. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Cai, X.; Kim, D.; Sridhara, K.; Fuhrer, M.S. High mobility ambipolar MoS2 field-effect transistors: Substrate and dielectric effects. Appl. Phys. Lett. 2013, 102, 042104. [Google Scholar] [CrossRef]
- Lian, S.; Kodaimati, M.S.; Dolzhnikov, D.S.; Calzada, R.; Weiss, E.A. Powering a CO2 Reduction Catalyst with Visible Light through Multiple Sub-picosecond Electron Transfers from a Quantum Dot. J. Am. Chem. Soc. 2017, 139, 8931–8938. [Google Scholar] [CrossRef]
- Li, X.-B.; Gao, Y.-J.; Wang, Y.; Zhan, F.; Zhang, X.-Y.; Kong, Q.-Y.; Zhao, N.-J.; Guo, Q.; Wu, H.-L.; Li, Z.-J.; et al. Self-Assembled Framework Enhances Electronic Communication of Ultrasmall-Sized Nanoparticles for Exceptional Solar Hydrogen Evolution. J. Am. Chem. Soc. 2017, 139, 4789–4796. [Google Scholar] [CrossRef]
- Wu, L.-Z.; Chen, B.; Li, Z.-J.; Tung, C.-H. Enhancement of the Efficiency of Photocatalytic Reduction of Protons to Hydrogen via Molecular Assembly. Acc. Chem. Res. 2014, 47, 2177–2185. [Google Scholar] [CrossRef]
- Zrazhevskiy, P.; Sena, M.; Gao, X. Designing multifunctional quantum dots for bioimaging, detection, and drug delivery. Chem. Soc. Rev. 2010, 39, 4326–4354. [Google Scholar] [CrossRef]
- Smith, A.M.; Nie, S. Semiconductor Nanocrystals: Structure, Properties, and Band Gap Engineering. Acc. Chem. Res. 2010, 43, 190–200. [Google Scholar] [CrossRef]
- Zheng, L.; Su, H.; Zhang, J.; Walekar, L.S.; Vafaei Molamahmood, H.; Zhou, B.; Long, M.; Hu, Y.H. Highly selective photocatalytic production of H2O2 on sulfur and nitrogen co-doped graphene quantum dots tuned TiO2. Appl. Catal. B 2018, 239, 475–484. [Google Scholar] [CrossRef]
- Diao, S.; Zhang, X.; Shao, Z.; Ding, K.; Jie, J.; Zhang, X. 12.35% efficient graphene quantum dots/silicon heterojunction solar cells using graphene transparent electrode. Nano Energy 2017, 31, 359–366. [Google Scholar] [CrossRef]
- Di, J.; Chen, C.; Yang, S.-Z.; Chen, S.; Duan, M.; Xiong, J.; Zhu, C.; Long, R.; Hao, W.; Chi, Z.; et al. Isolated single atom cobalt in Bi3O4Br atomic layers to trigger efficient CO2 photoreduction. Nat. Commun. 2019, 10, 2840. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Li, J.; Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2018, 2, 65–81. [Google Scholar] [CrossRef]
- Liu, J. Catalysis by Supported Single Metal Atoms. ACS Catal. 2017, 7, 34–59. [Google Scholar] [CrossRef]
- Li, X.; Bi, W.; Zhang, L.; Tao, S.; Chu, W.; Zhang, Q.; Luo, Y.; Wu, C.; Xie, Y. Single-Atom Pt as Co-Catalyst for Enhanced Photocatalytic H2 Evolution. Adv. Mater. 2016, 28, 2427–2431. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Li, Z.; Garcia, H. Catalysis and photocatalysis by metal organic frameworks. Chem. Soc. Rev. 2018, 47, 8134–8172. [Google Scholar] [CrossRef] [PubMed]
- Nasalevich, M.A.; Van der Veen, M.; Kapteijn, F.; Gascon, J. Metal-organic frameworks as heterogeneous photocatalysts: Advantages and challenges. CrystEngComm 2014, 16, 4919–4926. [Google Scholar] [CrossRef]
- Corma, A.; García, H.; Llabrés i Xamena, F.X. Engineering Metal Organic Frameworks for Heterogeneous Catalysis. Chem. Rev. 2010, 110, 4606–4655. [Google Scholar] [CrossRef]
- Baloyi, J.; Seadira, T.; Raphulu, M.; Ochieng, A. Preparation, Characterization and Growth Mechanism of Dandelion-like TiO2 Nanostructures and their Application in Photocatalysis towards Reduction of Cr(VI). Mater. Today 2015, 2, 3973–3987. [Google Scholar] [CrossRef]
- Goodeve, C.F.; Kitchener, J.A. The mechanism of photosensitisation by solids. J. Chem. Soc. Faraday Trans. 1938, 34, 902–908. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Y.; Feng, C. Application of Photocatalytic Technology in Environmental Safety. Procedia Eng. 2012, 45, 993–997. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Higashimoto, S. Titanium-dioxide-based visible-light-sensitive photocatalysis: Mechanistic insight and applications. Catalysts 2019, 9, 201. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Torimoto, T.; Okawa, Y.; Takeda, N.; Yoneyama, H. Effect of activated carbon content in TiO2-loaded activated carbon on photodegradation behaviors of dichloromethane. J. Photochem. Photobiol. C 1997, 103, 153–157. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Kawi, S.; Ray, M.B. Photocatalytic degradation of orange II by TiO2 catalysts supported on adsorbents. Catal. Today 2004, 98, 431–439. [Google Scholar] [CrossRef]
- Szczepanik, B. Photocatalytic degradation of organic contaminants over clay–TiO2 nanocomposites: A review. Appl. Clay Sci. 2017, 141, 227–239. [Google Scholar] [CrossRef]
- Yang, J.; Du, J.; Li, X.; Liu, Y.; Jiang, C.; Qi, W.; Zhang, K.; Gong, C.; Li, R.; Luo, M. Highly hydrophilic TiO2 nanotubes network by alkaline hydrothermal method for photocatalysis degradation of methyl orange. Nanomaterials 2019, 9, 526. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.-Q.; Fu, X.; Zhang, N.; Xu, Y.-J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhan, X.; Wang, F.; Safdar, M.; He, J. Visible light driven type II heterostructures and their enhanced photocatalysis properties: A review. Nanoscale 2013, 5, 8326–8339. [Google Scholar] [CrossRef] [PubMed]
- Lazar, M.A.; Daoud, W.A. Achieving selectivity in TiO2-based photocatalysis. RSC Adv. 2013, 3, 4130–4140. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Z.; Liu, A.; Gu, X.; Ge, C.; Gao, F.; Dong, L. Engineering the TiO2—Graphene Interface to Enhance Photocatalytic H2 Production. ChemSusChem 2014, 7, 618–626. [Google Scholar] [CrossRef]
- Chen, J.I.; Von Freymann, G.; Choi, S.Y.; Kitaev, V.; Ozin, G.A. Amplified photochemistry with slow photons. Adv. Mater. 2006, 18, 1915–1919. [Google Scholar] [CrossRef]
- Li, H.; Bian, Z.; Zhu, J.; Zhang, D.; Li, G.; Huo, Y.; Li, H.; Lu, Y. Mesoporous Titania Spheres with Tunable Chamber Stucture and Enhanced Photocatalytic Activity. J. Am. Chem. Soc. 2007, 129, 8406–8407. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Deng, Z.; Chen, F.; Zhang, J.; Chen, H.; Anpo, M.; Huang, J.; Zhang, L. Hydrothermal doping method for preparation of Cr3+–TiO2 photocatalysts with concentration gradient distribution of Cr3+. Appl. Catal. B 2006, 62, 329–335. [Google Scholar] [CrossRef]
- Mathew, S.; Ganguly, P.; Rhatigan, S.; Kumaravel, V.; Byrne, C.; Hinder, S.J.; Bartlett, J.; Nolan, M.; Pillai, S.C. Cu-doped TiO2: Visible light assisted photocatalytic antimicrobial activity. Appl. Sci. 2018, 8, 2067. [Google Scholar] [CrossRef]
- He, S.; Huang, J.; Goodsell, J.L.; Angerhofer, A.; Wei, W.D. Plasmonic Nickel–TiO2 Heterostructures for Visible-Light-Driven Photochemical Reactions. Angew. Chem. Int. Ed. 2019, 58, 6038–6041. [Google Scholar] [CrossRef]
- Jia, T.; Fu, F.; Yu, D.; Cao, J.; Sun, G. Facile synthesis and characterization of N-doped TiO2/C nanocomposites with enhanced visible-light photocatalytic performance. Appl. Surf. Sci. 2018, 430, 438–447. [Google Scholar] [CrossRef]
- Zhou, F.; Song, H.; Wang, H.; Komarneni, S.; Yan, C. N-doped TiO2/sepiolite nanocomposites with enhanced visible-light catalysis: Role of N precursors. Appl. Clay Sci. 2018, 166, 9–17. [Google Scholar] [CrossRef]
- Romero Saez, M.; Jaramillo, L.; Saravanan, R.; Benito, N.; Pabón, E.; Mosquera, E.; Gracia Caroca, F. Notable photocatalytic activity of TiO2-polyethylene nanocomposites for visible light degradation of organic pollutants. EXPRESS Polym. Lett. 2017, 11, 899–909. [Google Scholar] [CrossRef]
- Saravanan, R.; Aviles, J.; Gracia, F.; Mosquera, E.; Gupta, V.K. Crystallinity and lowering band gap induced visible light photocatalytic activity of TiO2/CS (Chitosan) nanocomposites. Int. J. Biol. Macromol. 2018, 109, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Raziq, F.; Sun, L.; Wang, Y.; Zhang, X.; Humayun, M.; Ali, S.; Bai, L.; Qu, Y.; Yu, H.; Jing, L. Synthesis of Large Surface-Area g–C3N4 Comodified with MnOx and Au–TiO2 as Efficient Visible-Light Photocatalysts for Fuel Production. Adv. Energy Mater. 2018, 8, 1701580. [Google Scholar] [CrossRef]
- Delsouz Khaki, M.R.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Evaluating the efficiency of nano-sized Cu doped TiO2/ZnO photocatalyst under visible light irradiation. J. Mol. Liq. 2018, 258, 354–365. [Google Scholar] [CrossRef]
- Liu, X.; Xing, Z.; Zhang, Y.; Li, Z.; Wu, X.; Tan, S.; Yu, X.; Zhu, Q.; Zhou, W. Fabrication of 3D flower-like black N–TiO2-x@MoS2 for unprecedented-high visible-light-driven photocatalytic performance. Appl. Catal. B 2017, 201, 119–127. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, M.; Yu, W.; Zhang, Q.; Xie, H.; Sun, Z.; Shao, Q.; Guo, X.; Hao, L.; Zheng, Y. Heterostructured TiO2/WO3 nanocomposites for photocatalytic degradation of toluene under visible light. J. Electrochem. Soc. 2017, 164, H1086. [Google Scholar] [CrossRef]
- Yao, G.-Y.; Liu, Q.-L.; Zhao, Z.-Y. Studied Localized Surface Plasmon Resonance Effects of Au Nanoparticles on TiO2 by FDTD Simulations. Catalysts 2018, 8, 236. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, X.; Liu, J.; Tian, Z.; Dai, L.; He, B.; Han, C.; Wu, Y.; Zeng, Z.; Hu, Z. On the role of localized surface plasmon resonance in UV-Vis light irradiated Au/TiO2 photocatalysis systems: Pros and cons. Nanoscale 2015, 7, 4114–4123. [Google Scholar] [CrossRef]
- Schaub, R.; Wahlström, E.; Rønnau, A.; Lægsgaard, E.; Stensgaard, I.; Besenbacher, F. Oxygen-Mediated Diffusion of Oxygen Vacancies on the TiO2110) Surface. Science 2003, 299, 377. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, L.C.; Gong, Y.; Niu, P.; Wang, J.Q.; Gu, L.; Chen, X.; Liu, G.; Wang, L.; Cheng, H.M. An Unusual Strong Visible-Light Absorption Band in Red Anatase TiO2 Photocatalyst Induced by Atomic Hydrogen-Occupied Oxygen Vacancies. Adv. Mater. 2018, 30, 1704479. [Google Scholar] [CrossRef]
- Wu, T.; Niu, P.; Yang, Y.; Yin, L.-C.; Tan, J.; Zhu, H.; Irvine, J.T.S.; Wang, L.; Liu, G.; Cheng, H.-M. Homogeneous Doping of Substitutional Nitrogen/Carbon in TiO2 Plates for Visible Light Photocatalytic Water Oxidation. Sci. Rep. 2019, 29, 1901943. [Google Scholar]
- Cao, Y.-Q.; Zhao, X.-R.; Chen, J.; Zhang, W.; Li, M.; Zhu, L.; Zhang, X.-J.; Wu, D.; Li, A.-D. TiOxNy Modified TiO2 Powders Prepared by Plasma Enhanced Atomic Layer Deposition for Highly Visible Light Photocatalysis. Sci. Rep. 2018, 8, 12131. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.A.; Bahnemann, D.W.; Bannat, I.; Wark, M. Gold nanoparticles on mesoporous interparticle networks of titanium dioxide nanocrystals for enhanced photonic efficiencies. J. Phys. Chem. 2009, 113, 7429–7435. [Google Scholar] [CrossRef]
- Forsyth, M.; MacFarlane, D.R.; Best, A.; Adebahr, J.; Jacobsson, P.; Hill, A.J. The effect of nano-particle TiO2 fillers on structure and transport in polymer electrolytes. Solid State Ion. 2002, 147, 203–211. [Google Scholar] [CrossRef]
- Xiu, Z.; Guo, M.; Zhao, T.; Pan, K.; Xing, Z.; Li, Z.; Zhou, W. Recent advances in Ti3+ self-doped nanostructured TiO2 visible light photocatalysts for environmental and energy applications. Chem. Eng. J. 2020, 382, 123011. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, L.; Kim, J.K.; Ma, M.; Veerappan, G.; Lee, C.-L.; Kong, K.-J.; Lee, H.; Park, J.H. An order/disorder/water junction system for highly efficient co-catalyst-free photocatalytic hydrogen generation. Energy Environ. Sci. 2016, 9, 499–503. [Google Scholar] [CrossRef]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Bianchi, C.L.; Psaro, R.; Dal Santo, V. Effect of Nature and Location of Defects on Bandgap Narrowing in Black TiO2 Nanoparticles. J. Am. Chem. Soc. 2012, 134, 7600–7603. [Google Scholar] [CrossRef]
- Hejazi, S.; Mohajernia, S.; Osuagwu, B.; Zoppellaro, G.; Andryskova, P.; Tomanec, O.; Kment, S.; Zbořil, R.; Schmuki, P. On the Controlled Loading of Single Platinum Atoms as a Co-Catalyst on TiO2 Anatase for Optimized Photocatalytic H2 Generation. Adv. Mater. 2020, 32, 1908505. [Google Scholar] [CrossRef]
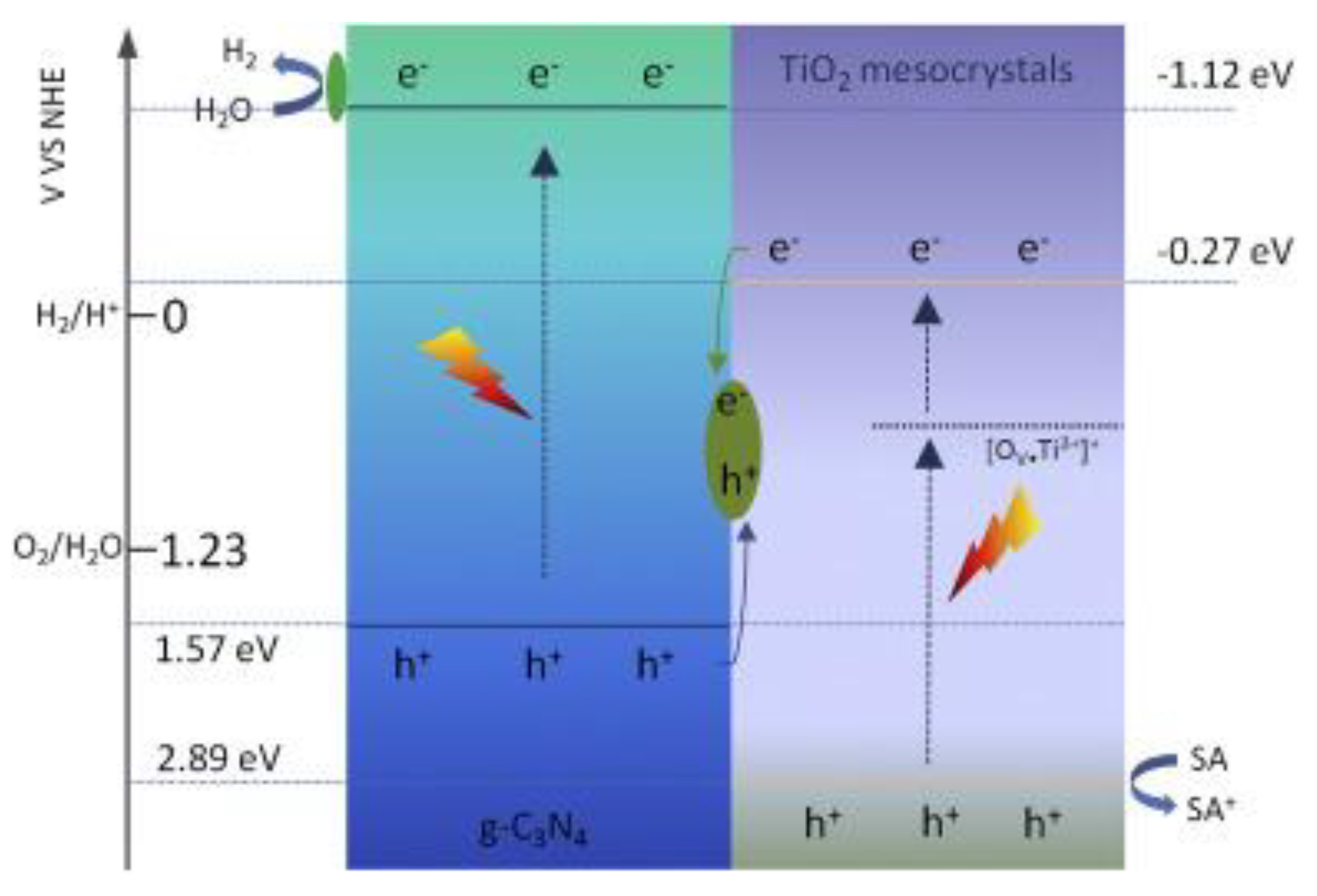
- Yu, X.; Fan, X.; An, L.; Liu, G.; Li, Z.; Liu, J.; Hu, P. Mesocrystalline Ti3+ TiO2 hybridized g–C3N4 for efficient visible-light photocatalysis. Carbon 2018, 128, 21–30. [Google Scholar] [CrossRef]
- Nguyen, C.T.K.; Quang Tran, N.; Seo, S.; Hwang, H.; Oh, S.; Yu, J.; Lee, J.; Anh Le, T.; Hwang, J.; Kim, M.; et al. Highly efficient nanostructured metal-decorated hybrid semiconductors for solar conversion of CO2 with almost complete CO selectivity. Mater. Today 2020. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nanocrystals. Science 2011, 331, 746. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cai, J.; Mao, J.; Li, S.; Shen, J.; Gao, S.; Huang, J.; Wang, X.; Parkin, I.P.; Lai, Y. Defective black Ti3+ self-doped TiO2 and reduced graphene oxide composite nanoparticles for boosting visible-light driven photocatalytic and photoelectrochemical activity. Appl. Surf. Sci. 2019, 467, 45–55. [Google Scholar] [CrossRef]
- Tan, S.; Xing, Z.; Zhang, J.; Li, Z.; Wu, X.; Cui, J.; Kuang, J.; Zhu, Q.; Zhou, W. Ti3+–TiO2/g–C3N4 mesostructured nanosheets heterojunctions as efficient visible-light-driven photocatalysts. J. Catal. 2018, 357, 90–99. [Google Scholar] [CrossRef]
- Sasikala, R.; Shirole, A.; Sudarsan, V.; Sakuntala, T.; Sudakar, C.; Naik, R.; Bharadwaj, S.R. Highly dispersed phase of SnO2 on TiO2 nanoparticles synthesized by polyol-mediated route: Photocatalytic activity for hydrogen generation. Int. J. Hydrog. Energy 2009, 34, 3621–3630. [Google Scholar] [CrossRef]
- Justicia, I.; Ordejón, P.; Canto, G.; Mozos, J.L.; Fraxedas, J.; Battiston, G.A.; Gerbasi, R.; Figueras, A. Designed Self-Doped Titanium Oxide Thin Films for Efficient Visible-Light Photocatalysis. Adv. Mater. 2002, 14, 1399–1402. [Google Scholar] [CrossRef]
- Buso, D.; Pacifico, J.; Martucci, A.; Mulvaney, P. Gold-nanoparticle-doped TiO2 semiconductor thin films: Optical characterization. Adv. Funct. Mater. 2007, 17, 347–354. [Google Scholar] [CrossRef]
- Zhu, G.; Shan, Y.; Lin, T.; Zhao, W.; Xu, J.; Tian, Z.; Zhang, H.; Zheng, C.; Huang, F. Hydrogenated blue titania with high solar absorption and greatly improved photocatalysis. Nanoscale 2016, 8, 4705–4712. [Google Scholar] [CrossRef]
- Qiu, J.; Li, S.; Gray, E.; Liu, H.; Gu, Q.-F.; Sun, C.; Lai, C.; Zhao, H.; Zhang, S. Hydrogenation Synthesis of Blue TiO2 for High-Performance Lithium-Ion Batteries. J. Phys. Chem. 2014, 118, 8824–8830. [Google Scholar]
- Sun, L.; Li, Z.; Li, Z.; Hu, Y.; Chen, C.; Yang, C.; Du, B.; Sun, Y.; Besenbacher, F.; Yu, M. Design and mechanism of core-shell TiO2 nanoparticles as a high-performance photothermal agent. Nanoscale 2017, 9, 16183–16192. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Wang, W.; Peng, T.; Liang, J.; Li, P.; Pan, D.; Fan, Q.; Wu, W. Visible light driven Ti3+ self-doped TiO2 for adsorption-photocatalysis of aqueous U(VI). Environ. Pollut. 2020, 262, 114373. [Google Scholar] [CrossRef]
- Cheng, D.; Li, Y.; Yang, L.; Luo, S.; Yang, L.; Luo, X.; Luo, Y.; Li, T.; Gao, J.; Dionysiou, D.D. One-step reductive synthesis of Ti3+ self–doped elongated anatase TiO2 nanowires combined with reduced graphene oxide for adsorbing and degrading waste engine oil. J. Hazard. Mater. 2019, 378, 120752. [Google Scholar] [CrossRef] [PubMed]
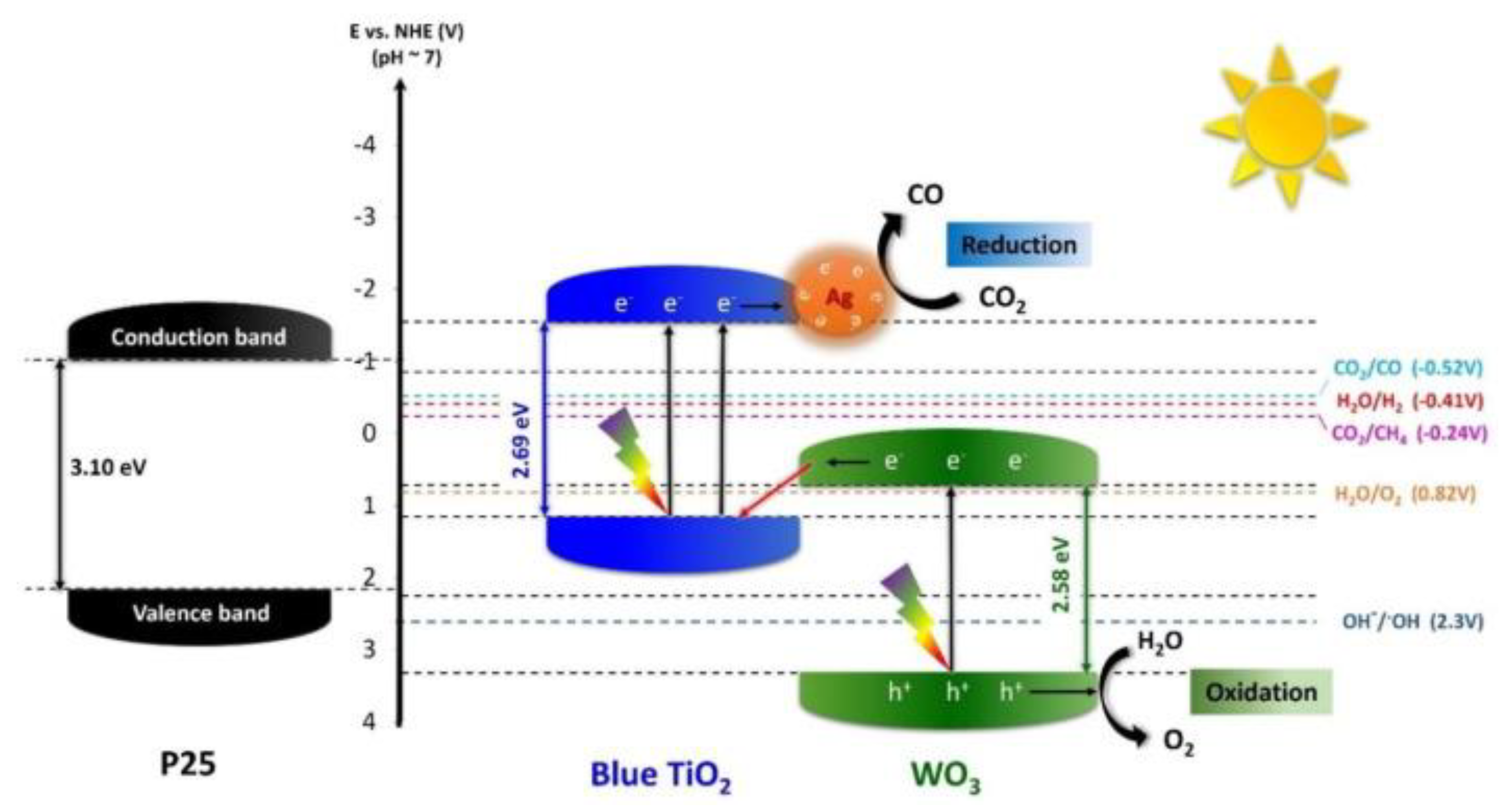
- Li, J.-J.; Weng, B.; Cai, S.-C.; Chen, J.; Jia, H.-P.; Xu, Y.-J. Efficient promotion of charge transfer and separation in hydrogenated TiO2/WO3 with rich surface-oxygen-vacancies for photodecomposition of gaseous toluene. J. Hazard. Mater. 2018, 342, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Zhu, Q.; Peng, Y.; Lin, L.; Fan, C.-M.; Gao, G.-Q.; Wang, R.-X.; Xu, A.-W. Stable blue TiO2−x nanoparticles for efficient visible light photocatalysts. J. Mater. Chem. 2014, 2, 4429–4437. [Google Scholar] [CrossRef]
- Fang, W.; Dappozze, F.; Guillard, C.; Zhou, Y.; Xing, M.; Mishra, S.; Daniele, S.; Zhang, J. Zn-Assisted TiO2–x Photocatalyst with Efficient Charge Separation for Enhanced Photocatalytic Activities. J. Phys. Chem. 2017, 121, 17068–17076. [Google Scholar] [CrossRef]
- Yin, H.; Lin, T.; Yang, C.; Wang, Z.; Zhu, G.; Xu, T.; Xie, X.; Huang, F.; Jiang, M. Gray TiO2 Nanowires Synthesized by Aluminum-Mediated Reduction and Their Excellent Photocatalytic Activity for Water Cleaning. Chem. Eur. 2013, 19, 13313–13316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Pei, Q.; Chen, W.; Liu, L.; He, T.; Chen, P. Room temperature synthesis of reduced TiO2 and its application as a support for catalytic hydrogenation. RSC Adv. 2017, 7, 4306–4311. [Google Scholar] [CrossRef]
- Zhang, Y.; Xing, Z.; Liu, X.; Li, Z.; Wu, X.; Jiang, J.; Li, M.; Zhu, Q.; Zhou, W. Ti3+ Self-Doped Blue TiO2(B) Single-Crystalline Nanorods for Efficient Solar-Driven Photocatalytic Performance. ACS Appl. Mater. Interfaces 2016, 8, 26851–26859. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Sun, M.; Yuan, X.; Zhu, Y.; Lin, X.; Anandan, S. One-step hydrothermal synthesis of N/Ti3+ co-doping multiphasic TiO2/BiOBr heterojunctions towards enhanced sonocatalytic performance. Ultrason Sonochem 2018, 49, 69–78. [Google Scholar] [CrossRef]
- Hwang, H.M.; Oh, S.; Shim, J.-H.; Kim, Y.-M.; Kim, A.; Kim, D.; Kim, J.; Bak, S.; Cho, Y.; Bui, V.Q.; et al. Phase-Selective Disordered Anatase/Ordered Rutile Interface System for Visible-Light-Driven, Metal-Free CO2 Reduction. ACS Appl. Mater. Interfaces 2019, 11, 35693–35701. [Google Scholar] [CrossRef]
- Bak, S.; Lee, S.M.; Hwang, H.M.; Lee, H. Phase-selective modulation of TiO2 for visible light-driven CH arylation: Tuning of absorption and adsorptivity. J. Mol. Catal. B Enzym. 2019, 471, 71–76. [Google Scholar] [CrossRef]
- Kim, Y.; Hwang, H.M.; Wang, L.; Kim, I.; Yoon, Y.; Lee, H. Solar-light photocatalytic disinfection using crystalline/amorphous low energy bandgap reduced TiO2. Sci. Rep. 2016, 6, 25212. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Lee, H.K.; Moon, J.T.; Joo, J.B. Synthesis of Spherical TiO2 Particles with Disordered Rutile Surface for Photocatalytic Hydrogen Production. Catalysts 2019, 9, 491. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, S.; Mo, L.e.; Huang, Y.; Tian, H.; Hu, L.; Huo, Z.; Dai, S.; Kong, F.; Pan, X. Charge Recombination and Band-Edge Shift in the Dye-Sensitized Mg2+-Doped TiO2 Solar Cells. J. Phys. Chem. 2011, 115, 16418–16424. [Google Scholar]
- Cao, J.; Zhang, Y.; Tong, H.; Li, P.; Kako, T.; Ye, J. Selective local nitrogen doping in a TiO2 electrode for enhancing photoelectrochemical water splitting. ChemComm 2012, 48, 8649–8651. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhang, Y.; Liu, L.; Ye, J. A p-type Cr-doped TiO2 photo-electrode for photo-reduction. ChemComm 2013, 49, 3440–3442. [Google Scholar] [CrossRef]
- Gul, I.; Sayed, M.; Shah, N.S.; Ali Khan, J.; Polychronopoulou, K.; Iqbal, J.; Rehman, F. Solar light responsive bismuth doped titania with Ti3+ for efficient photocatalytic degradation of flumequine: Synergistic role of peroxymonosulfate. Chem. Eng. J. 2020, 384, 123255. [Google Scholar] [CrossRef]
- Liu, H.; Shen, B.; Xing, M.; Zhang, J.; Tian, B. Vacuum-activated Co2+ and Ti3+ co-modified TiO2 with stable and enhanced photocatalytic activity. Res. Chem. Intermed. 2016, 42, 3459–3471. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, M.; Liu, X.; Wang, X.; Liu, S.; Han, B.; Liu, B.; Shi, H. Copper modified Ti3+ self-doped TiO2 photocatalyst for highly efficient photodisinfection of five agricultural pathogenic fungus. Chem. Eng. J. 2020, 387, 124171. [Google Scholar] [CrossRef]
- Zhou, F.; Yan, C.; Sun, Q.; Komarneni, S. TiO2/Sepiolite nanocomposites doped with rare earth ions: Preparation, characterization and visible light photocatalytic activity. Microporous Mesoporous Mater. 2019, 274, 25–32. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Dai, Y.; Whangbo, M.-H. Plasmonic photocatalysts: Harvesting visible light with noble metal nanoparticles. Phys. Chem. Chem. Phys. 2012, 14, 9813–9825. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Huang, B.; Dai, Y.; Lou, Z.; Wang, G.; Zhang, X.; Qin, X. Progress on extending the light absorption spectra of photocatalysts. Phys. Chem. Chem. Phys. 2014, 16, 2758–2774. [Google Scholar] [CrossRef]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Chen, J.; Li, Z.-Y.; Au, L.; Hartland, G.V.; Li, X.; Marquez, M.; Xia, Y. Gold nanostructures: Engineering their plasmonic properties for biomedical applications. Chem. Soc. Rev. 2006, 35, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Rycenga, M.; Cobley, C.M.; Zeng, J.; Li, W.; Moran, C.H.; Zhang, Q.; Qin, D.; Xia, Y. Controlling the Synthesis and Assembly of Silver Nanostructures for Plasmonic Applications. Chem. Rev. 2011, 111, 3669–3712. [Google Scholar] [CrossRef] [PubMed]
- Bigall, N.C.; Härtling, T.; Klose, M.; Simon, P.; Eng, L.M.; Eychmüller, A. Monodisperse Platinum Nanospheres with Adjustable Diameters from 10 to 100 nm: Synthesis and Distinct Optical Properties. Nano Lett. 2008, 8, 4588–4592. [Google Scholar] [CrossRef]
- Hutter, E.; Fendler, J.H. Exploitation of Localized Surface Plasmon Resonance. Adv. Mater. 2004, 16, 1685–1706. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, L.; Yu, Y.; Gao, F.; Wang, W.; Chen, D.; Zhao, X. Facile one-pot preparation of Ti3+, N co-doping TiO2 nanotube arrays and enhanced photodegradation activities by tuning tube lengths and diameters. Catal. Today 2019. [Google Scholar] [CrossRef]
- Cao, Y.; Xing, Z.; Shen, Y.; Li, Z.; Wu, X.; Yan, X.; Zou, J.; Yang, S.; Zhou, W. Mesoporous black Ti3+/N–TiO2 spheres for efficient visible-light-driven photocatalytic performance. Chem. Eng. J. 2017, 325, 199–207. [Google Scholar] [CrossRef]
- Ji, L.; Zhou, X.; Schmuki, P. Sulfur and Ti3+ co-Doping of TiO2 Nanotubes Enhance Photocatalytic H2 Evolution without the Use of Any co-catalyst. Chem.: Asian J. 2019, 14, 2724–2730. [Google Scholar]
- Li, M.; Xing, Z.; Jiang, J.; Li, Z.; Yin, J.; Kuang, J.; Tan, S.; Zhu, Q.; Zhou, W. Surface plasmon resonance-enhanced visible-light-driven photocatalysis by Ag nanoparticles decorated S–TiO2−x nanorods. J. Taiwan Inst. Chem. Eng. 2018, 82, 198–204. [Google Scholar] [CrossRef]
- Yan, X.; Xing, Z.; Cao, Y.; Hu, M.; Li, Z.; Wu, X.; Zhu, Q.; Yang, S.; Zhou, W. In-situ C-N-S-tridoped single crystal black TiO2 nanosheets with exposed {001} facets as efficient visible-light-driven photocatalysts. Appl. Catal. B 2017, 219, 572–579. [Google Scholar] [CrossRef]
- Irie, H.; Watanabe, Y.; Hashimoto, K. Nitrogen-Concentration Dependence on Photocatalytic Activity of TiO2-xNx Powders. J. Phys. Chem. B 2003, 107, 5483–5486. [Google Scholar] [CrossRef]
- Wang, X.; Cui, Y.; Li, T.; Lei, M.; Li, J.; Wei, Z. Recent Advances in the Functional 2D Photonic and Optoelectronic Devices. Adv. Opt. Mater. 2019, 7, 1801274. [Google Scholar] [CrossRef]
- Wei, H.; McMaster, W.A.; Tan, J.Z.Y.; Chen, D.; Caruso, R.A. Tricomponent brookite/anatase TiO2/g–C3N4 heterojunction in mesoporous hollow microspheres for enhanced visible-light photocatalysis. J. Mater. Chem. 2018, 6, 7236–7245. [Google Scholar] [CrossRef]
- Li, S.-Y.; Liu, Z.-L.; Xiang, G.-X.; Ma, B.-H.; Meng, X.-D.; He, Y.-L. Influence of calcination temperature on the photocatalytic performance of the hierarchical TiO2 pinecone-like structure decorated with CdS nanoparticles. Ceram. Int. 2019, 45, 767–776. [Google Scholar] [CrossRef]
- Zhao, F.-M.; Pan, L.; Wang, S.; Deng, Q.; Zou, J.-J.; Wang, L.; Zhang, X. Ag3PO4/TiO2 composite for efficient photodegradation of organic pollutants under visible light. Appl. Surf. Sci. 2014, 317, 833–838. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Li, Y.-L.; Jiu, B.-B.; Gong, F.-L.; Chen, J.; Fang, S.; Zhang, H. Highly enhanced photocatalytic H2 evolution of Cu2O microcube by coupling with TiO2 nanoparticles. Nat. Nanotechnol. 2019, 30. [Google Scholar] [CrossRef]
- Chen, X.; Ye, J.; Ouyang, S.; Kako, T.; Li, Z.; Zou, Z. Enhanced Incident Photon-to-Electron Conversion Efficiency of Tungsten Trioxide Photoanodes Based on 3D-Photonic Crystal Design. ACS Nano 2011, 5, 4310–4318. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, M.; Li, L.; Pi, Y.; Yan, G.; Yang, L. A Direct Z-Scheme Van Der Waals Heterojunction (WO3·H2O/g–C3N4) for High Efficient Overall Water Splitting under Visible-Light. Sol. RRL 2018, 2, 1800148. [Google Scholar] [CrossRef]
- Pan, J.; Jiang, Z.; Feng, S.; Zhao, C.; Dong, Z.; Wang, B.; Wang, J.; Song, C.; Zheng, Y.; Li, C. The enhanced photocatalytic hydrogen production of the fusiform g–C3N4 modification CaTiO3 nano-heterojunction. Int. J. Hydrog. Energy 2018, 43, 19019–19028. [Google Scholar] [CrossRef]
- Zhao, T.; Xing, Z.; Xiu, Z.; Li, Z.; Chen, P.; Zhu, Q.; Zhou, W. Synergistic effect of surface plasmon resonance, Ti3+ and oxygen vacancy defects on Ag/MoS2/TiO2-x ternary heterojunctions with enhancing photothermal catalysis for low-temperature wastewater degradation. J. Hazard. Mater. 2019, 364, 117–124. [Google Scholar] [CrossRef]
- Ma, L.; Han, H.; Pan, L.; Tahir, M.; Wang, L.; Zhang, X.; Zou, J.-J. Fabrication of TiO2 nanosheets via Ti3+ doping and Ag3PO4 QD sensitization for highly efficient visible-light photocatalysis. RSC Adv. 2016, 6, 63984–63990. [Google Scholar] [CrossRef]
- Zhao, T.; Xing, Z.; Xiu, Z.; Li, Z.; Shen, L.; Cao, Y.; Hu, M.; Yang, S.; Zhou, W. CdS quantum dots/Ti3+–TiO2 nanobelts heterojunctions as efficient visible-light-driven photocatalysts. Mater. Res. Bull. 2018, 103, 114–121. [Google Scholar] [CrossRef]
- Sun, B.; Zhou, W.; Li, H.; Ren, L.; Qiao, P.; Li, W.; Fu, H. Synthesis of Particulate Hierarchical Tandem Heterojunctions toward Optimized Photocatalytic Hydrogen Production. Adv. Mater. 2018, 30, 1804282. [Google Scholar] [CrossRef]
- Xiu, Z.; Xing, Z.; Li, Z.; Wu, X.; Yan, X.; Hu, M.; Cao, Y.; Yang, S.; Zhou, W. Ti3+–TiO2/Ce3+-CeO2 Nanosheet heterojunctions as efficient visible-light-driven photocatalysts. Mater. Res. Bull. 2018, 100, 191–197. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, X.; Hu, Q.; Liang, X.; Tian, T.; Lin, J.; Yue, M.; Ding, Y. FeOx Derived from an Iron-Containing Polyoxometalate Boosting the Photocatalytic Water Oxidation Activity of Ti3+-Doped TiO2. ACS Appl. Mater. Interfaces 2019, 11, 23135–23143. [Google Scholar] [CrossRef]
- Pan, J.; Dong, Z.; Jiang, Z.; Zhao, C.; Wang, B.; Zhao, W.; Wang, J.; Song, C.; Zheng, Y.; Li, C. MoS2 Quantum Dots Modified Black Ti3+–TiO2/g–C3N4 Hollow Nanosphere Heterojunction toward Photocatalytic Hydrogen Production Enhancement. Solar RRL 2019, 3, 1900337. [Google Scholar] [CrossRef]
- Cao, Y.; Xing, Z.; Li, Z.; Wu, X.; Hu, M.; Yan, X.; Zhu, Q.; Yang, S.; Zhou, W. Mesoporous black TiO2-x/Ag nanospheres coupled with g–C3N4 nanosheets as 3D/2D ternary heterojunctions visible light photocatalysts. J. Hazard. Mater. 2018, 343, 181–190. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, P.; Wang, Z.; Rao, Y.; Cao, J.-j.; Pu, S.; Ho, W.; Lee, S.C. Protonated g–C3N4/Ti3+ self-doped TiO2 nanocomposite films: Room-temperature preparation, hydrophilicity, and application for photocatalytic NOx removal. Appl. Catal. B 2019, 240, 122–131. [Google Scholar] [CrossRef]
- Ye, M.-Y.; Zhao, Z.-H.; Hu, Z.-F.; Liu, L.-Q.; Ji, H.-M.; Shen, Z.-R.; Ma, T.-Y. 0D/2D Heterojunctions of Vanadate Quantum Dots/Graphitic Carbon Nitride Nanosheets for Enhanced Visible-Light-Driven Photocatalysis. Angew. Chem. Int. Ed. 2017, 56, 8407–8411. [Google Scholar] [CrossRef] [PubMed]
- Huo, N.; Gupta, S.; Konstantatos, G. MoS2–HgTe Quantum Dot Hybrid Photodetectors beyond 2 µm. Adv. Mater. 2017, 29, 1606576. [Google Scholar] [CrossRef] [PubMed]
- Lian, Z.; Xu, P.; Wang, W.; Zhang, D.; Xiao, S.; Li, X.; Li, G. C60-Decorated CdS/TiO2 Mesoporous Architectures with Enhanced Photostability and Photocatalytic Activity for H2 Evolution. ACS Appl. Mater. Interfaces 2015, 7, 4533–4540. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Yang, X.; Zhu, J.; Li, H. Highly active and stable CdS–TiO2 visible photocatalyst prepared by in situ sulfurization under supercritical conditions. Appl. Catal. B 2011, 106, 69–75. [Google Scholar] [CrossRef]
- Yu, X.; Li, Z.; Liu, J.; Hu, P. TaOC chemical bond enhancing charge separation between Ta4+ doped Ta2O5 quantum dots and cotton-like g–C3N4. Appl. Catal. B 2017, 205, 271–280. [Google Scholar] [CrossRef]
- Zhou, P.; Yu, J.; Jaroniec, M. All-Solid-State Z-Scheme Photocatalytic Systems. Adv. Mater. 2014, 26, 4920–4935. [Google Scholar] [CrossRef]
- Zhang, L.J.; Li, S.; Liu, B.K.; Wang, D.J.; Xie, T.F. Highly Efficient CdS/WO3 Photocatalysts: Z-Scheme Photocatalytic Mechanism for Their Enhanced Photocatalytic H2 Evolution under Visible Light. ACS Catal. 2014, 4, 3724–3729. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, X.; Liu, L.; Jiang, S.; Li, Y.; Guo, L.; Yan, S.; Tai, X. Heterogeneous Bimetallic Cu–Ni Nanoparticle-Supported Catalysts in the Selective Oxidation of Benzyl Alcohol to Benzaldehyde. Catalysts 2019, 9, 538. [Google Scholar] [CrossRef]











| Catalysts | Light | Application Target | Efficiency | Ref. |
|---|---|---|---|---|
| Plasmonic Ni/Ti3+ TiO2/SiO2 nanospheres | 300 W Xe lamp | Methylene blue | 0.11 ± 0.04 μmol·L−1·min−1 | [69] |
| Pt single atom-anatase(001) Ti3+ TiO2 nano sheets | 325 nm, 365 nm | Hydrogen evolution reaction | 400 µmol·h−1·g−1 | [89] |
| Zn-assisted Ti3+ TiO2 | 500 W Tungsten halogen lamp | Formic acid | 100% 90 min−1 | [106] |
| Li-EDA TiO2–Pt | 150 W Xe lamp | Hydrogen evolution reaction | 350 µmol·h−1·g−1 | [114] |
| Bi3+/Ti3+ TiO2 | Xenon lamp | Flumequine under HSO5− | 65% | [118] |
| Co2+/Ti3+ TiO2 | 300 W Xe lamp | Acid Orange 7 | 100% 5 h−1 | [119] |
| Cu/Ti3+ TiO2 | 300 W Xe lamp | F. graminearum and B. dothidea spores | 100% | [120] |
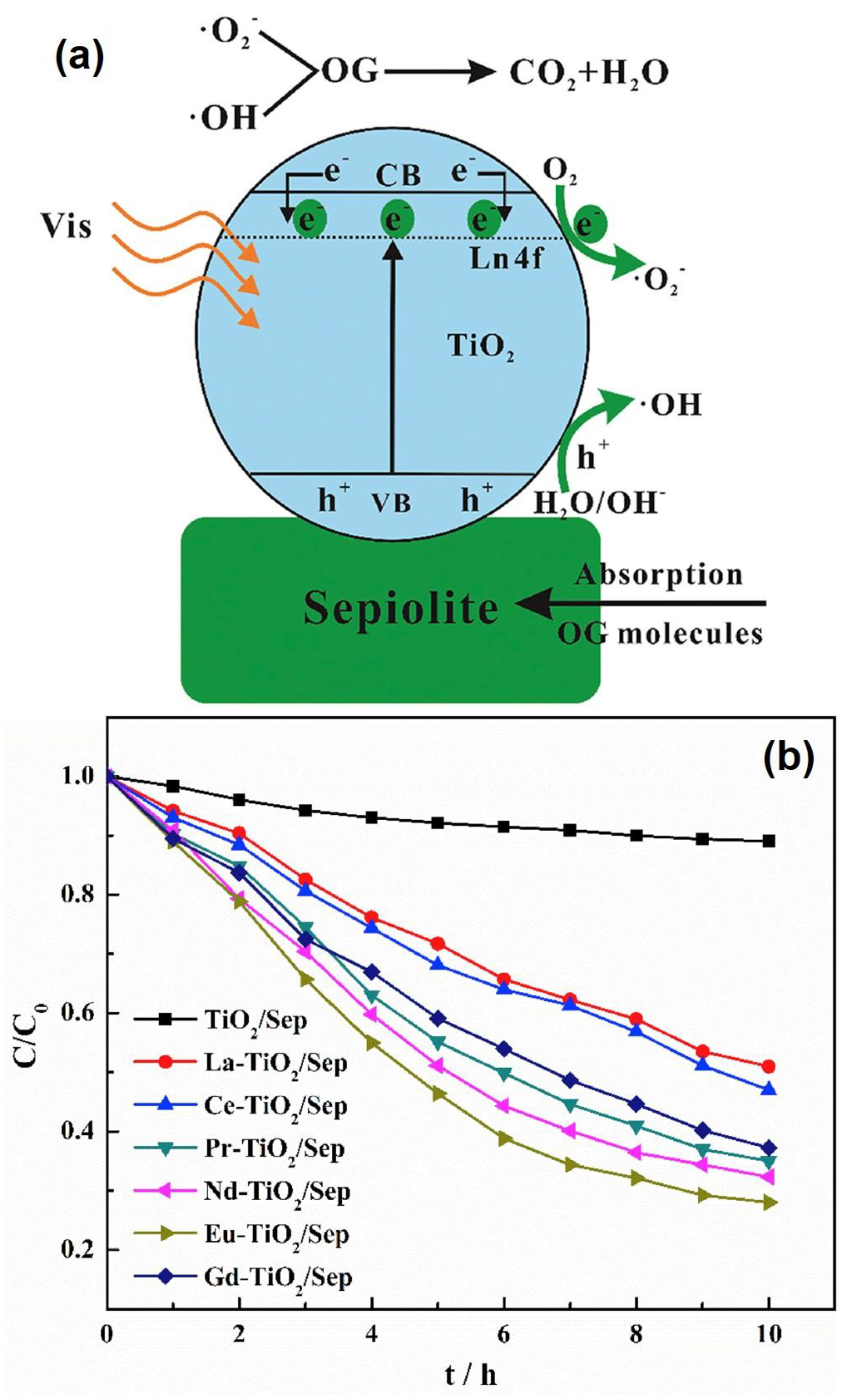
| RE-doped/Ti3+ TiO2/Sep | 300 W Xe lamp | Orange G | 72% | [121] |
| Catalysts | Light | Application Target | Efficiency | Ref. |
|---|---|---|---|---|
| N/Ti3+/C TiO2 | 500 W Xe lamp | Rhodamine B | 100% 1 h−1 | [70] |
| N/Ti3+ TiO2 nanotube | 400 W Halogen lamp | Rhodamine B | 100% 4 h−1 | [130] |
| N/Ti3+ TiO2 spheres | 300 W Xe lamp | Methylene orange | 100% 3 h−1 | [131] |
| S/Ti3+ TiO2 | 150 W Xenon lamp | Hydrogen evolution reaction | 22.5 µL·h−1·cm2 | [132] |
| S/Ti3+ TiO2–Ag nanorods | Xe lamp | Hydrogen evolution reaction | 209.2 μmol·h−1·g−1 | [133] |
| C–N–S-tridoped Ti3+ TiO2 | 300 W Xe lamp | Hydrogen evolution reaction | 149.7 μmol·h−1·g−1 | [134] |
| Catalysis | Light | Application Target | Efficiency | Ref. |
|---|---|---|---|---|
| Ti3+ TiO2/gC3N4 | Xe lamp | Hydrogen evolution reaction | 3748.46 μmol·h−1·g−1 | [90] |
| Ti3+ TiO2/WO3–Ag | Solar | CO2 to CO | 1166.72 μmol·h−1·g−1 | [91] |
| Ti3+ TiO2/rGO | 500 W Xe lamp | Methylene blue | 100% 2 h−1 | [93] |
| Ti3+ TiO2/meso-gC3N4 | 300 W Xenon lamp | Phenol | 100% 90 min−1 | [94] |
| Ti3+ TiO2/WO3 | 420 nm cutoff | Toluene | 100% 60 min−1 | [103] |
| Ti3+ TiO2 nanowires/rGO | 300 W Xe lamp | Waste oil | 100% 5 h−1 | [102] |
| N/Ti3+ TiO2/BiOBr | 120 mW lamp | Methylene blue | 100% 50 min−1 | [110] |
| Ti3+ TiO2/MoS2/Ag | 500 W Xe lamp | Bisphenol A | 100% 2 h−1 | [144] |
| Ti3+ TiO2/Ag3PO4 QD | 300 W Xe lamp | Methylene orange | 100% 100 min−1 | [145] |
| Ti3+ TiO2/CdS QD | 300 W Xe lamp | Methylene blue | 100% 150 min −1 | [146] |
| Ti3+ hollow TiO2/MoS2/CdS | 300 W Xe lamp | Hydrogen evolution reaction | 8950 μmol·h−1·g−1 | [147] |
| N/Ti3+ TiO2/MoS2 | 300 W Xe lamp | Methylene orange | 100% 2 h−1 | [76] |
| Ti3+ TiO2/Ce3+ CeO2 | 300 W Xe lamp | Methylene blue, Methylene orange | 100% 3 h −1 | [148] |
| Ti3+ TiO2/FeOx | 420 nm LED | Oxygen evolution reaction | 410 μmol·h−1·g−1 | [149] |
| Ti3+ TiO2/g–C3N4 hollow nanosphere/MoS2 QD | 300 W Xe lamp | Hydrogen evolution reaction | 1524.37 μmol·h−1·g−1 | [150] |
| Ti3+ TiO2/g–C3N4 nanospheres/Ag | 300 W Xe lamp | Methylene orange | 100% 3 h−1 | [151] |
| Ti3+ TiO2/g–C3N4 | Xe lamp | NOx | 75% | [152] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na, S.; Seo, S.; Lee, H. Recent Developments of Advanced Ti3+-Self-Doped TiO2 for Efficient Visible-Light-Driven Photocatalysis. Catalysts 2020, 10, 679. https://doi.org/10.3390/catal10060679
Na S, Seo S, Lee H. Recent Developments of Advanced Ti3+-Self-Doped TiO2 for Efficient Visible-Light-Driven Photocatalysis. Catalysts. 2020; 10(6):679. https://doi.org/10.3390/catal10060679
Chicago/Turabian StyleNa, Siyoung, Sohyeon Seo, and Hyoyoung Lee. 2020. "Recent Developments of Advanced Ti3+-Self-Doped TiO2 for Efficient Visible-Light-Driven Photocatalysis" Catalysts 10, no. 6: 679. https://doi.org/10.3390/catal10060679
APA StyleNa, S., Seo, S., & Lee, H. (2020). Recent Developments of Advanced Ti3+-Self-Doped TiO2 for Efficient Visible-Light-Driven Photocatalysis. Catalysts, 10(6), 679. https://doi.org/10.3390/catal10060679