Facile One-Pot Biogenic Synthesis of Cu-Co-Ni Trimetallic Nanoparticles for Enhanced Photocatalytic Dye Degradation
Abstract
:1. Introduction
2. Results and Discussion
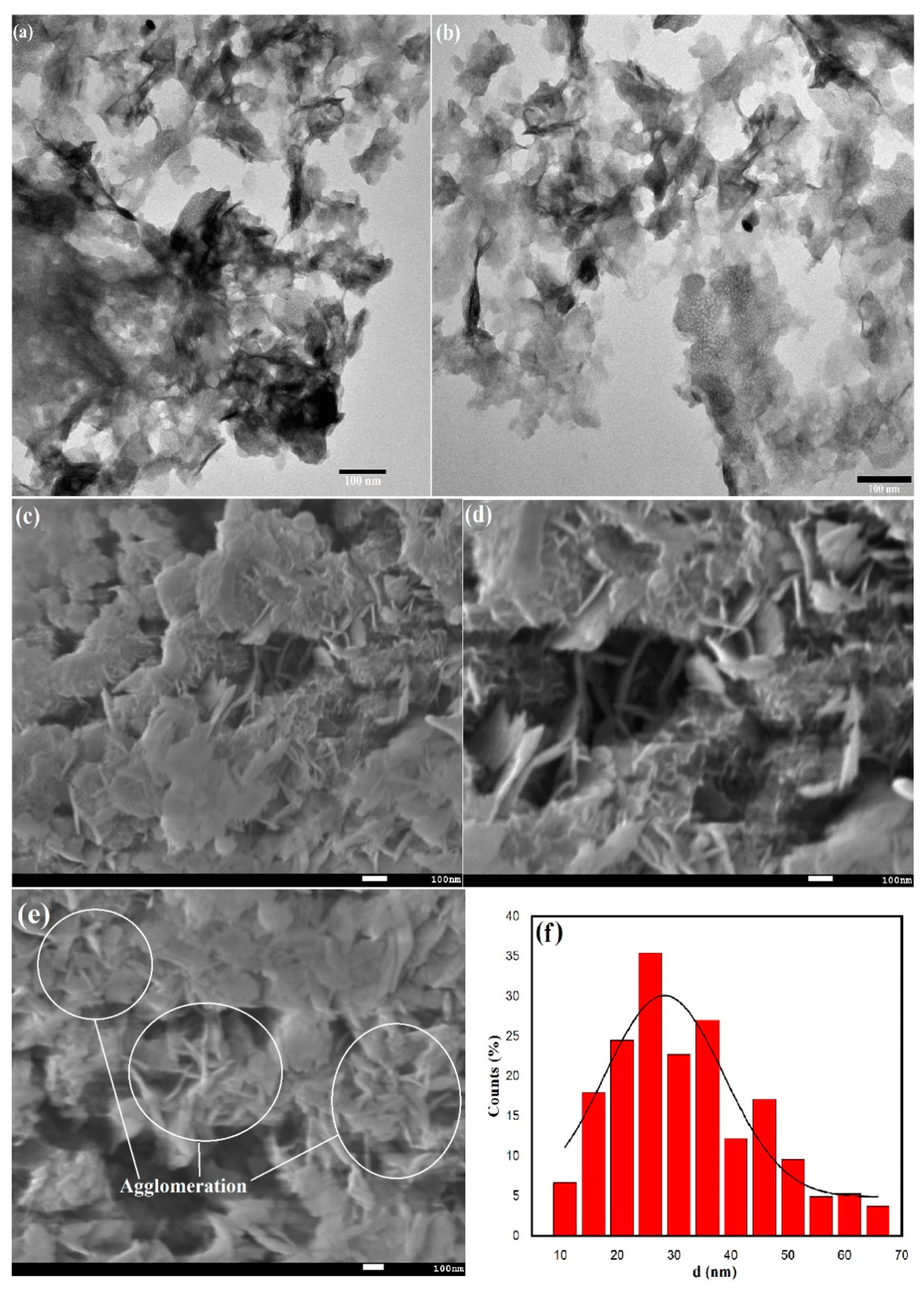
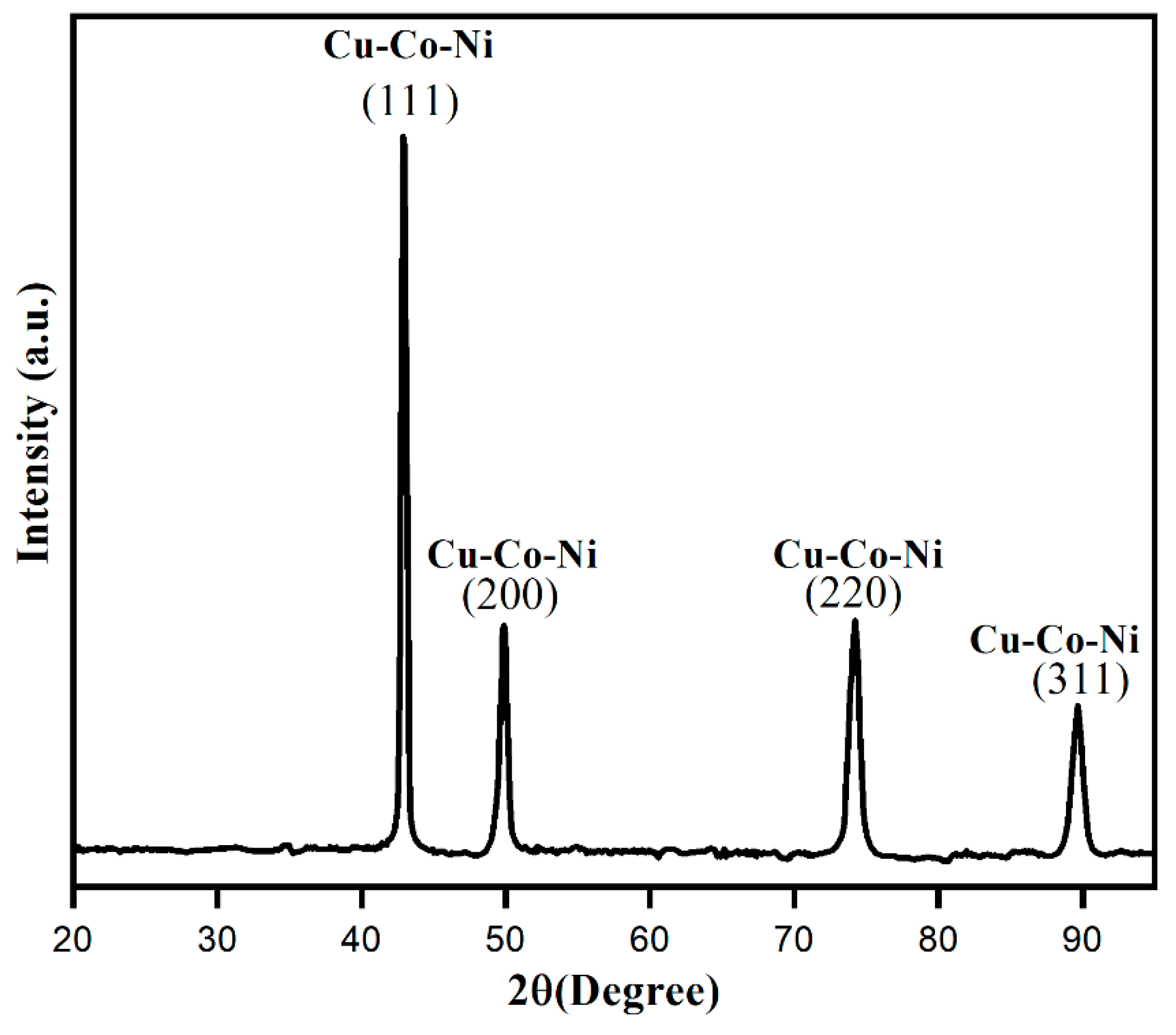
2.1. Surface Morphological Characterization of Cu-Co-Ni Trimetallic Nanoparticles
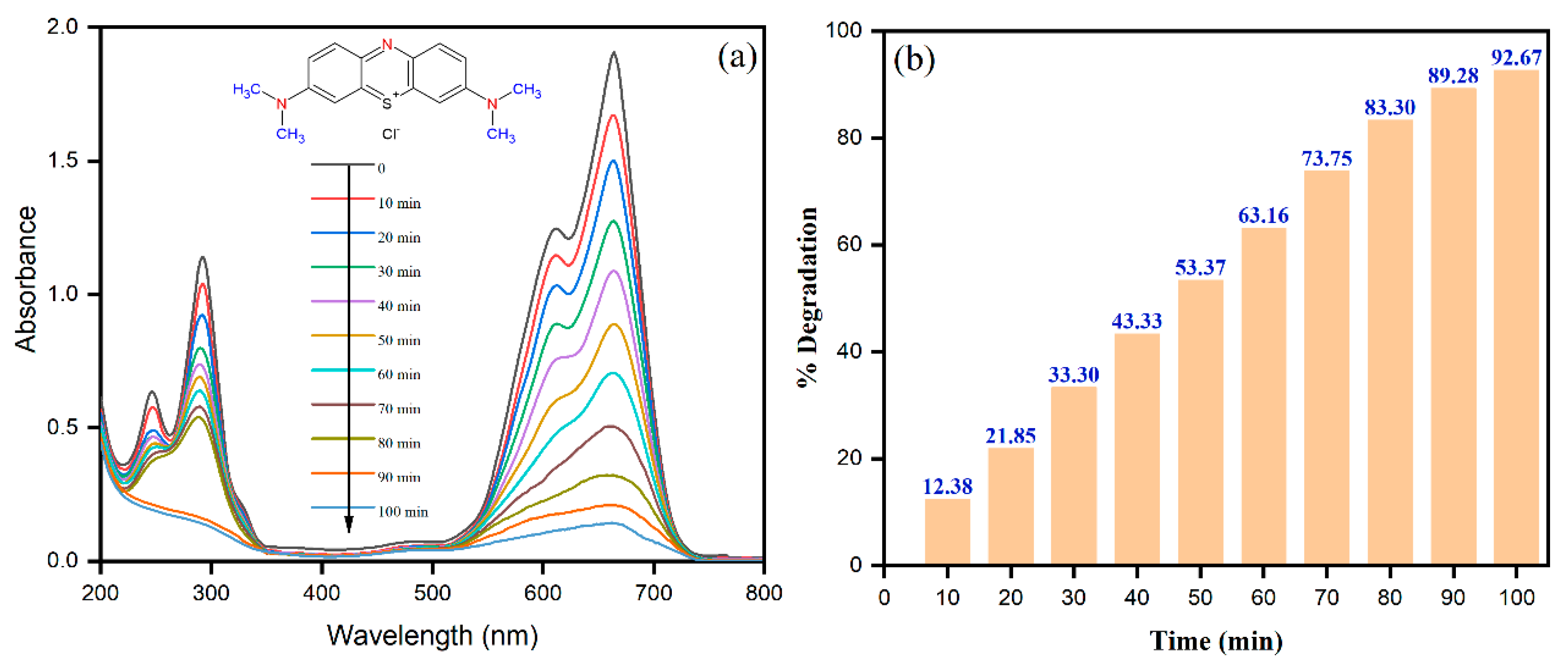
2.2. Photochemical Activities Cu-Co-Ni Nanoparticles
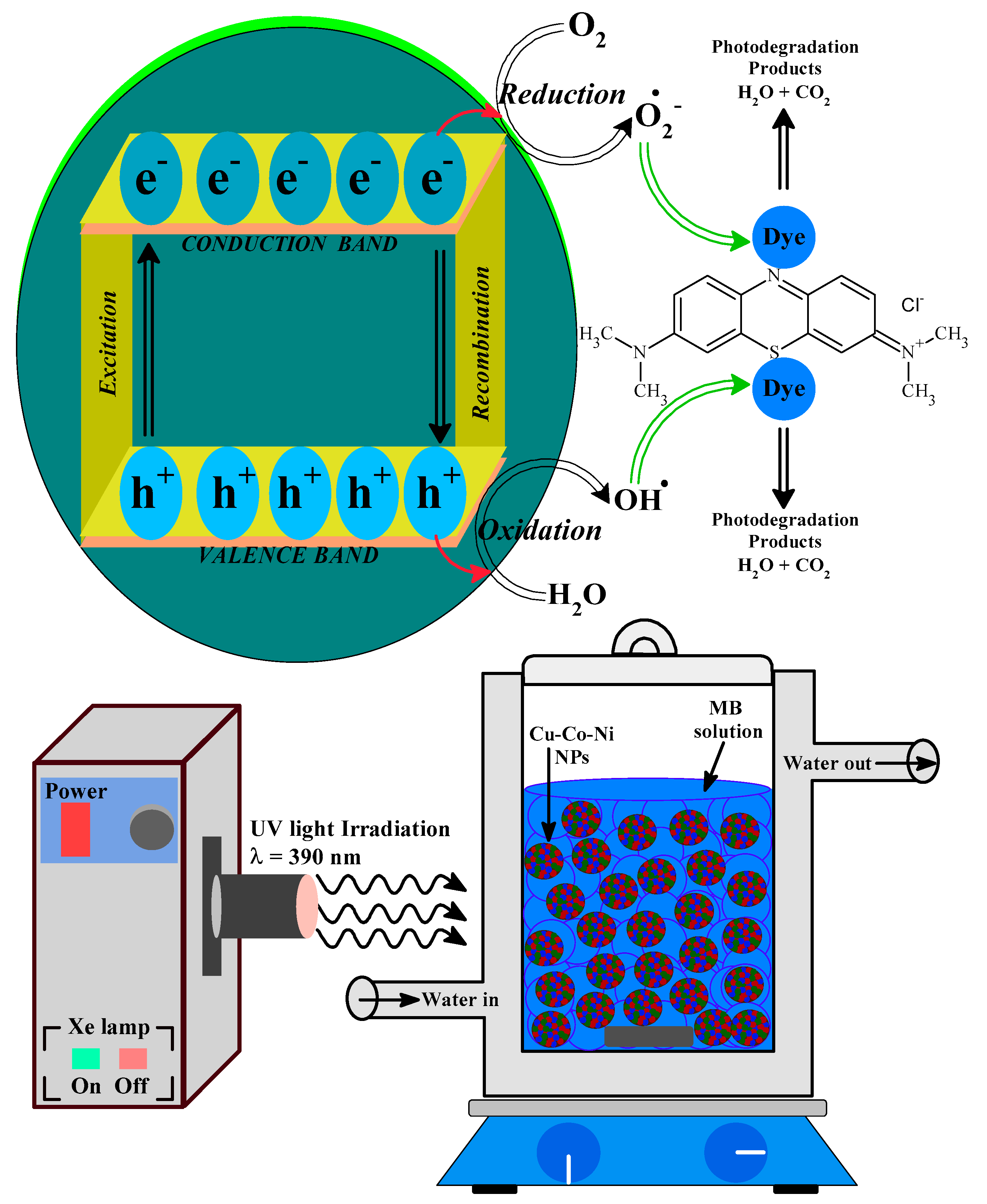
2.3. The Mechanism behind the Photocatalytic-Degradation of MB
2.4. Stability and Reuse of the Photocatalyst
3. Experimental
3.1. Preparation of Origanum Vulgare Leaves Extract
3.2. Biogenic Synthesis of Trimetallic Nanoparticles
3.3. Characterization
3.4. Photocatalytic Degradation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khanal, S.; Bhattarai, N.; Velázquez-Salazar, J.J.; Bahena, D.; Soldano, G.; Ponce, A.; Mariscal, M.M.; Mejía-Rosales, S.; José-Yacamán, M. Trimetallic nanostructures: The case of AgPd–Pt multiply twinned nanoparticles. Nanoscale 2013, 5, 12456–12463. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-L.; Xu, Q. Recent progress in synergistic catalysis over heterometallic nanoparticles. J. Mater. Chem. 2011, 21, 13705–13725. [Google Scholar] [CrossRef]
- Aranishi, K.; Jiang, H.-L.; Akita, T.; Haruta, M.; Xu, Q. One-step synthesis of magnetically recyclable Au/Co/Fe triple-layered core-shell nanoparticles as highly efficient catalysts for the hydrolytic dehydrogenation of ammonia borane. Nano Res. 2011, 4, 1233–1241. [Google Scholar] [CrossRef]
- Pandey, P.; Pandey, G. One-pot two-step rapid synthesis of 3-aminopropyltrimethoxysilane-mediated highly catalytic Ag@(PdAu) trimetallic nanoparticles. Catal. Sci. Technol. 2016, 6, 3911–3917. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, J.; Ye, H.; Li, L.; Wang, H.; Li, X.; Zhang, X.; Li, H. Ni0.5Cu0.5Co2O4 Nanocomposites, Morphology, Controlled Synthesis, and Catalytic Performance in the Hydrolysis of Ammonia Borane for Hydrogen Production. Nanomaterials 2019, 9, 1334. [Google Scholar] [CrossRef] [Green Version]
- Yadav, N.; Jaiswal, A.K.; Dey, K.K.; Yadav, V.B.; Nath, G.; Srivastava, A.K.; Yadav, R.R. Trimetallic Au/Pt/Ag based nanofluid for enhanced antibacterial response. Mater. Chem. Phys. 2018, 218, 10–17. [Google Scholar] [CrossRef]
- Akbarzadeh, H.; Abbaspour, M.; Mehrjouei, E.; Kamrani, M. AgPd@ Pt nanoparticles with different morphologies of cuboctahedron, icosahedron, decahedron, octahedron, and Marks-decahedron: Insights from atomistic simulations. Inorg. Chem. Front. 2018, 5, 870–878. [Google Scholar] [CrossRef]
- Ge, S.; Zhang, Y.; Zhang, L.; Liang, L.; Liu, H.; Yan, M.; Huang, J.; Yu, J. Ultrasensitive electrochemical cancer cells sensor based on trimetallic dendritic Au@ PtPd nanoparticles for signal amplification on lab-on-paper device. Sens. Actuators B Chem. 2015, 220, 665–672. [Google Scholar] [CrossRef]
- Wu, P.; Ding, P.; Ye, X.; Li, L.; He, X.; Wang, K. One-pot synthesized Cu/Au/Pt trimetallic nanoparticles as a novel enzyme mimic for biosensing applications. RSC Adv. 2019, 9, 14982–14989. [Google Scholar] [CrossRef] [Green Version]
- Nie, F.; Ga, L.; Ai, J.; Wang, Y. Trimetallic PdCuAu Nanoparticles for Temperature Sensing and Fluorescence Detection of H2O2 and Glucose. Front. Chem. 2020, 8, 244. [Google Scholar] [CrossRef] [Green Version]
- Khan, Z. Chitosan capped Au@ Pd@ Ag trimetallic nanoparticles: Synthesis, stability, capping action and adsorbing activities. Int. J. Biol. Macromol. 2020, 153, 545–560. [Google Scholar] [CrossRef]
- Tang, M.; Luo, S.; Wang, K.; Du, H.; Sriphathoorat, R.; Shen, P. Simultaneous formation of trimetallic Pt-Ni-Cu excavated rhombic dodecahedrons with enhanced catalytic performance for the methanol oxidation reaction. Nano Res. 2018, 11, 4786–4795. [Google Scholar] [CrossRef]
- dos Santos Araújo, P.; Belini, G.B.; Mambrini, G.P.; Yamaji, F.M.; Waldman, W.R. Thermal degradation of calcium and sodium alginate: A greener synthesis towards calcium oxide micro/nanoparticles. Int. J. Biol. Macromol. 2019, 140, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Finotelli, P.; Coaquira, J.; Rocha-Leão, M.; Diaz-Aguila, C.; Baggio-Saitovitch, E.; Rossi, A. In situ synthesis and magnetic studies of iron oxide nanoparticles in calcium-alginate matrix for biomedical applications. Mater. Sci. Eng. C 2008, 28, 253–257. [Google Scholar] [CrossRef]
- Kawano, T.; Niidome, Y.; Mori, T.; Katayama, Y.; Niidome, T. PNIPAM gel-coated gold nanorods for targeted delivery responding to a near-infrared laser. Bioconjugate Chem. 2009, 20, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Allaedini, G.; Tasirin, S.M.; Aminayi, P. Synthesis of Fe–Ni–Ce trimetallic catalyst nanoparticles via impregnation and co-precipitation and their application to dye degradation. Chem. Pap. 2016, 70, 231–242. [Google Scholar] [CrossRef]
- Loganathan, B.; Karthikeyan, B. Au core Pd/Pt shell in trimetallic Au/Pd/Pt colloidal nanocomposites–physicochemical characterization study. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 944–952. [Google Scholar] [CrossRef]
- Ding, K.; Li, Y.; Zhao, Y.; Zhao, J.; Chen, Y.; Wang, Q. Preparation of a trimetallic Pd1NiyAl0.5 composite nanoparticle by a hydrothermal method for ethanol oxidation reaction. Int. J. Electrochem. Sci. 2015, 10, 8844–8857. [Google Scholar]
- Zhang, H.; Watanabe, T.; Okumura, M.; Haruta, M.; Toshima, N. Catalytically highly active top gold atom on palladium nanocluster. Nat. Mater. 2012, 11, 49–52. [Google Scholar] [CrossRef]
- Zhang, H.; Watanabe, T.; Okumura, M.; Haruta, M.; Toshima, N. Crown Jewel catalyst: How neighboring atoms affect the catalytic activity of top Au atoms? J. Catal. 2013, 305, 7–18. [Google Scholar] [CrossRef]
- Du, J.; Cheng, F.; Si, M.; Liang, J.; Tao, Z.; Chen, J. Nanoporous Ni-based catalysts for hydrogen generation from hydrolysis of ammonia borane. Int. J. Hydrogen Energy 2013, 38, 5768–5774. [Google Scholar] [CrossRef]
- Cai, X.-L.; Liu, C.-H.; Liu, J.; Lu, Y.; Zhong, Y.-N.; Nie, K.-Q.; Xu, J.-L.; Gao, X.; Sun, X.-H.; Wang, S.-D. Synergistic effects in CNTs-PdAu/Pt trimetallic nanoparticles with high electrocatalytic activity and stability. Nano Micro Lett. 2017, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Ralston, W.T.; Liu, W.-C.; Alayoglu, S.; Melaet, G. Bimetallic Cobalt Nanoparticles (Co–M): Synthesis, Characterization, and Application in the Fischer–Tropsch Process. Top. Catal. 2018, 61, 1002–1015. [Google Scholar] [CrossRef]
- Bhunia, K.; Khilari, S.; Pradhan, D. Trimetallic PtAuNi alloy nanoparticles as an efficient electrocatalyst for the methanol electrooxidation reaction. Dalton Trans. 2017, 46, 15558–15566. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Bhogal, S.; Naushad, M.; Kumar, A.; Stadler, F.J. Microwave assisted fabrication of La/Cu/Zr/carbon dots trimetallic nanocomposites with their adsorptional vs. photocatalytic efficiency for remediation of persistent organic pollutants. J. Photochem. Photobiol. A Chem. 2017, 347, 235–243. [Google Scholar] [CrossRef]
- Li, B.; Chan, S.H. PtFeNi tri-metallic alloy nanoparticles as electrocatalyst for oxygen reduction reaction in proton exchange membrane fuel cells with ultra-low Pt loading. Int. J. Hydrogen Energy 2013, 38, 3338–3345. [Google Scholar] [CrossRef]
- Shen, Y.-Y.; Sun, Y.; Zhou, L.-N.; Li, Y.-J.; Yeung, E.S. Synthesis of ultrathin PtPdBi nanowire and its enhanced catalytic activity towards p-nitrophenol reduction. J. Mater. Chem. A 2014, 2, 2977–2984. [Google Scholar] [CrossRef]
- Singh, R.; Soni, R. Improved catalytic activity of laser generated bimetallic and trimetallic nanoparticles. J. Nanosci. Nanotechnol. 2014, 14, 6872–6879. [Google Scholar] [CrossRef]
- Yang, P.; Xu, A.-D.; Xia, J.; He, J.; Xing, H.-L.; Zhang, X.-M.; Wei, S.-Y.; Wang, N.-N. Facile synthesis of highly catalytic activity Ni–Co–Pd–P composite for reduction of the p-Nitrophenol. Appl. Catal. A Gen. 2014, 470, 89–96. [Google Scholar] [CrossRef]
- Sahoo, A.; Tripathy, S.K.; Dehury, N.; Patra, S. A porous trimetallic Au@Pd@Ru nanoparticle system: Synthesis, characterisation and efficient dye degradation and removal. J. Mater. Chem. A 2015, 3, 19376–19383. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, L.; Cao, Y.; Du, S.; Cheng, Z.; Zhang, S. Fabrication of catalytically active Au/Pt/Pd trimetallic nanoparticles by rapid injection of NaBH4. Mater. Res. Bull. 2014, 49, 393–398. [Google Scholar] [CrossRef]
- Matin, M.A.; Jang, J.-H.; Kwon, Y.-U. One-pot sonication-assisted polyol synthesis of trimetallic core–shell (Pd, Co)@ Pt nanoparticles for enhanced electrocatalysis. Int. J. Hydrogen Energy 2014, 39, 3710–3718. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Ping, Y.; Yan, J.-M.; Wang, H.-L.; Jiang, Q. Hydrogen generation from formic acid decomposition at room temperature using a NiAuPd alloy nanocatalyst. Int. J. Hydrogen Energy 2014, 39, 4850–4856. [Google Scholar] [CrossRef]
- Tayal, J.; Rawat, B.; Basu, S. Bi-metallic and tri-metallic Pt–Sn/C, Pt–Ir/C, Pt–Ir–Sn/C catalysts for electro-oxidation of ethanol in direct ethanol fuel cell. Int. J. Hydrogen Energy 2011, 36, 14884–14897. [Google Scholar] [CrossRef]
- Venkatesan, P.; Santhanalakshmi, J. Synthesis, characterization and catalytic activity of trimetallic nanoparticles in the Suzuki C–C coupling reaction. J. Mol. Catal. A Chem. 2010, 326, 99–106. [Google Scholar] [CrossRef]
- Khan, Z. Trimetallic nanoparticles: Synthesis, characterization and catalytic degradation of formic acid for hydrogen generation. Int. J. Hydrogen Energy 2019, 44, 11503–11513. [Google Scholar] [CrossRef]
- Albeladi, S.S.R.; Malik, M.A.; Al-thabaiti, S.A. Facile biofabrication of silver nanoparticles using Salvia officinalis leaf extract and its catalytic activity towards Congo red dye degradation. J. Mater. Res. Technol. 2020, 9, 10031–10044. [Google Scholar] [CrossRef]
- Anpo, M.; Dohshi, S.; Kitano, M.; Hu, Y.; Takeuchi, M.; Matsuoka, M. The preparation and characterization of highly efficient titanium oxide–based photofunctional materials. Annu. Rev. Mater. Res. 2005, 35, 1–27. [Google Scholar] [CrossRef]
- Ghosh, M.; Lohrasbi, M.; Chuang, S.S.; Jana, S.C. Mesoporous titanium dioxide nanofibers with a significantly enhanced photocatalytic activity. ChemCatChem 2016, 8, 2525–2535. [Google Scholar] [CrossRef]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous photocatalysis: Recent advances and applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef] [Green Version]
- Schubert, J.S.; Popovic, J.; Haselmann, G.M.; Nandan, S.P.; Wang, J.; Giesriegl, A.; Cherevan, A.S.; Eder, D. Immobilization of Co, Mn, Ni and Fe oxide co-catalysts on TiO2 for photocatalytic water splitting reactions. J. Mater. Chem. A 2019, 7, 18568–18579. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, M.; Liu, J.; Chuang, S.S.; Jana, S.C. Fabrication of hierarchical V2O5 nanorods on TiO2 nanofibers and their enhanced photocatalytic activity under visible light. ChemCatChem 2018, 10, 3305–3318. [Google Scholar] [CrossRef]
- Zhen, W.; Guo, Y.; Wu, Y.; Lu, G. Co–P/graphene alloy catalysts doped with Cu and Ni for efficient photocatalytic hydrogen generation. New J. Chem. 2017, 41, 13804–13811. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent advances for dyes removal using novel adsorbents: A review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El Nemr, A. Health and environmental impacts of dyes: Mini review. Am. J. Environ. Sci. Eng. 2017, 1, 64–67. [Google Scholar]
- Herath, A.C.; Rajapakse, R.; Wicramasinghe, A.; Karunaratne, V. Photodegradation of triphenylamino methane (magenta) by photosensitizer in oxygenated solutions. Environ. Sci. Technol. 2009, 43, 176–180. [Google Scholar] [CrossRef]
- Carmen, Z.; Daniela, S. Textile organic dyes–characteristics, polluting effects and separation/elimination procedures from industrial effluents–a critical overview. In Organic Pollutants Ten Years after the Stockholm Convention-Environmental and Analytical Update, 2012; IntechOpen: London, UK, 2012; p. 32373. [Google Scholar]
- Tang, W.Z.; An, H. UV/TiO2 photocatalytic oxidation of commercial dyes in aqueous solutions. Chemosphere 1995, 31, 4157–4170. [Google Scholar] [CrossRef]
- Panakoulias, T.; Kalatzis, P.; Kalderis, D.; Katsaounis, A. Electrochemical degradation of Reactive Red 120 using DSA and BDD anodes. J. Appl. Electrochem. 2010, 40, 1759–1765. [Google Scholar] [CrossRef]
- Valenti, L.E.; Giacomelli, C.E. Stability of silver nanoparticles: Agglomeration and oxidation in biological relevant conditions. J. Nanopart. Res. 2017, 19, 156. [Google Scholar] [CrossRef]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef] [Green Version]
- Sankar, R.; Karthik, A.; Prabu, A.; Karthik, S.; Shivashangari, K.S.; Ravikumar, V. Origanum vulgare mediated biosynthesis of silver nanoparticles for its antibacterial and anticancer activity. Colloids Surf. B Biointerfaces 2013, 108, 80–84. [Google Scholar] [CrossRef]
- Alshehri, A.A.; Malik, M.A. Biogenic fabrication of ZnO nanoparticles using Trigonella foenum-graecum (Fenugreek) for proficient photocatalytic degradation of methylene blue under UV irradiation. J. Mater. Sci. Mater. Electron. 2019, 30, 16156–16173. [Google Scholar] [CrossRef]
- Jayarambabu, N.; Akshaykranth, A.; Rao, T.V.; Rao, K.V.; Kumar, R.R. Green synthesis of Cu nanoparticles using Curcuma longa extract and their application in antimicrobial activity. Mater. Lett. 2020, 259, 126813. [Google Scholar] [CrossRef]
- Pandian, C.J.; Palanivel, R.; Dhananasekaran, S. Green synthesis of nickel nanoparticles using Ocimum sanctum and their application in dye and pollutant adsorption. Chin. J. Chem. Eng. 2015, 23, 1307–1315. [Google Scholar] [CrossRef]
- Guo, K.; Hansen, V.F.; Li, H.; Yu, Z. Monodispersed nickel and cobalt nanoparticles in desulfurization of thiophene for in-situ upgrading of heavy crude oil. Fuel 2018, 211, 697–703. [Google Scholar] [CrossRef]
- Karpuz, A.; Kockar, H.; Alper, M. Study of electrolyte pH in production of Cu–Co–Ni ternary alloys and its effect on microstructural and magnetic properties. IEEE Trans. Magn. 2014, 50, 1–4. [Google Scholar] [CrossRef]
- Singh, S.; Srivastava, P.; Singh, G. Synthesis, characterization of Co–Ni–Cu trimetallic alloy nanocrystals and their catalytic properties, Part–91. J. Alloys Compd. 2013, 562, 150–155. [Google Scholar] [CrossRef]
- Mondal, B.; Basumallick, A.; Chattopadhyay, P. Correlation of microstructure and magnetic properties in Cu–Co–Ni alloys. Mater. Sci. Eng. B 2010, 166, 174–179. [Google Scholar] [CrossRef]
- David, L.; Moldovan, B. Green Synthesis of Biogenic Silver Nanoparticles for Efficient Catalytic Removal of Harmful Organic Dyes. Nanomaterials 2020, 10, 202. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Wang, M.; Xu, C.; Chen, S.; Fu, X. Significantly enhanced visible-light photocatalytic activity of g-C3N4 via ZnO modification and the mechanism study. J. Mol. Catal. A Chem. 2013, 368, 9–15. [Google Scholar] [CrossRef]
- Gupta, N.K.; Ghaffari, Y.; Kim, S.; Bae, J.; Kim, K.S.; Saifuddin, M. Photocatalytic Degradation of Organic Pollutants over MFe2O4 (M = Co, Ni, Cu, Zn) Nanoparticles at Neutral pH. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Alam, U.; Khan, A.; Ali, D.; Bahnemann, D.; Muneer, M. Comparative photocatalytic activity of sol–gel derived rare earth metal (La, Nd, Sm and Dy)-doped ZnO photocatalysts for degradation of dyes. RSC Adv. 2018, 8, 17582–17594. [Google Scholar] [CrossRef] [Green Version]
- Kong, J.-Z.; Zhai, H.-F.; Zhang, W.; Wang, S.-S.; Zhao, X.-R.; Li, M.; Li, H.; Li, A.-D.; Wu, D. Visible light-driven photocatalytic performance of N-doped ZnO/gC3N4 nanocomposites. Nanoscale Res. Lett. 2017, 12, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tammina, S.K.; Mandal, B.K.; Kadiyala, N.K. Photocatalytic degradation of methylene blue dye by nonconventional synthesized SnO2 nanoparticles. Environ. Nanotechnol. Monit. Manag. 2018, 10, 339–350. [Google Scholar] [CrossRef]
- Li, S.; Lin, Q.; Liu, X.; Yang, L.; Ding, J.; Dong, F.; Li, Y.; Irfan, M.; Zhang, P. Fast photocatalytic degradation of dyes using low-power laser-fabricated Cu2O–Cu nanocomposites. RSC Adv. 2018, 8, 20277–20286. [Google Scholar] [CrossRef] [Green Version]
- Suresh, M.; Sivasamy, A. Fabrication of graphene nanosheets decorated by nitrogen-doped ZnO nanoparticles with enhanced visible photocatalytic activity for the degradation of Methylene Blue dye. J. Mol. Liq. 2020, 317, 114112. [Google Scholar] [CrossRef]
- Xu, L.; Srinivasakannan, C.; Peng, J.; Yan, M.; Zhang, D.; Zhang, L. Microfluidic reactor synthesis and photocatalytic behavior of Cu@ Cu2O nanocomposite. Appl. Surf. Sci. 2015, 331, 449–454. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Ahamad, T.; Al-Saeedi, S.I.; Al-Senani, G.M.; Al-Kadhi, N.S.; Stadler, F.J. Facile fabrication of Zr2Ni1Cu7 trimetallic nanoalloy and its composite with Si3N4 for visible light assisted photodegradation of methylene blue. J. Mol. Liq. 2018, 272, 170–179. [Google Scholar] [CrossRef]
- Gorduk, S.; Avciata, O.; Avciata, U. Photocatalytic degradation of methylene blue under visible light irradiation by non-peripherally tetra substituted phthalocyanine-TiO2 nanocomposites. Inorg. Chim. Acta 2018, 471, 137–147. [Google Scholar] [CrossRef]
- Chen, J.; Xing, Z.; Han, J.; Su, M.; Li, Y.; Lu, A. Enhanced degradation of dyes by Cu-Co-Ni nanoparticles loaded on amino-modified octahedral metal–organic framework. J. Alloys Compd. 2020, 834, 155106. [Google Scholar] [CrossRef]
- Lin, J.; Luo, Z.; Liu, J.; Li, P. Photocatalytic degradation of methylene blue in aqueous solution by using ZnO-SnO2 nanocomposites. Mater. Sci. Semicond. Process. 2018, 87, 24–31. [Google Scholar] [CrossRef]
- Kazemi, F.; Mohamadnia, Z.; Kaboudin, B.; Karimi, Z. Photodegradation of methylene blue with a titanium dioxide/polyacrylamide photocatalyst under sunlight. J. Appl. Polym. Sci. 2016, 133, 43386. [Google Scholar] [CrossRef]
- Hou, C.; Hu, B.; Zhu, J. Photocatalytic degradation of methylene blue over TiO2 pretreated with varying concentrations of NaOH. Catalysts 2018, 8, 575. [Google Scholar] [CrossRef] [Green Version]
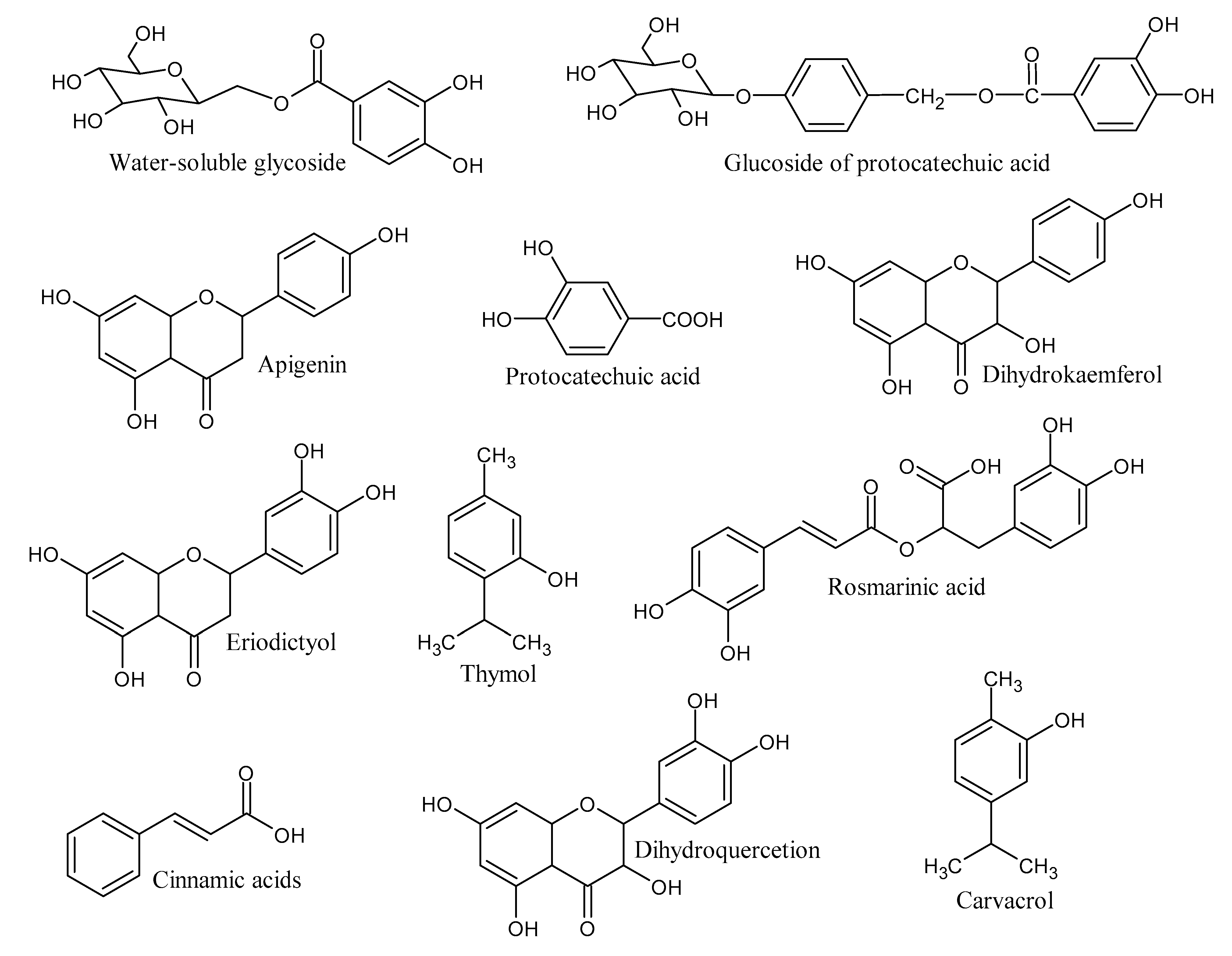
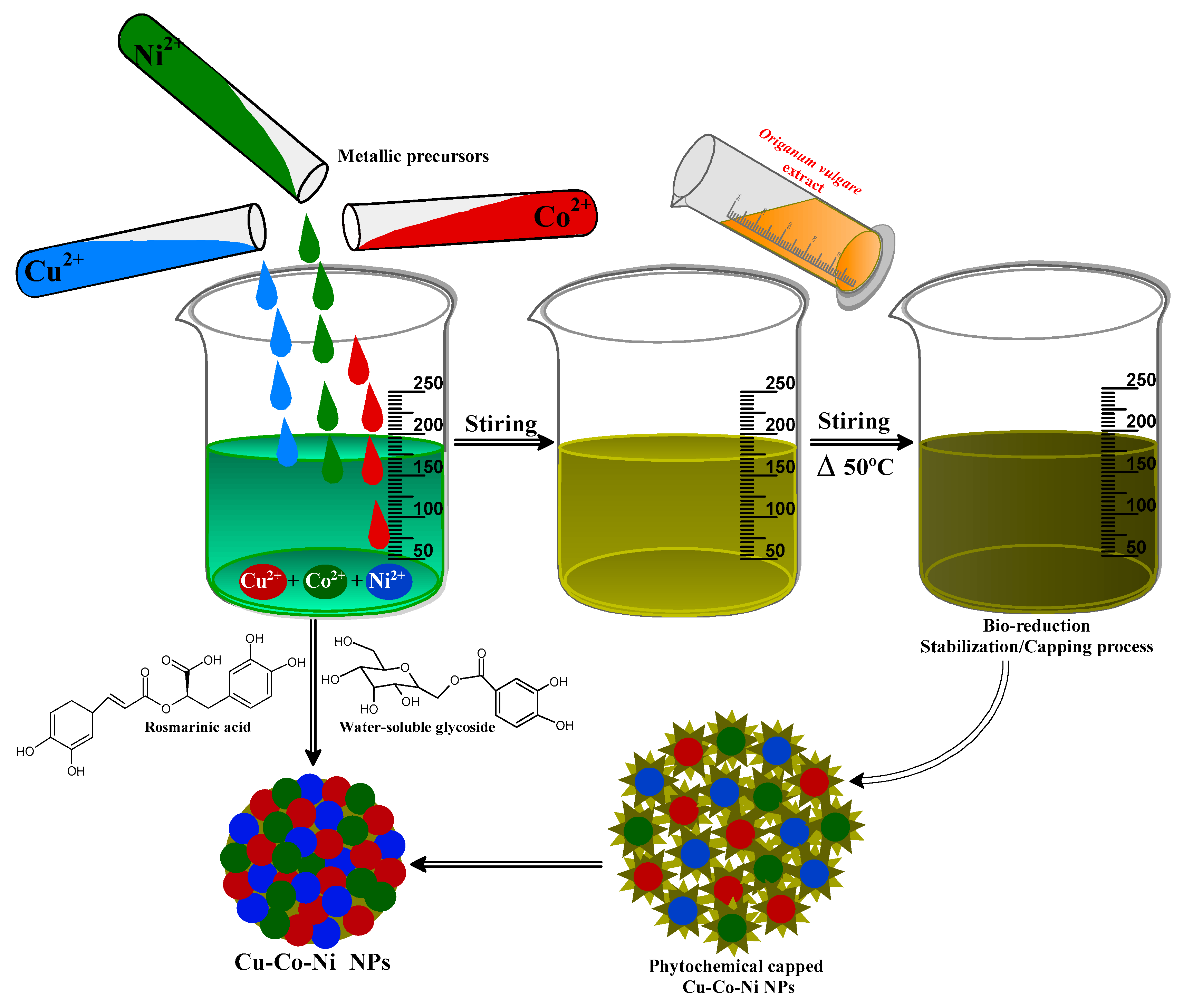
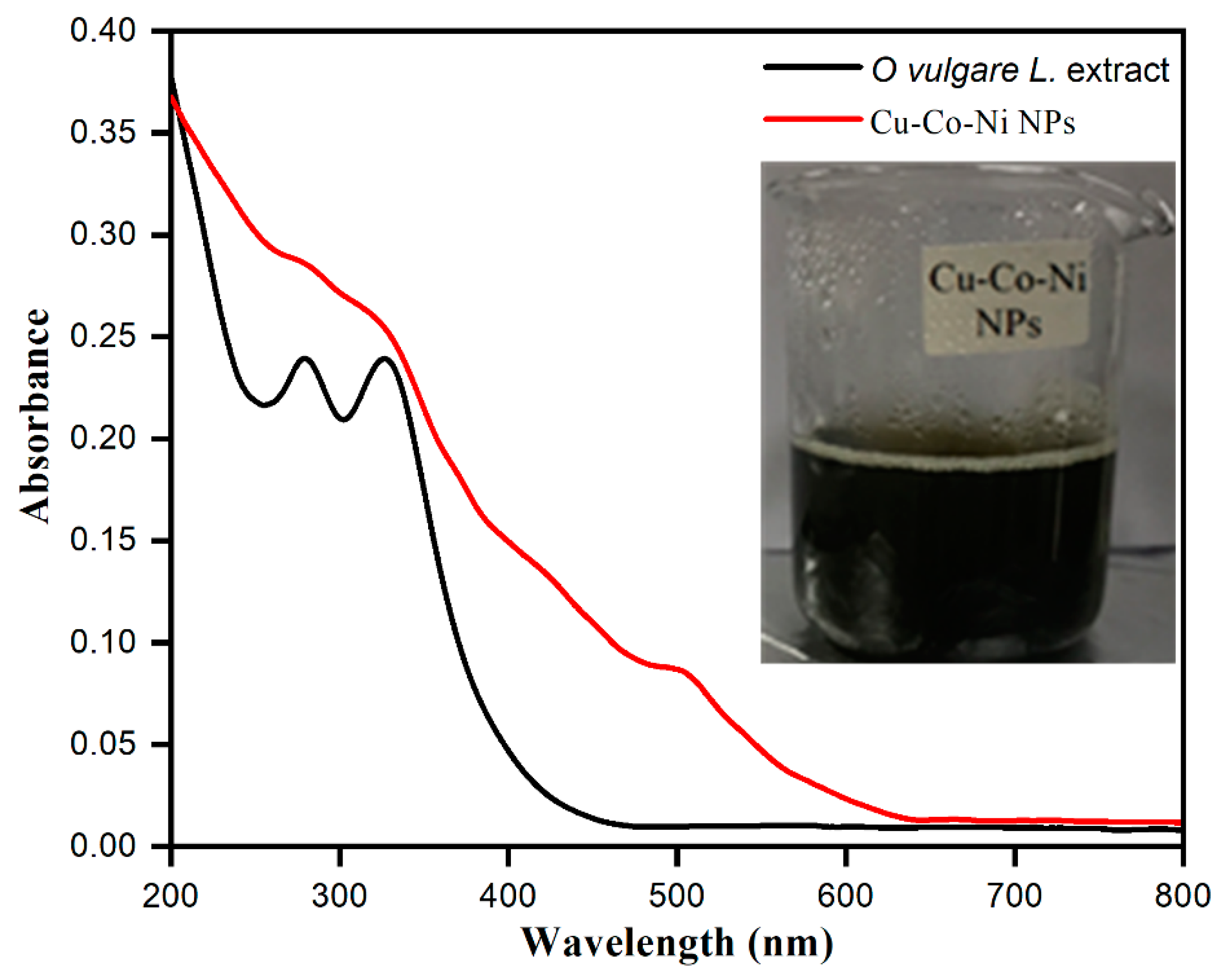
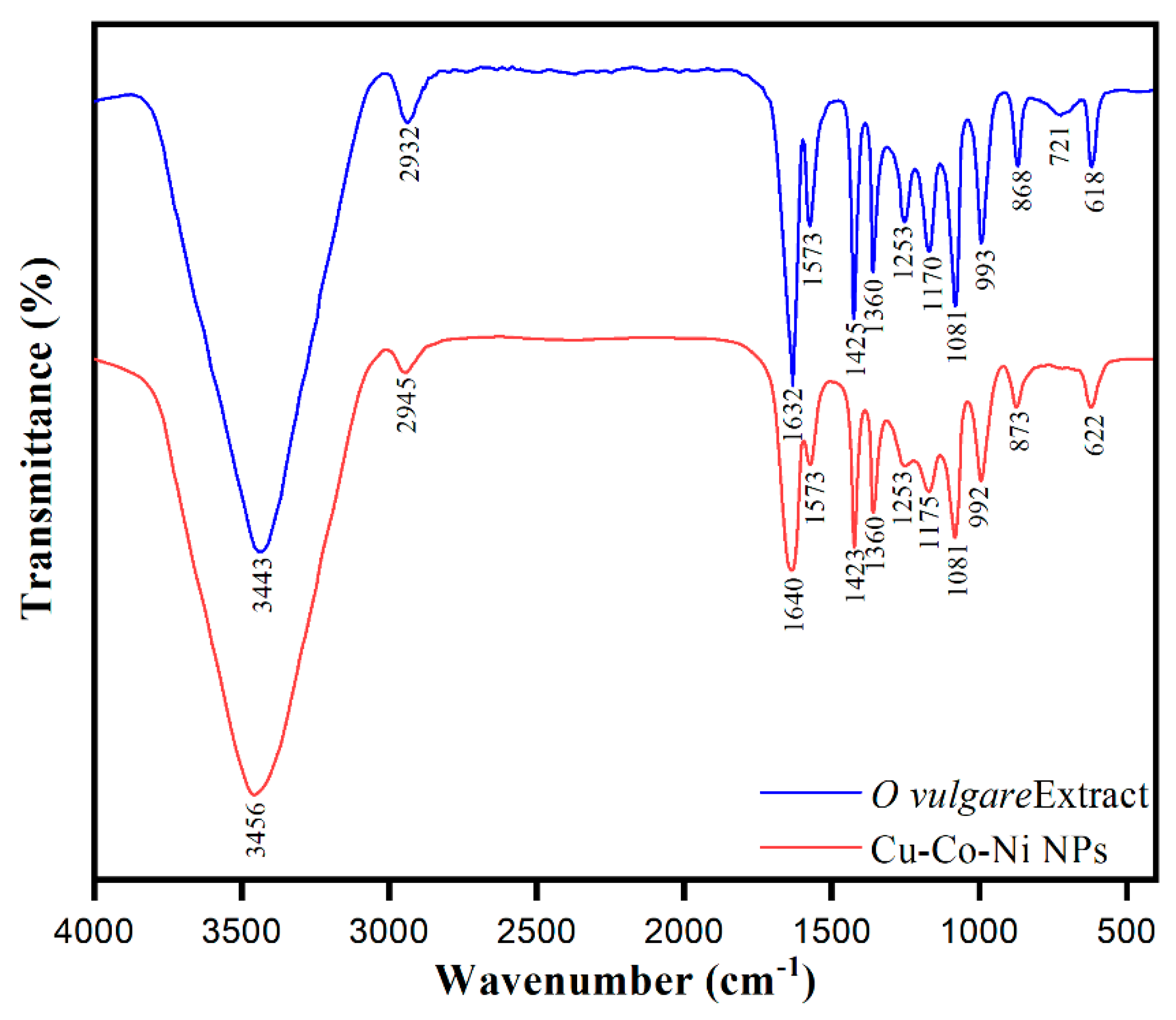


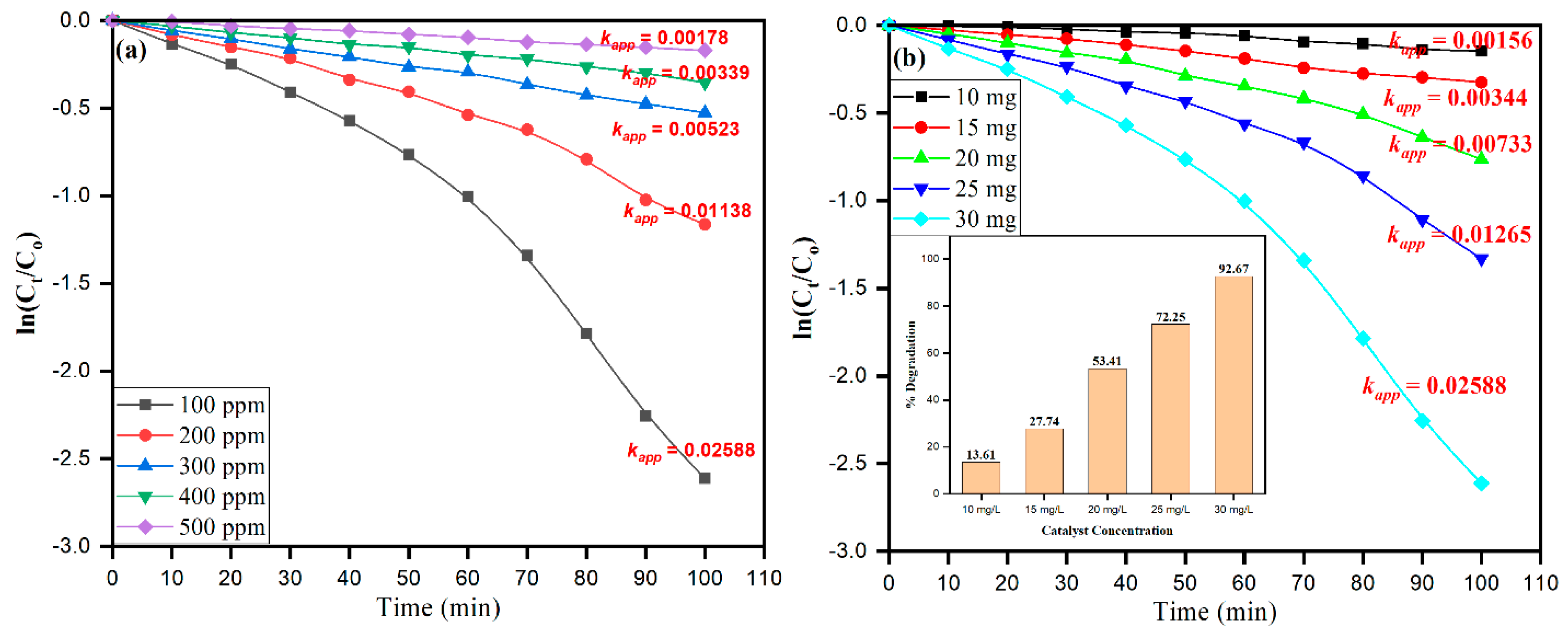

| [MB] (ppm) | Rate Constant (min−1) | % Degradation | R2 | [Cu-Co-Ni] (mg) | % Degradation | Rate Constant (min−1) | R2 |
|---|---|---|---|---|---|---|---|
| 100 | 0.02588 | 92.67 | 0.935 | 10 | 13.61 | 0.00156 | 0.942 |
| 200 | 0.01138 | 68.75 | 0.961 | 15 | 27.74 | 0.00344 | 0.990 |
| 300 | 0.00523 | 40.93 | 0.997 | 20 | 53.41 | 0.00733 | 0.996 |
| 400 | 0.00339 | 29.89 | 0.991 | 25 | 72.25 | 0.01265 | 0.947 |
| 500 | 0.00178 | 15.73 | 0.993 | 30 | 92.67 | 0.02588 | 0.935 |
| Photocatalyst | Irradiation Time (min) | % Degradation | Irradiation Source | References |
|---|---|---|---|---|
| ZnO | 90 | 88.37 | UV Light | [53] |
| SnO2 | 30 | 90 | UV Light | [65] |
| Cu2O–Cu | 50 | 90.1 | Visible light | [66] |
| RGO-N-ZnO | 120 | 98.5 | Visible light | [67] |
| Cu@Cu2O | 50 | 96.5 | UV Light | [68] |
| Zr2Ni1Cu7/Zr2Ni1Cu7/Si3N4 | 90 | 92 | Visible light | [69] |
| Pc-TiO2 | 100–140 | 100 | Visible light | [70] |
| NH2-MIL-101(Fe)@CuCoNi | 120 | 99 | Visible light | [71] |
| ZnO-SnO2 | 60 | 96.53 | UV Light | [72] |
| TiO2/PAAm | 300 | 95 | Sunlight | [73] |
| Cu-Co-Ni | 100 | 92.67 | UV Light | Present work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshehri, A.A.; Malik, M.A. Facile One-Pot Biogenic Synthesis of Cu-Co-Ni Trimetallic Nanoparticles for Enhanced Photocatalytic Dye Degradation. Catalysts 2020, 10, 1138. https://doi.org/10.3390/catal10101138
Alshehri AA, Malik MA. Facile One-Pot Biogenic Synthesis of Cu-Co-Ni Trimetallic Nanoparticles for Enhanced Photocatalytic Dye Degradation. Catalysts. 2020; 10(10):1138. https://doi.org/10.3390/catal10101138
Chicago/Turabian StyleAlshehri, Abdulmohsen Ali, and Maqsood Ahmad Malik. 2020. "Facile One-Pot Biogenic Synthesis of Cu-Co-Ni Trimetallic Nanoparticles for Enhanced Photocatalytic Dye Degradation" Catalysts 10, no. 10: 1138. https://doi.org/10.3390/catal10101138




