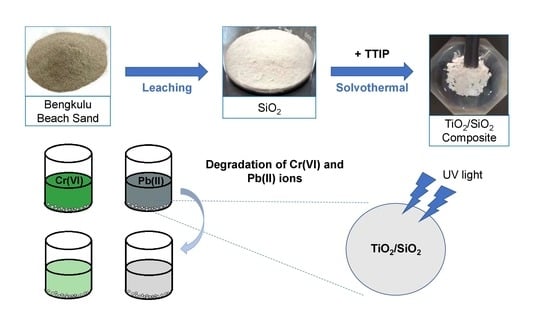
Synthesis of Titanium Dioxide/Silicon Dioxide from Beach Sand as Photocatalyst for Cr and Pb Remediation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Catalyst
2.2. Photocatalytic Activity
3. Materials and Methods
3.1. Materials
3.2. Extraction of Silica
3.3. The Synthesis of TiO2/SiO2 Composite
3.4. Characterization of Catalysis
3.5. Photocatalytic Activity Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sane, P.; Chaudhari, S.; Nemade, P.; Sontakke, S. Photocatalytic reduction of chromium(VI) using combustion synthesized TiO2. J. Environ. Chem. Eng. 2018, 6, 68–73. [Google Scholar] [CrossRef]
- Ojemaye, M.O.; Okoh, O.O.; Okoh, A.I. Performance of NiFe2O4-SiO2-TiO2 Magnetic Photocatalyst for the Effective Photocatalytic Reduction of Cr(VI) in Aqueous Solutions. J. Nanomater. 2017, 2017, 5264910. [Google Scholar] [CrossRef] [Green Version]
- Kotz, J.C.; Treichel, P.M.; Townsend, J.R. Chemistry and Chemical Reactivity, 7th ed.; Thomson Brooks/Cole: Pacific Grove, CA, USA, 2009. [Google Scholar]
- Said, N.I. Pengolahan Air Limbah Domestik di DKI Jakarta “Tinjauan Permasalahan, Strategi dan Teknologi Pengolahan”, 1st ed.; Badan Pengkajian dan Penerapan Teknologi: Jakarta, Indonesia, 2008; 606p.
- Eddy, D.R.; Rahayu, I.; Hartati, Y.W.; Firdaus, M.L.; Bakti, H.H. Photocatalytic activity of gadolinium doped TiO2 particles for decreasing heavy metal chromium concentration. J. Phys. Conf. Ser. 2018, 1080, 012013. [Google Scholar] [CrossRef]
- Bi, J.; Wang, J.; Huang, X.; Tao, Q.; Chen, M.; Wang, T.; Hao, H. Enhanced removal of Pb (II) and organics by titanate in a designed simultaneous process. Sep. Purif. Technol. 2020, 251, 117339. [Google Scholar] [CrossRef]
- Besançon, M.; Michelin, L.; Josien, L.; Vidal, L.; Assaker, K.; Bonne, M.; Lebeau, B.; Blin, J.L. Influence of the porous texture of SBA-15 mesoporous silica on the anatase formation in TiO2-SiO2 nanocomposites. New J. Chem. 2016, 40, 4386–4397. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, F.; Jiang, Y.; Li, F.; Wei, C. The effect of calcination temperature on the structure and activity of TiO2/SiO2 composite catalysts derived from titanium sulfate and fly ash acid sludge. Colloids Surf. A Physicochem. Eng. Asp. 2018, 554, 81–85. [Google Scholar] [CrossRef]
- Eddy, D.R.; Puri, F.N.; Noviyanti, A.R. Synthesis and Photocatalytic Activity of Silica-based Sand Quartz as the Supporting TiO2 Photocatalyst. Procedia Chem. 2015, 17, 55–58. [Google Scholar] [CrossRef] [Green Version]
- Munasir; Sulton, A.; Triwikantoro; Zainuri, M.; Darminto. Synthesis of silica nanopowder produced from Indonesian natural sand via alkalifussion route. AIP Conf. Proc. 2013, 1555, 28–31. [Google Scholar]
- Ishmah, S.N.; Permana, M.D.; Firdaus, M.L.; Eddy, D.R. Extraction of Silica from Bengkulu Beach Sand using Alkali Fusion Method. PENDIPA J. Sci. Edu. 2020, 4, 1–5. [Google Scholar] [CrossRef]
- Firdaus, M.L.; Madina, F.E.; Yulia, F.S.; Elvia, R.; Soraya, N.I.; Eddy, D.R.; Cid-Andres, A.P. Silica Extraction from Beach Sand for Dyes Removal: Isotherms, Kinetics and Thermodynamics. Rasayan J. Chem. 2020, 13, 249–254. [Google Scholar] [CrossRef]
- Zhang, Y.; Weidenkaff, A.; Reller, A. Mesoporous Structure and Phase Transition of Nanosrystalline TiO2. Mater. Lett. 2002, 54, 375–381. [Google Scholar] [CrossRef]
- Sikong, L.; Damchan, J.; Kooptarnond, K.; Niyomwas, S. Effect of doped SiO2 and calcinations temperature on phase transformation of TiO2 photocatalyst prepared by sol-gel method. Songklanakarin J. Sci. Technol. 2008, 30, 385–391. [Google Scholar]
- Devi, L.G.; Rajashekhar, K.E. A kinetic model based on non-linear regression analysis is proposed for the degradation of phenol under UV/solar light using nitrogen doped TiO2. J. Mol Catal. A Chem. 2011, 334, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Zheng, R.Y.; Xie, J.L.; Zhu, Y.X.; Xie, Y.C. Synthesis and characterization of phosphor and nitrogen co-doped titania. Appl. Catal. B Environ. 2007, 76, 196–202. [Google Scholar] [CrossRef]
- Sirimahachai, U.; Ndiege, N.; Chandrasekharan, R.; Wongnawa, S.; Shannon, M.A. Nanosized TiO2 particles decorated on SiO2 spheres: Synthesis and photocatalytic activities. J. Sol-Gel Sci. Technol. 2010, 56, 3–6. [Google Scholar] [CrossRef]
- Sellapan, R. Mechanisms of Enhanced Activity of Model TiO2/Carbon and TiO2/Metal Nanocomposite Photocatalysts; Department of Applied Physics Chalmers University: Gotebrog, Sweden, 2013. [Google Scholar]
- Pu, S.; Hou, Y.; Chen, H.; Deng, D.; Yang, Z.; Xue, S.; Zhu, R.; Diao, Z.; Chu, W. An efficient photocatalyst for fast reduction of Cr(VI) by ultra-trace silver enhanced titania in aqueous solution. Catalysts 2018, 8, 251. [Google Scholar] [CrossRef] [Green Version]
- Zaccariello, G.; Moretti, E.; Storaro, L.; Riello, P.; Canton, P.; Gombac, V.; Montini, T.; Rodríguez-Castellón, E.; Benedetti, A. TiO2-mesoporous silica nanocomposites: Cooperative effect in the photocatalytic degradation of dyes and drugs. RSC Adv. 2014, 4, 37826–37837. [Google Scholar] [CrossRef]
- Gao, M.; Zhu, L.; Ong, W.L.; Wang, J.; Ho, G.W. Structural design of TiO2-based photocatalyst for H2 production and degradation applications. Catal. Sci. Technol. 2015, 5, 4703–4726. [Google Scholar] [CrossRef]
- Pal, A.; Jana, T.K.; Chatterjee, K. Silica supported TiO2 nanostructures for highly efficient photocatalytic application under visible light irradiation. Mat. Res. Bull. 2016, 76, 353–357. [Google Scholar] [CrossRef]







| Photocatalysts | Phase (%) | ||
|---|---|---|---|
| Anatase | Rutile | Amorphous SiO2 | |
| TiO2 P25 | 85.7 | 14.3 | - |
| TiO2:SiO2 (1:1) | 49.0 | 6.5 | 44.4 |
| TiO2:SiO2 (3:1) | 69.0 | 8.3 | 22.6 |
| TiO2:SiO2 (7:1) | 97.9 | 0.9 | 1.2 |
| Photocatalysts | Crystallite Size (nm) | ||
|---|---|---|---|
| Anatase | Rutile | Amorphous SiO2 | |
| TiO2 P25 | 32.84 | 22.59 | - |
| TiO2:SiO2 (1:1) | 8.79 | 9.01 | 2.57 |
| TiO2:SiO2 (3:1) | 11.79 | 7.16 | 2.59 |
| TiO2:SiO2 (7:1) | 11.58 | - | - |
| Bond Type | SiO2 | TiO2 P25 | TiO2:SiO2 (1:1) | TiO2:SiO2 (3:1) | TiO2:SiO2 (7:1) |
|---|---|---|---|---|---|
| O–H streching | 3467 cm−1 | 3411 cm−1 | 3429 cm−1 | 3411 cm−1 | 3429 cm−1 |
| O–H bending | 1638 cm−1 | 1635 cm−1 | 1634 cm−1 | 1631 cm−1 | 1636 cm−1 |
| Si–O–Si | 1091 cm−1 | - | 1100 cm−1 | 1105 cm−1 | 1102 cm−1 |
| Ti–O–Ti | - | 667 cm−1 | 711 cm−1 | 671 cm−1 | 667 cm−1 |
| Ti–O–Si | - | - | 942 cm−1 | 958 cm−1 | 942 cm−1 |
| Particle Size | TiO2/SiO2 Ratio | ||
|---|---|---|---|
| 1:1 | 3:1 | 7:1 | |
| Median (nm) | 797.7 | 628.9 | 719.0 |
| Mode (nm) | 698.5 | 620.1 | 696.4 |
| Z-Average (nm) | 557.4 | 616.9 | 683.9 |
| Photocatalysts | k (min−1) | r0 (mg/L·min) | R2 |
|---|---|---|---|
| Photocatalysis of Cr(VI): | |||
| TiO2 P25 | 0.0060 | 0.0180 | 0.9301 |
| TiO2:SiO2 (1:1) | 0.0084 | 0.0252 | 0.9462 |
| TiO2:SiO2 (3:1) | 0.0101 | 0.0303 | 0.9645 |
| TiO2:SiO2 (7:1) | 0.0162 | 0.0486 | 0.9559 |
| Photocatalysis of Pb(II): | |||
| TiO2 P25 | 0.0085 | 0.3400 | 0.9626 |
| TiO2:SiO2 (1:1) | 0.0105 | 0.4200 | 0.9224 |
| TiO2:SiO2 (3:1) | 0.0135 | 0.5400 | 0.9060 |
| TiO2:SiO2 (7:1) | 0.0143 | 0.5720 | 0.9443 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eddy, D.R.; Ishmah, S.N.; Permana, M.D.; Firdaus, M.L. Synthesis of Titanium Dioxide/Silicon Dioxide from Beach Sand as Photocatalyst for Cr and Pb Remediation. Catalysts 2020, 10, 1248. https://doi.org/10.3390/catal10111248
Eddy DR, Ishmah SN, Permana MD, Firdaus ML. Synthesis of Titanium Dioxide/Silicon Dioxide from Beach Sand as Photocatalyst for Cr and Pb Remediation. Catalysts. 2020; 10(11):1248. https://doi.org/10.3390/catal10111248
Chicago/Turabian StyleEddy, Diana Rakhmawaty, Soraya Nur Ishmah, Muhamad Diki Permana, and M. Lutfi Firdaus. 2020. "Synthesis of Titanium Dioxide/Silicon Dioxide from Beach Sand as Photocatalyst for Cr and Pb Remediation" Catalysts 10, no. 11: 1248. https://doi.org/10.3390/catal10111248