Fine Characterization of Natural SiO2-Doped Catalyst Derived from Mussel Shell with Potential Photocatalytic Performance for Organic Dyes
Abstract
:1. Introduction
2. Results
2.1. Morphology Analysis
2.2. Chemical Composition Analysis
2.3. Optical Absorption Tests
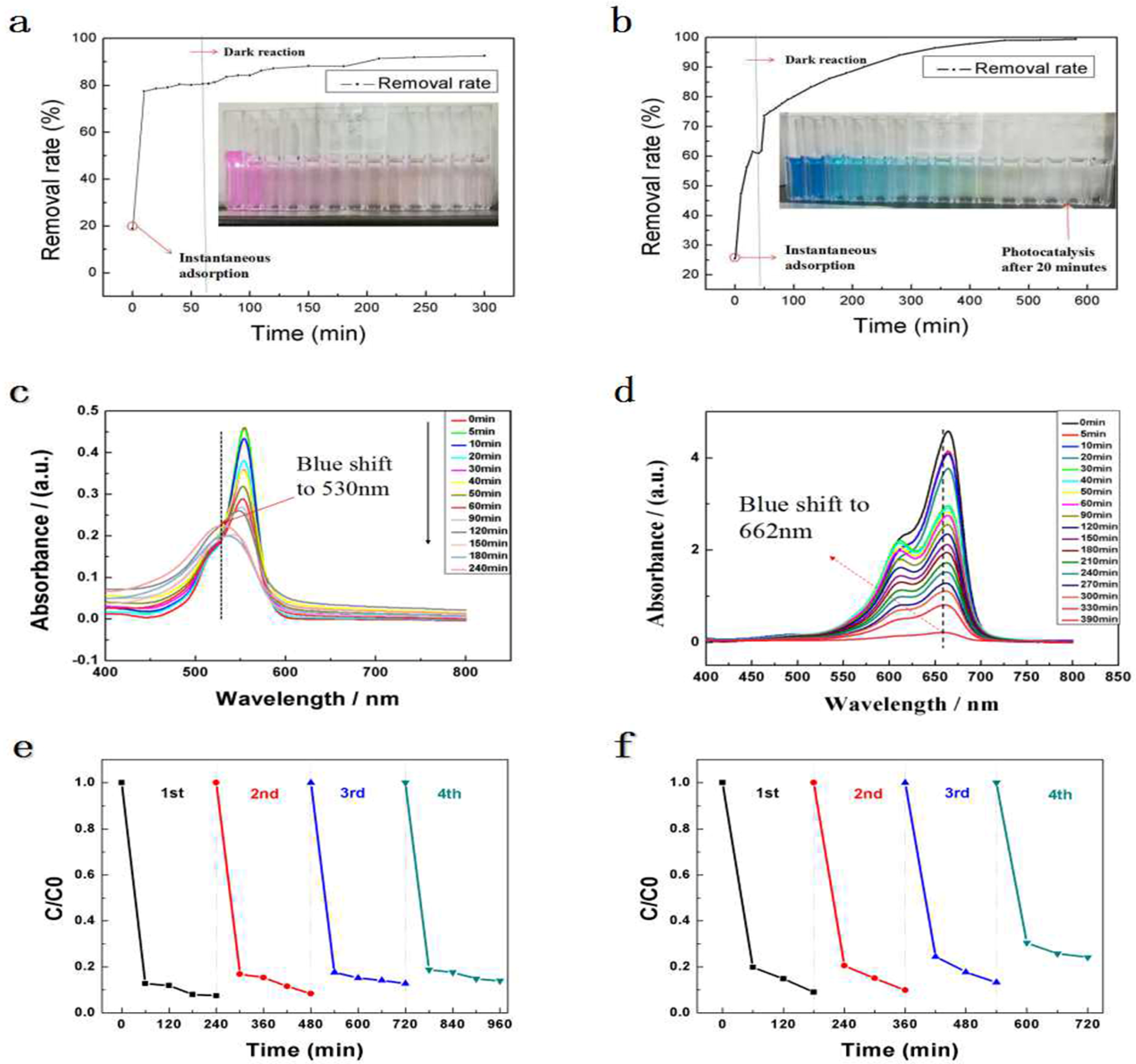
2.4. Photocatalytic Performance
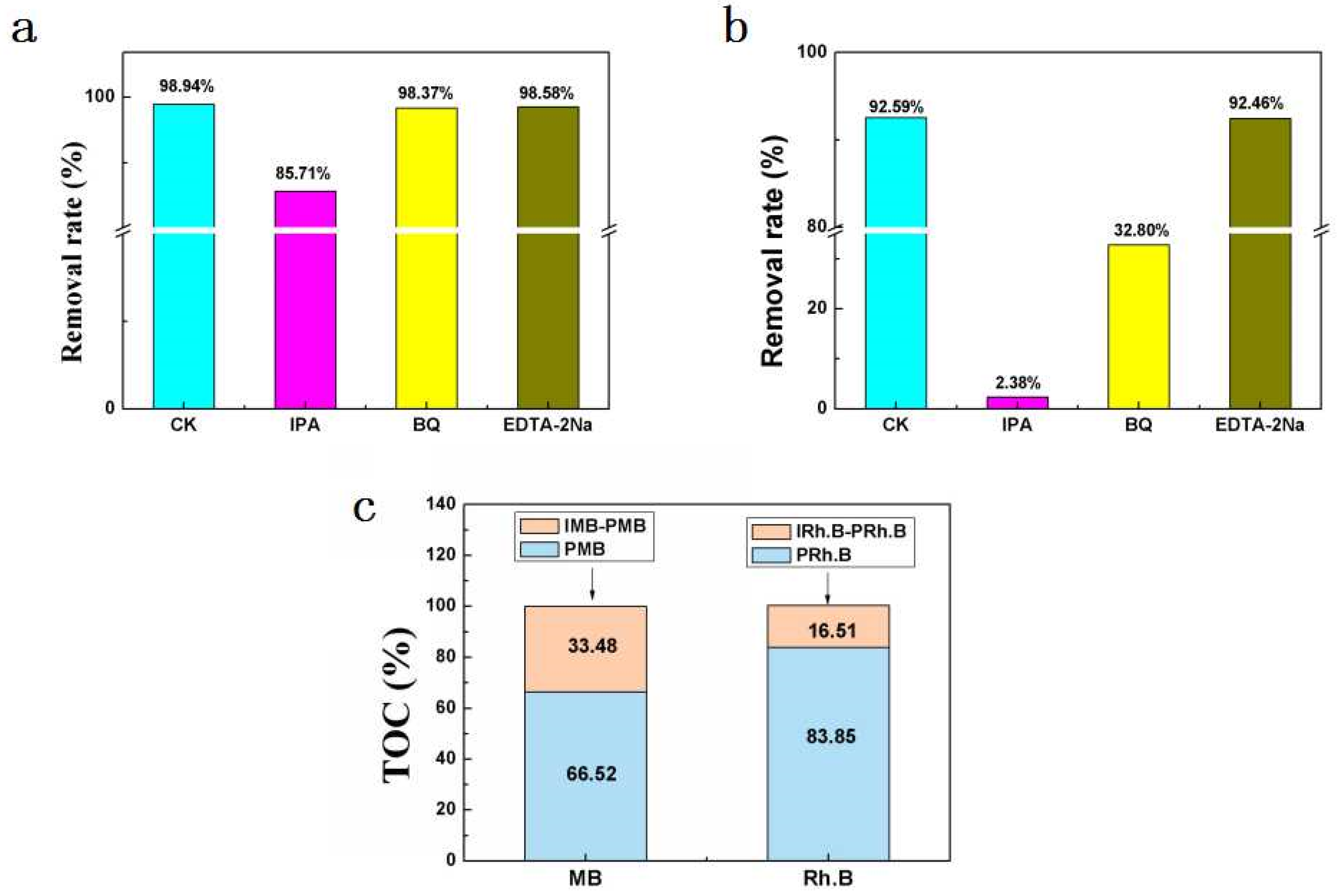
2.5. Active Species Detection
2.6. Total Organic Carbon (TOC)Analysis of Degradation Products
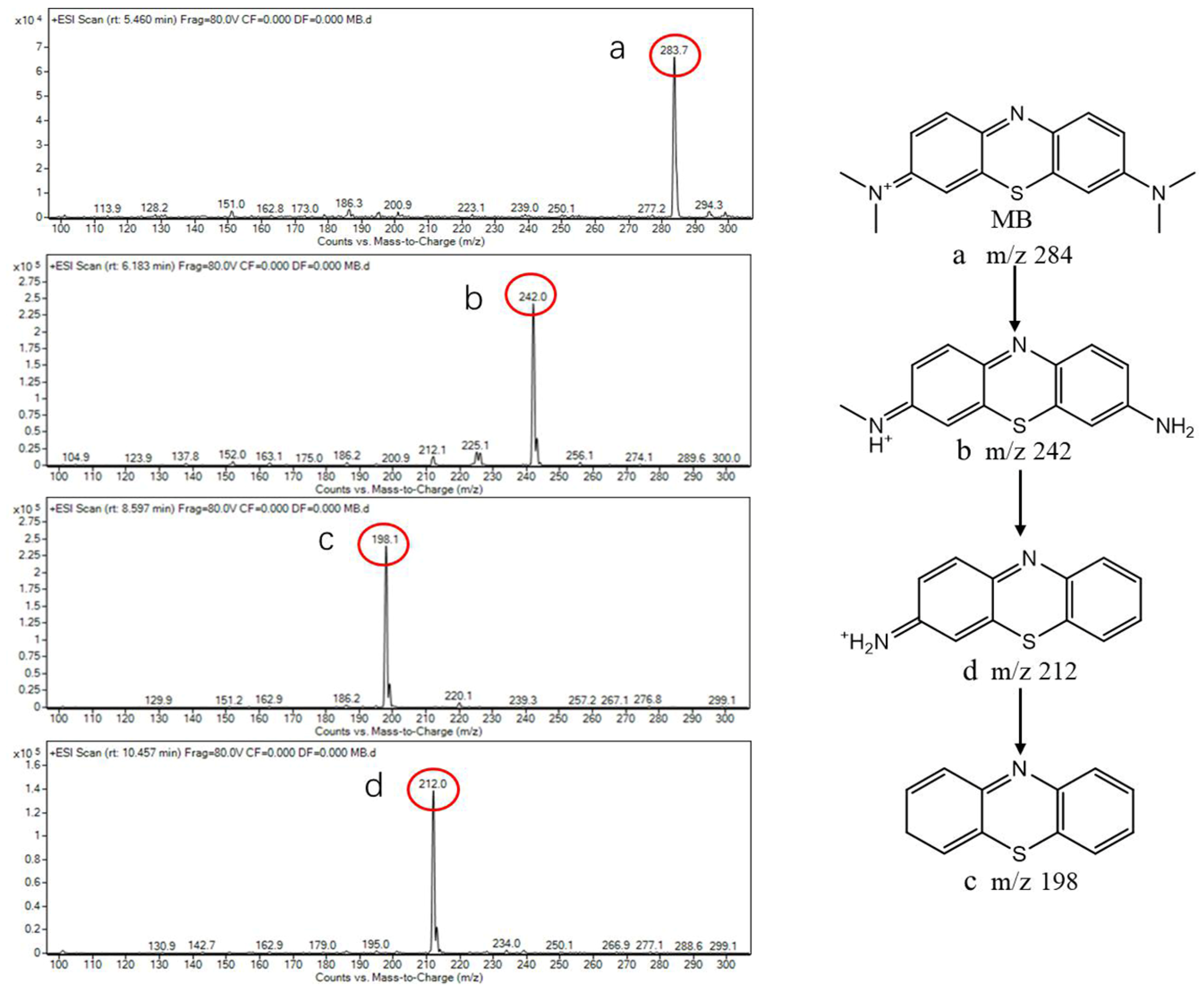
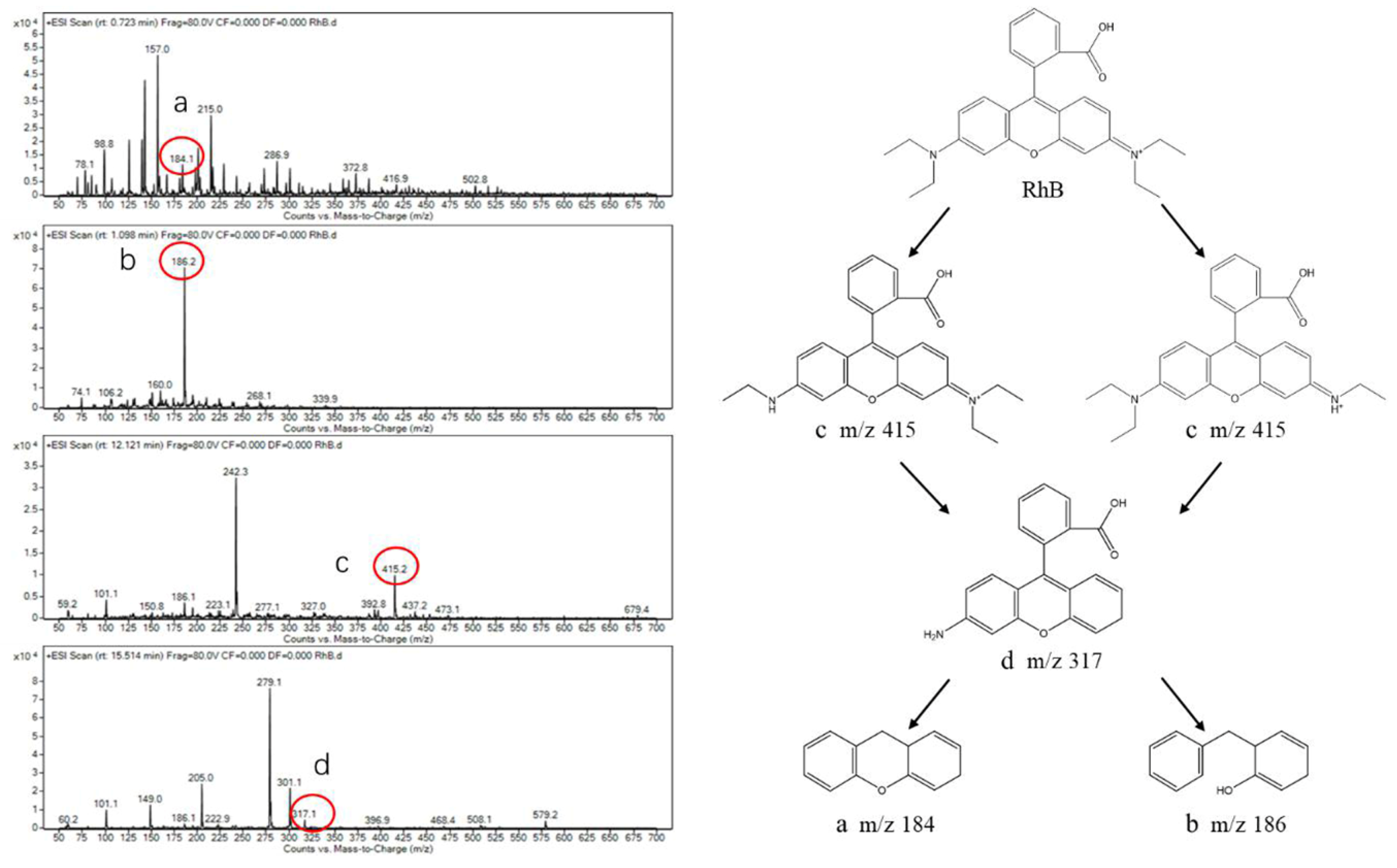
2.7. Photocatalytic Degradation Products Analysis
3. Materials and Methods
3.1. Initial Materials
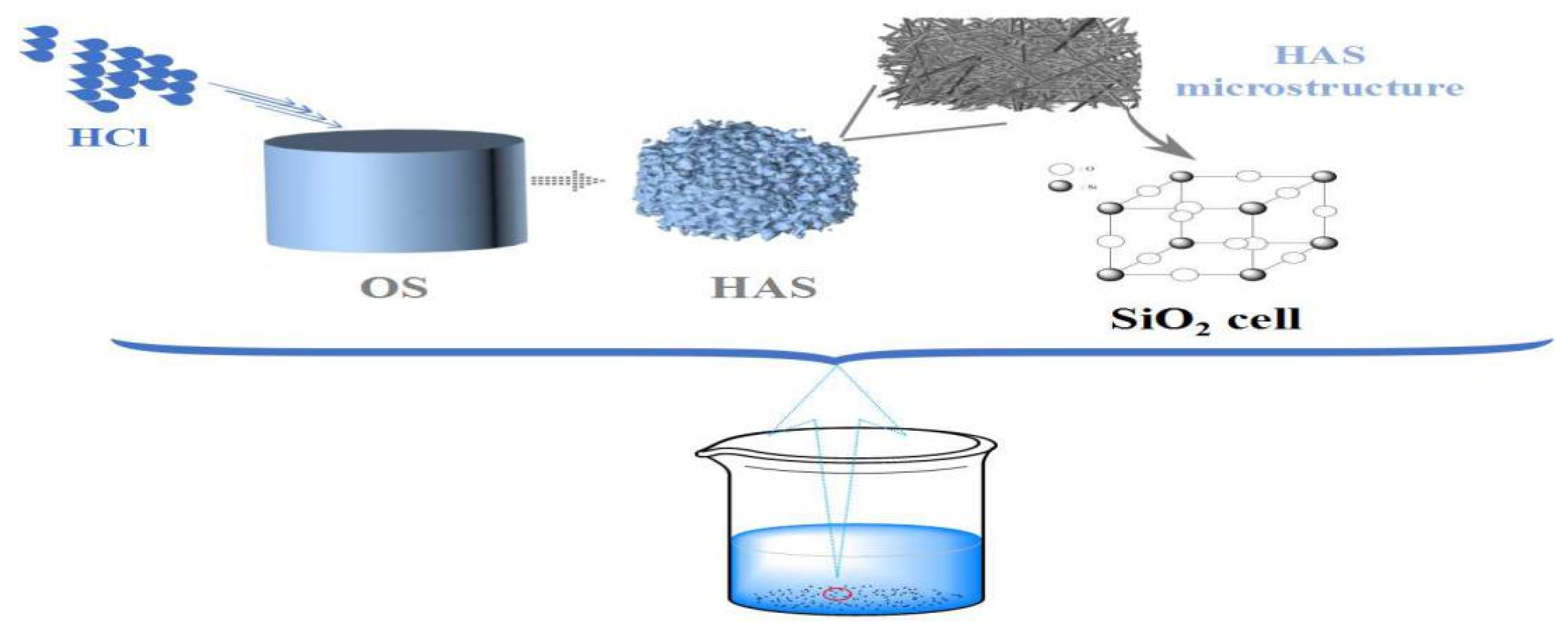
3.2. Mussel Shell Photocatalyst Preparation
3.3. Characterization
3.4. Photodegradation of Organic Dyes
3.5. Active Species Detection
3.6. Analysis of the Degradation Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zheng, Y.; Lin, L.H.; Wang, B.; Wang, X.C. Graphitic carbon nitride polymers toward sustainable photoredox catalysis. Angew. Chem. Int. Ed. 2015, 54, 12868–12884. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Basu, S. Highly reusable visible light active hierarchical porous WO3 /SiO2 monolith in centimeter length scale for enhanced photocatalytic degradation of toxic pollutants. Sep. Purif. Technol. 2020, 231, 115916. [Google Scholar] [CrossRef]
- Vieillard, J.; Bouazizi, N.; Mohammad, N.M. CuO nanosheets modified with amine and thiol grafting for high catalytic and antibacterial activities. Ind. Eng. Chem. Res. 2019, 58, 10179–10189. [Google Scholar] [CrossRef]
- Qin, N.; Wei, W.; Huang, C. An efficient strategy for the fabrication of cus as a highly excellent and recyclable photocatalyst for the degradation of organic dyes. Catalysts 2019, 10, 40. [Google Scholar] [CrossRef] [Green Version]
- Sapkota, K.P.; Lee, I.; Hanif, M.A.; Islam, M.A.; Akter, J.; Hahn, J.R. Enhanced visible-light photocatalysis of nanocomposites of copper oxide and single-walled carbon nanotubes for the degradation of methylene blue. Catalysts 2020, 10, 297. [Google Scholar] [CrossRef] [Green Version]
- Zucker, I.; Lester, Y.; Avisar, D.; Hübner, M.; Jekel, U.; Weinberger, Y. Influence of wastewater particles on ozone degradation of trace organic contaminants. Environ. Sci. Technol. 2015, 49, 301–308. [Google Scholar] [CrossRef]
- Wang, W.; Tade, M.O.; Shao, Z. Research progress of perovskite materials in photocatalysis-and photovoltaics-related energy conversion and environmental treatment. Chem. Soc. Rev. 2015, 44, 5371–5408. [Google Scholar] [CrossRef]
- Wang, F.; Li, Q.; Xu, D. Recent progress in semiconductor-based nanocomposite photocatalysts for solar-to-chemical energy conversion. Adv. Energy. Mater. 2017, 7, 1700529. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.; Lee, M.J.; Kim, M.S.; Lee, S.; Kim, Y.K.; Lee, D.H.; Chung, J.H. Infrared plus visible light and heat from natural sunlight participate in the expression of MMPs and type I procollagen as well as infiltration of inflammatory cell in human skin in vivo. J. Dermatol. Sci. 2008, 50, 123–133. [Google Scholar] [CrossRef]
- He, K.; Chen, G.; Zeng, G.; Chen, A.; Huang, Z.; Shi, J.; Huang, T.; Peng, M.; Hu, L. Three-dimensional graphene supported catalysts for organic dyes degradation. Appl. Catal. B-Environ. 2018, 228, 19–28. [Google Scholar] [CrossRef]
- Wang, M.; Tan, G.; Zhang, D.; Li, B.; Lv, L.; Wang, Y.; Ren, H.; Zhang, X.L.; Xia, A.; Liu, Y. Defect-mediated Z-scheme BiO2-x/Bi2O2.75 photocatalyst for full spectrum solar-driven organic dyes degradation. Appl. Catal. B-Environ. 2019, 254, 98–112. [Google Scholar] [CrossRef]
- Alansi, A.M.; Qunaibit, M.A.; Alde, L.O.; Qahtan, T.F.; Saleh, T.A. Visible-light responsive BiOBr nanoparticles loaded on reduced graphene oxide for photocatalytic degradation of dye. J. Mol. Liq. 2018, 253, 297–304. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-doped titanium dioxide as visible-light-sensitive photocatalyst: Designs, developments, and prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef] [PubMed]
- Ani, I.J.; Akpan, U.G.; Olutoye, M.A.; Hameed, B.H. Photocatalytic degradation of pollutants in petroleum refinery wastewater by TiO2- and ZnO-based photocatalysts: Recent development. J. Clean. Prod. 2018, 205, 930–954. [Google Scholar] [CrossRef]
- Li, H.J.; Tu, W.G.; Zhou, Y.; Zou, Z.G. Z-scheme photocatalytic systems for promoting photocatalytic performance: Recent progress and future challenges. Adv. Sci. 2016, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Wang, J.; Ma, J.; Yang, H.; Zhang, J.; Lv, K.; Peng, T. A novel bodipy-based mof photocatalyst for efficient visible-light-driven hydrogen evolution. J. Mater. Chem. A 2019, 7, 10439–10445. [Google Scholar] [CrossRef]
- Malengreaux, C.M.; Pirard, S.L.; Mahy, J.G.; Herlistschke, M.; Klobes, B.; Hermann, R.; Heinrichs, B.; Bartlett, J.R. Study of the photocatalytic activity of Fe3+, Cr3+, La3+ and Eu3+ single-doped and co-doped TiO2 catalysts produced by aqueous sol-gel processing. J. Alloys Compd. 2017, 691, 726–738. [Google Scholar] [CrossRef] [Green Version]
- Ohno, T.; Akiyoshi, M.; Umebayashi, T.; Asai, K.; Mitsui, T.; Matsumura, M. Preparation of S-doped TiO2 photocatalysts and their photocatalyticactivities under visible light. Appl. Catal. A 2004, 265, 115–121. [Google Scholar] [CrossRef]
- Khan, M.; Cao, W. Preparation of Y-doped TiO2 by hydrothermal method and investigation of its visible light photocatalytic activity by the degradation of methylene blue. J. Mol. Catal. A Chem. 2013, 376, 71–77. [Google Scholar] [CrossRef]
- Zuas, O.; Abimanyu, H.; Wibowo, W. Synthesis and characterization of nanostructured CeO2with dyes adsorption property. Process. Appl. Ceram. 2014, 8, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Jiang, D.; Cai, L.; Ji, L.; Zhang, H.; Song, W. Nano-Bi2MoO6/calcined mussel shell composites with enhanced photocatalytic performance under visible-light irradiation. Micro Nano Lett. 2018, 13, 1021–1025. [Google Scholar] [CrossRef]
- Liu, C.; Chen, L.; Ding, D.; Cai, T. From rice straw to magnetically recoverable nitrogen doped biochar: Efficient activation of peroxymonosulfate for the degradation of metolachlor. Appl. Catal. B-Environ. 2019, 254, 312–320. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, H.; Cai, L.; Guo, J.; Wang, Y.; Ji, L.; Song, W. Preparation and characterization of macroalgae biochar nanomaterials with highly efficient adsorption and photodegradation ability. Materials 2018, 11, 1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Z.; Yang, H.; Liu, Q. Natural Seashell Waste as an Efficient and Low-Cost Catalyst for the Synthesis of Arylmethylenemalonitriles. CLEAN–Soil Air Water 2019, 47, 1900129. [Google Scholar] [CrossRef]
- Morris, J.P.; Backeljau, T.; Chapelle, G. Shells from aquaculture: A valuable biomaterial. Not a nuisance waste product. Rev. Aquac. 2019, 11, 42–57. [Google Scholar] [CrossRef] [Green Version]
- Wijsman, J.W.M.; Troost, K.; Fang, J.; Roncarati, A. Global production of marine bivalves. Trends and challenges. In Goods and Services of Marine Bivalves; Springer: New York, NY, USA; Cham, Switzerland, 2019; pp. 7–26. [Google Scholar]
- Tokeshi, M.; Ota, N.; Kawai, T. A comparative study of morphometry in shell-bearing molluscs. J. Zool. 2000, 251, 31–38. [Google Scholar] [CrossRef]
- Paradelo, R.; Conde-Cid, M.; Cutillas-Barreiro, L.; Arias-Estévez, M.; Nóvoa-Muñoz, J.C.; Álvarez-Rodríguez, E.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A. Phosphorus removal from wastewater using mussel shell: Investigation on retention mechanisms. Ecol. Eng. 2016, 97, 558–566. [Google Scholar] [CrossRef]
- Peinemann, J.C.; Krenz, L.M.M.; Pleissner, D. Is seashell powder suitable for phosphate recovery from fermentation broth? New. Biotechnol. 2019, 49, 43–47. [Google Scholar] [CrossRef]
- Osorio-López, C.; Seco-Reigosa, N.; Garrido-Rodríguez, B.; Cutillas-Barreiro, L.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A. As (V) adsorption on forest and vineyard soils and pyritic material with or without mussel shell: Kinetics and fractionation. J. Taiwan Inst. Chem. Eng. 2014, 45, 1007–1014. [Google Scholar] [CrossRef]
- Otero, M.; Cutillas-Barreiro, L.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Cr (VI) sorption/desorption on untreated and mussel-shell-treated soil materials: Fractionation and effects of pH and chromium concentration. Solid Earth 2015, 6, 373–382. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.S.; Wu, D.Y.; Wang, S.S. Antibacterial properties of bio-based polyester composites achieved through modification with thermally treated waste scallop shell. ACS Appl. Bio Mater. 2019, 25, 2262–2270. [Google Scholar] [CrossRef]
- Melo, P.M.A.; Macêdo, O.B.; Barbosa, G.P.; Ueki, M.M.; Silva, L.B. High-density polyethylene/mollusk shell-waste composites: Effects of particle size and coupling agent on morphology, mechanical and thermal properties. J. Mater. Res. Technol. 2019, 8, 1915–1925. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, H.; Wang, T.; Wang, B.; Wei, W.; Li, H.; Yuan, S.; An, T.; Zhao, H.; Yu, J.; et al. Nature-based catalyst for visible-light-driven photocatalytic CO2 reduction. Energ. Environ. Sci. 2018, 11, 2382–2389. [Google Scholar] [CrossRef] [Green Version]
- Cai, L.; Zhou, Y.; Wang, Z.; Chen, J.; Ji, L.; Guo, J.; Liu, J. Preparation and evaluation of a hierarchical Bi2MoO6/MSB composite for visible-light-driven photocatalytic performance. RSC. Adv. 2019, 9, 38280–38288. [Google Scholar] [CrossRef] [Green Version]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Shen, Y.; Li, H.; Zhu, W.; Ho, S.H.; Yuan, W.; Chen, J.; Xie, Y. Microalgal-biochar immobilized complex: A novel efficient biosorbent for cadmium removal from aqueous solution. Bioresour. Technol. 2017, 244, 1031–1038. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M. Gas adsorption characterization of ordered organic- inorganic nanocomposite materials. Chem. Mater. 2001, 13, 3169–3183. [Google Scholar] [CrossRef]
- Zhang, Q.Z.; Deng, J.J.; Xu, Z.H.; Chaker, M.; Ma, D.L. High-efficiency broadband C3N4 photocatalysts: Synergistic effects from upconversion and plasmons. ACS Catal. 2017, 7, 6225–6234. [Google Scholar] [CrossRef]
- Gangaraju, D.; Sridhar, V.; Lee, I.; Park, H. Graphene–carbon nanotube–Mn3O4, mesoporous nano-alloys as high capacity anodes for lithium-ion batteries. J. Alloys Compd. 2016, 699, 106–111. [Google Scholar] [CrossRef]
- Mueller, R.; Kammler, H.K.; Wegner, K.; Pratsinis, S.E. OH surface density of SiO2 and TiO2 by thermogravimetric analysis. Langmuir 2003, 19, 160–165. [Google Scholar] [CrossRef]
- Zhou, F.; Shi, R.; Zhu, Y. Significant enhancement of the visible photocatalytic degradation performances of γ-Bi2MoO6, nanoplate by graphene hybridization. J. Mol. Catal. A Chem. 2011, 340, 77–82. [Google Scholar] [CrossRef]
- Cai, L.; Gong, J.; Liu, J.; Zhang, H.; Song, W.; Ji, L. Facile preparation of nano-Bi2MoO6/diatomite composite for enhancing photocatalytic performance under visible light irradiation. Materials 2018, 11, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Cao, W.; Su, Y.; Wang, Y.; Wang, X. Synthesis, characterization and photocatalytic performance of novel visible-light-induced ag/bioi. Appl. Catal. B Environ. 2012, 111–112, 271–279. [Google Scholar] [CrossRef]
- Li, S.; Hu, S.; Zhang, J.; Jiang, W.; Liu, J. Facile synthesis of Fe2O3 nanoparticles anchored on Bi2MoO6 microflowers with improved visible light photocatalytic activity. J. Colloid Interface Sci. 2017, 497, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Zhang, M.; Yao, C.; Liu, C.; Xu, S.; Li, Z. High performance visible-light driven photocatalysts of Bi2MoO6-g-C3N4 with controllable solvothermal fabrication. J. Photochem. Photobiol. A 2017, 332, 357–363. [Google Scholar] [CrossRef]
- Fu, H.; Pan, C.; Yao, W.; Zhu, Y. Visible-light-induced degradation of rhodamine b by nanosized Bi2Wo6. J. Phys. Chem. B 2005, 109, 22432. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Chen, Z.; Yang, Y.; Peng, J.; Zheng, T. Effects and mechanism of SiO2 on photocatalysis and super hydrophilicity of TiO2 films prepared by sol-gel method. Mater. Res. Express. 2019, 64, 046409. [Google Scholar] [CrossRef]
- Xu, H.; Li, H.; Sun, G.; Xia, J.; Wu, C.; Ye, Z.; Zhang, Q. Photocatalytic activity of La2O3-modified silver vanadates catalyst for Rhodamine B dye degradation under visible light irradiation. Chem. Eng. J. 2010, 160, 33–41. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Thomas, M.; Natarajan, K.; Bajaj, H.C.; Tayade, R.J. Study on UV-LED/TiO2 process for degradation of Rhodamine B dye. Chem. Eng. J. 2011, 169, 126–134. [Google Scholar] [CrossRef]
- Kasinathan, K.; Kennedy, J.; Elayaperumal, M.; Henini, M.; Malik, M. Photodegradation of organic pollutants RhB dye using UV simulated sunlight on ceria based TiO2 nanomaterials for antibacterial applications. Sci. Rep. UK 2016, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Laura, M.; Darinka, F.; Matej, B.; Selestina, G. Efficiency of differently processed membranes based on cellulose as cationic dye adsorbents. Nanomaterials 2020, 10, 642. [Google Scholar]
- Poudel, M.B.; Yu, C.; Kim, H.J. Synthesis of Conducting Bifunctional Polyaniline@Mn-TiO2 Nanocomposites for Supercapacitor Electrode and Visible Light Driven Photocatalysis. Catalysts 2020, 10, 546. [Google Scholar] [CrossRef]
- Tummino, M.; Laurenti, E.; Deganello, F.; Prevot, A.; Magnacca, G. Revisiting the catalytic activity of a doped SrFeO3 for water pollutants removal: Effect of light and temperature. Appl. Catal. B Environ. 2017, 207, 174–181. [Google Scholar] [CrossRef]

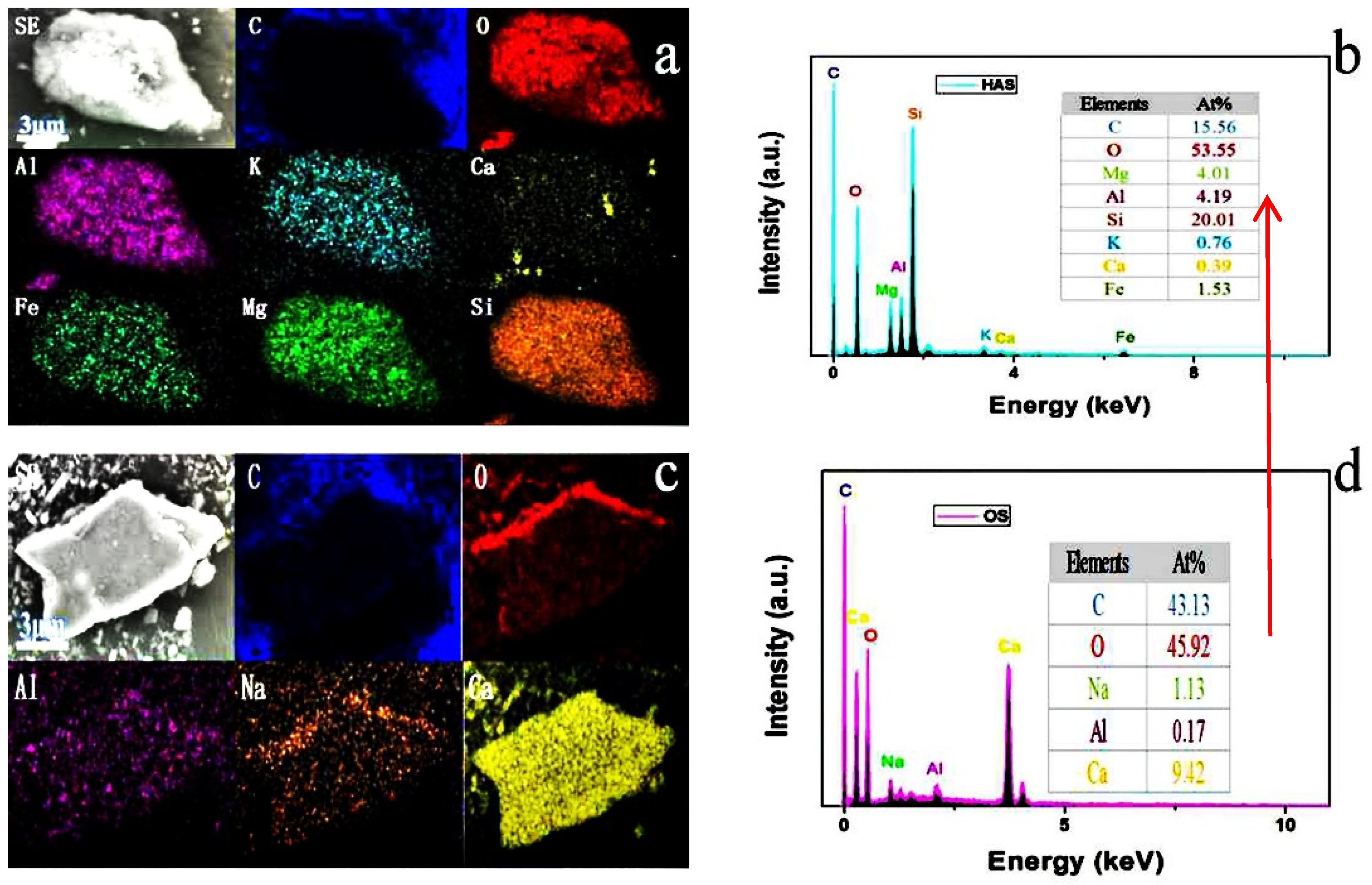
| Metal Element | Ca | Al | Si | Fe | K | Mg | Ti | Zr | Mn |
|---|---|---|---|---|---|---|---|---|---|
| HAS | 1621.5 | 33,437.8 | 233,580.0 | 27,243.6 | 7512.8 | 22,660.6 | 3622.1 | 779.7 | 127.4 |
| OS | 385,000.0 | 125.4 | 642.0 | 10.4 | 44.9 | 967.0 | - | - | 2.8 |
| Catalyst | Dye | Dye (mL) | Dye Conc. (mg/L) | Catalyst Content (g) | Reaction Time (min) | K (min−1) | Degradation Rate (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Bi2MoO6/Graphene | MB | 100 | 0.01 | 0.05 | 120 | 0.014 | 80 | [42] |
| TiO2@SiO2 | MO | 15 | 20 | 0.02 | 180 | - | 97.5 | [43] |
| BKBC | MB | 50 | 80 | 0.01 | 60 | 0.0079 | 61.39 | [23] |
| AKB | MB | 50 | 80 | 0.01 | 60 | 0.0073 | 94.12 | [23] |
| BiOI | MO | 50 | 10 | 0.05 | 240 | 0.0843 | 31.3 | [44] |
| BiOI | RB | 50 | 10 | 0.05 | 240 | 0.0502 | 17.8 | [44] |
| HAS | MB | 40 | 60 | 0.02 | 540 | 0.0059 | 99.14 | This study |
| HAS | RhB | 40 | 24 | 0.02 | 240 | 0.0040 | 92.59 | This study |
| Parameter | Condition |
|---|---|
| Column | Agilent ZORBAX SB-C18 |
| Eluent types | methanol-water (80:20, V/V) |
| Flow rate (mL/min) | 0.3 |
| Injection Volume (μL) | 5 |
| Nebulizer pressure (psi) | 70 |
| Column temperature (°C) | 25 |
| Capillary voltage (kV) mass range | 3.5 50–700 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Xia, L.; Chen, J.; Ji, L.; Zhou, Y.; Wang, Y.; Cai, L.; Guo, J.; Song, W. Fine Characterization of Natural SiO2-Doped Catalyst Derived from Mussel Shell with Potential Photocatalytic Performance for Organic Dyes. Catalysts 2020, 10, 1130. https://doi.org/10.3390/catal10101130
Wang Z, Xia L, Chen J, Ji L, Zhou Y, Wang Y, Cai L, Guo J, Song W. Fine Characterization of Natural SiO2-Doped Catalyst Derived from Mussel Shell with Potential Photocatalytic Performance for Organic Dyes. Catalysts. 2020; 10(10):1130. https://doi.org/10.3390/catal10101130
Chicago/Turabian StyleWang, Zhen, Liping Xia, Jinlong Chen, Lili Ji, Yarui Zhou, Yaning Wang, Lu Cai, Jian Guo, and Wendong Song. 2020. "Fine Characterization of Natural SiO2-Doped Catalyst Derived from Mussel Shell with Potential Photocatalytic Performance for Organic Dyes" Catalysts 10, no. 10: 1130. https://doi.org/10.3390/catal10101130





