Biochemical Degradation of Chitosan over Immobilized Cellulase and Supported Fenton Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Catalysts
2.1.1. N2 Adsorption–Desorption Analysis
2.1.2. FT-IR of Catalysts
2.1.3. High-Resolution Transmission Electron Microscopy and Scanning Electron Microscope Analysis of Catalysts
2.1.4. X-ray Diffraction of Catalysts
2.2. Catalytic Performances
2.2.1. Degradation of Chitosan Catalyzed by MCM-48, Fe-MCM-48, and Cellulase-MCM-48
2.2.2. Activity of Fe-MCM-48 Catalysts at Different Temperatures
2.2.3. Distribution of Degradation Products
2.2.4. FT-IR Analysis of Degradation Products
3. Experimental Section
3.1. Materials and Reagents
3.2. Preparation of the Modified MCM-48 Catalyst
3.2.1. Cellulase-MCM-48
3.2.2. Fe-MCM-48
3.3. Catalyst Characterization
3.4. Catalytic Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chang, K.L.B.; Tai, M.-C.; Cheng, F.-H. Kinetics and products of the degradation of chitosan by hydrogen peroxide. J. Agric. Food Chem. 2001, 49, 4845–4851. [Google Scholar] [CrossRef] [PubMed]
- Józef, S.; Nadia Ali, A.K. Production, properties, and some new applications of chitin and its derivatives. Crit. Rev. Food Sci. Nutr. 2003, 43, 145–171. [Google Scholar] [CrossRef]
- Tharanathan, R.N.; Kittur, F.S. Chitin—The undisputed biomolecule of great potential. C R C Crit. Rev. Food Technol. 2003, 43, 61–87. [Google Scholar] [CrossRef] [PubMed]
- Guojane, T.; Wu, Z.Y.; Su, W.H. Antibacterial activity of a chitooligosaccharide mixture prepared by cellulase digestion of shrimp chitosan and its application to milk preservation. J. Food Prot. 2000, 63, 747–752. [Google Scholar] [CrossRef]
- Kondo, Y.; Nakatani, A.; Hayashi, K.; Ito, M. Low molecular weight chitosan prevents the progression of low dose streptozotocin-induced slowly progressive diabetes mellitus in mice. Biol. Pharm. Bull. 2000, 23, 1458. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, W.; Zhang, Y.; Wang, X.; Li, Y. Chitosan Sulfate Inhibits Angiogenesis via Blocking VEGF/VEGFR2 Pathway and Suppress Tumor Growth in Vivo. Biomater. Sci. 2019, 7, 1584–1597. [Google Scholar] [CrossRef]
- Soe, Z.C.; Poudel, B.K.; Nguyen, H.T.; Thapa, R.K.; Ou, W.; Gautam, M.; Poudel, K.; Jin, S.G.; Jeong, J.H.; Ku, S.K. Folate-targeted nanostructured chitosan/chondroitin sulfate complex carriers for enhanced delivery of bortezomib to colorectal cancer cells. Asian J. Pharm. Sci. 2019, 14, 40–51. [Google Scholar] [CrossRef]
- Xu, Q.; Chao, Y.L.; Wan, Q.B. Health benefit application of functional oligosaccharides. Carbohydr. Polym. 2009, 77, 435–441. [Google Scholar] [CrossRef]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Kim, S.K.; Rajapakse, N. Enzymatic production and biological activities of chitosan oligosaccharides (COS): A review. Carbohydr. Polym. 2005, 62, 357–368. [Google Scholar] [CrossRef]
- Lee, M.Y.; Shinya, Y.; Kajiuchi, T.; Yang, J.W. Optimum conditions for the precipitation of chitosan oligomers with DP 5–7 in concentrated hydrochloric acid at low temperature. Process. Biochem. 1999, 34, 493–500. [Google Scholar] [CrossRef]
- Czechowska-Biskup, R.O.K.I.T.A.; Rokita, B.; Ulanski, P.; Rosiak, J.M. Radiation-induced and sonochemical degradation of chitosan as a way to increase its fat-binding capacity. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2005, 236, 383–390. [Google Scholar] [CrossRef]
- Wu, S. Preparation of water soluble chitosan by hydrolysis with commercial α-amylase containing chitosanase activity. Food Chem. 2011, 128, 769–772. [Google Scholar] [CrossRef]
- Popa-Nita, S.; Lucas, J.-M.; Ladavière, C.; David, L.; Domard, A. Mechanisms involved during the ultrasonically induced depolymerization of chitosan: Characterization and control. Biomacromolecules 2009, 10, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Liu, Y.; Hu, K.; Zhao, B. The depolymerization mechanism of chitosan by hydrogen peroxide. J. Mater. Sci. 2003, 38, 4709–4712. [Google Scholar] [CrossRef]
- Bu, F.; Bo, L.; Xu, G.F.; Wang, S.L. The research on progress of nonenzymic browning of chitooligomers and glucosamine. Food Ind. 2013, 30, 36–39. [Google Scholar] [CrossRef]
- Kang, B.; Dai, Y.-D.; Zhang, H.-Q.; Chen, D. Synergetic degradation of chitosan with gamma radiation and hydrogen peroxide. Polym. Degrad. Stab. 2007, 92, 359–362. [Google Scholar] [CrossRef]
- Wang, S.M.; Huang, Q.-Z.; Wang, Q.-S. Study on the synergetic degradation of chitosan with ultraviolet light and hydrogen peroxide. Carbohydr. Res. 2005, 340, 1143–1147. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Chen, S.; Fu, H.; Zhao, Y. Microwave-assisted degradation of chitosan with hydrogen peroxide treatment using Box-Behnken design for enhanced antibacterial activity. Int. J. Food Sci. Technol. 2018, 53, 156–165. [Google Scholar] [CrossRef]
- Yiu, H.H.P.; Wright, P.A.; Botting, N.P. Enzyme immobilisation using siliceous mesoporous molecular sieves. Microporous Mesoporous Mater. 2001, 44–45, 763–768. [Google Scholar] [CrossRef]
- Liu, L.K.; Zhong-Liang, S.U.; Lu, L.I.; Shi-Tao, Y.U. Immobilization of cellulase on amino-functionalized SBA-15 molecular sieve. J. Qingdao Univ. Sci. Technol. 2013, 6, 33–38. [Google Scholar]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Yao, Y.F.; Zhang, M.S.; Yang, Y.S. Synthesis of Nano-sized MCM-41 Mesoporous Molecular Sieve by Microwave Radiation Method. Acta Phys.-Chim. Sin. 2001, 17, 1117–1121. [Google Scholar] [CrossRef]
- Zhang, W.; Pinnavaia, T.J. Transition metal substituted derivatives of cubic MCM-48 mesoporous molecular sieves. Catal. Lett. 1996, 38, 261–265. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Q. Research progress and application prospect of enzyme immobilization on mesoporous silica-based materials. Petrochem. Technol. 2014, 43, 357–363. [Google Scholar]
- Han, T.; Shi, G.; Suzhe, L.I.; Liu, J. Study of the Determination Method of Protein Content in Chitosan. Chin. J. Med. Instrum. 2016, 40, 122–124. [Google Scholar]
- Kasaai, M.R. A review of several reported procedures to determine the degree of N-acetylation for chitin and chitosan using infrared spectroscopy. Carbohydr. Polym. 2008, 71, 497–508. [Google Scholar] [CrossRef]
- Xu, Z.P.; Yang, C.L.; Zhang, X.Q.; Wang, X.Z.; Huang, B.S. Determination of Chitosan Oligosaccharide Content by Acetylacetone Spectrophotometry Method. Adv. Mat. Res. 2012, 503–504, 543–547. [Google Scholar] [CrossRef]

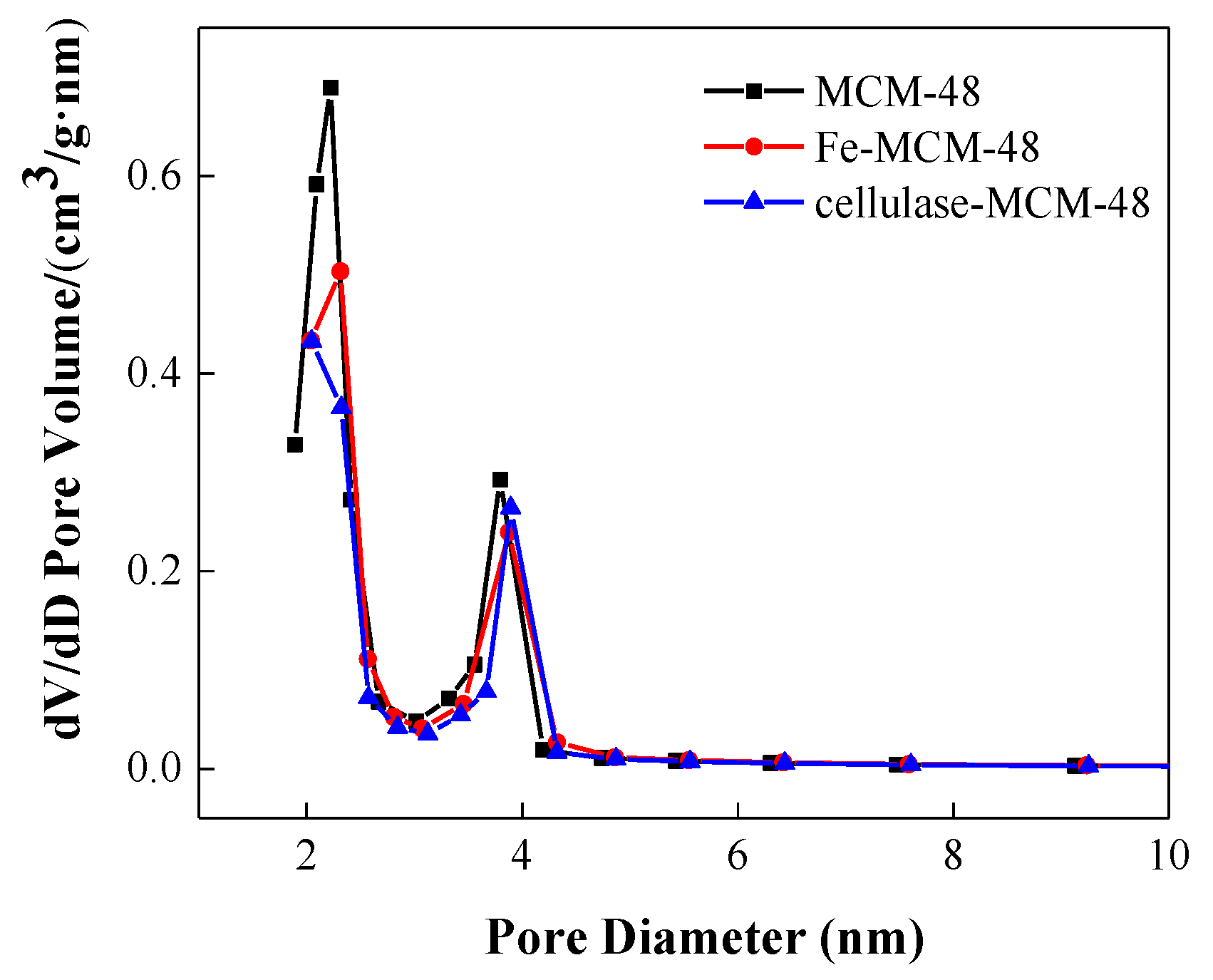
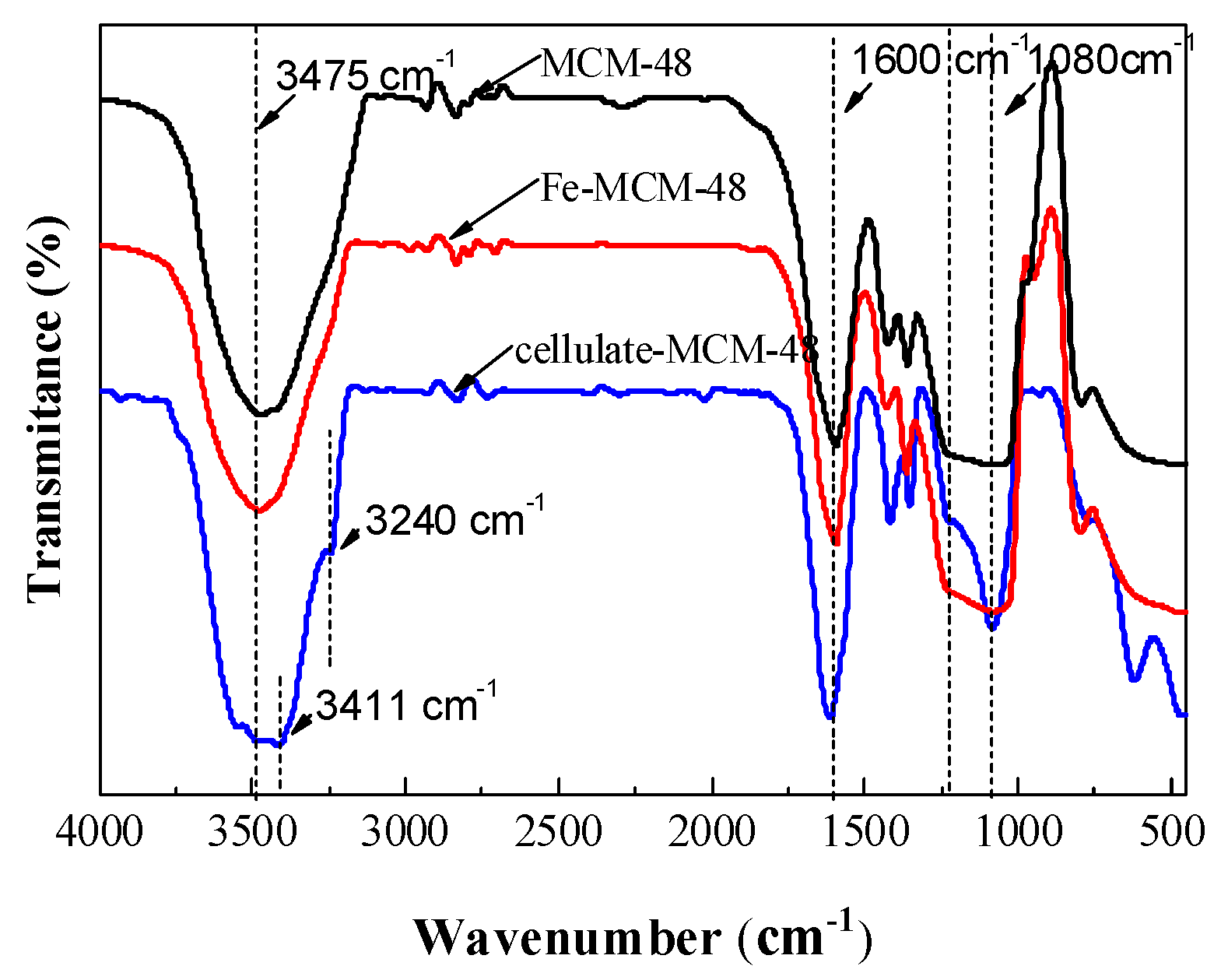
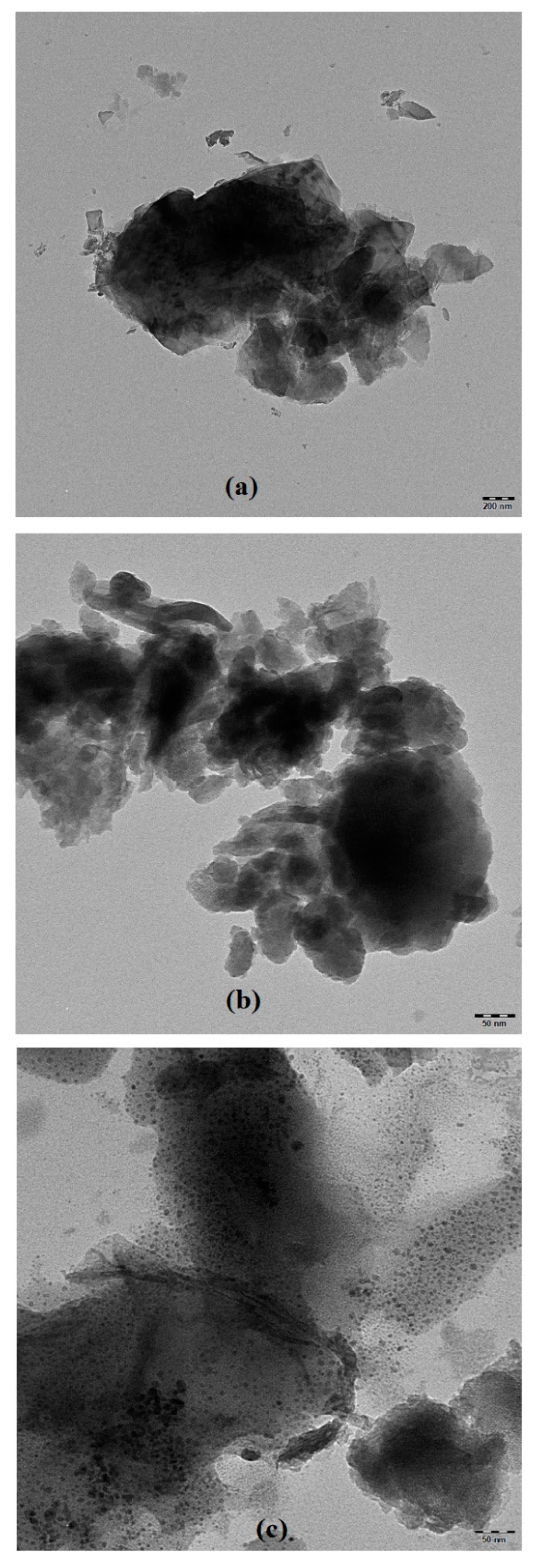

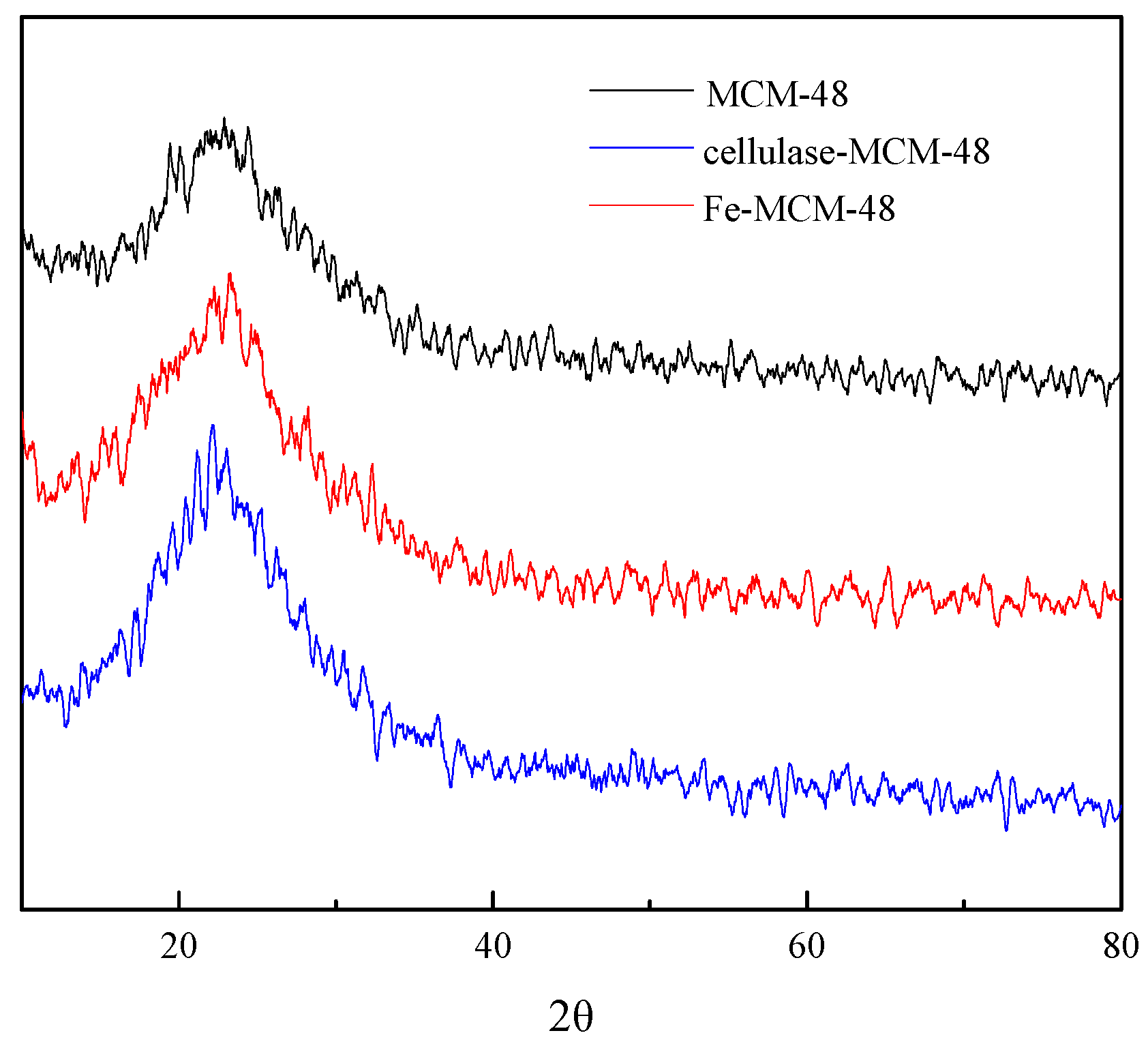


| Entry | Samples | Fe (wt %) | Enzyme (wt %) | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|---|---|---|
| 1 | MCM-48 | - | - | 797.7 | 0.65 | 3.04 |
| 2 | Fe-MCM-48 | 3.4% | - | 691.8 | 0.48 | 3.50 |
| 3 | Cellulase-MCM-48 | - | 6.4% | 589.5 | 0.39 | 3.72 |
| Entry | Catalyst | Product Yield (%) | Average Degree of Polymerization |
|---|---|---|---|
| 1 a | Fe-MCM-48 | 92 | 6.1 |
| 2 b | Cellulase-MCM-48 b | 95 | 29.2 |
| 3 c | Fe-MCM-48 and cellulase-MCM-48 c | 93 | 3.8 |
| Entry a | Temperature (°C) | Product Yield (%) | Average Degree of Polymerization |
|---|---|---|---|
| 1 | 40 | 94 | 7.5 |
| 2 | 50 | 95 | 6.1 |
| 3 | 60 | 93 | 5.9 |
| 4 | 70 | 90 | – b |
| Peak b | Retention Time/min | Peak Area | Molecular Mass | Proportion | Possible Components |
|---|---|---|---|---|---|
| 1 | 20.80 | 1.15 | 49 | 2.9% | - |
| 2 | 19.72 | 14.14 | 125 | 35.4% | Glucosamine |
| 3 | 19.51 | 3.20 | 170 | 8.0% | N-Acetyl-D-Glucosamine |
| 4 | 18.80 | 5.89 | 413 | 14.7% | Chitobiose Hydrochloride |
| 5 | 18.35 | 6.06 | 663 | 15.2% | Chitotriose Hydrochloride |
| 6 | 18.15 | 2.80 | 819 | 7.0% | Chitotetraose Hydrochloride |
| 7 | 17.87 | 1.21 | 1100 | 3.0% | Chitopentaose Hydrochloride |
| 8 | 17.71 | 1.39 | 1303 | 3.5% | Chitohexaose Hydrochloride |
| 9 | 17.52 | 1.49 | 1593 | 3.7% | - |
| 10 | 17.25 | 1.41 | 2120 | 3.5% | - |
| 11 | 16.89 | 0.18 | 3103 | 0.5% | - |
| Entry | Catalyst | Relative Content of Product (%) | |||||
|---|---|---|---|---|---|---|---|
| PD = 1 | PD = 2 | PD = 3 | PD = 4 | PD = 5 | PD = 6 | ||
| 1 | Fe-MCM-48 a | 22.1 | 9.7 | 10.7 | 11.5 | 19.0 | 18.4 |
| 2 | Cellulase-MCM-48 b | 0.77 | 0.60 | 0.76 | 0.71 | 0.71 | 0.66 |
| 3 | Fe-MCM-48 and cellulase-MCM-48 c | 31.8 | 12.0 | 17.7 | 16.6 | 10.4 | 7.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, H.; Mou, Z.; Liu, Z.; Li, F.; Yang, C. Biochemical Degradation of Chitosan over Immobilized Cellulase and Supported Fenton Catalysts. Catalysts 2020, 10, 604. https://doi.org/10.3390/catal10060604
Geng H, Mou Z, Liu Z, Li F, Yang C. Biochemical Degradation of Chitosan over Immobilized Cellulase and Supported Fenton Catalysts. Catalysts. 2020; 10(6):604. https://doi.org/10.3390/catal10060604
Chicago/Turabian StyleGeng, Huawei, Zonggang Mou, Ziyong Liu, Fuli Li, and Cheng Yang. 2020. "Biochemical Degradation of Chitosan over Immobilized Cellulase and Supported Fenton Catalysts" Catalysts 10, no. 6: 604. https://doi.org/10.3390/catal10060604





