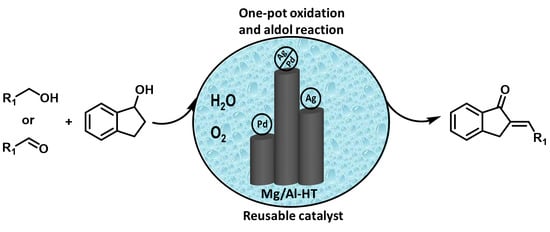
Hydrotalcite-Supported Ag/Pd Bimetallic Nanoclusters Catalyzed Oxidation and One-Pot Aldol Reaction in Water
Abstract
:1. Introduction
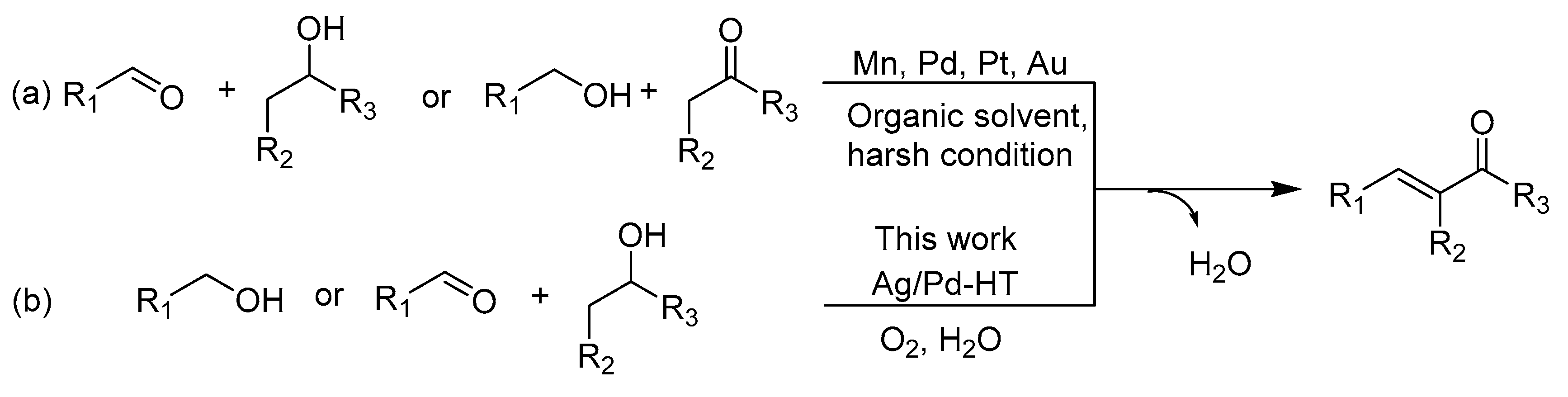
2. Results and Discussion
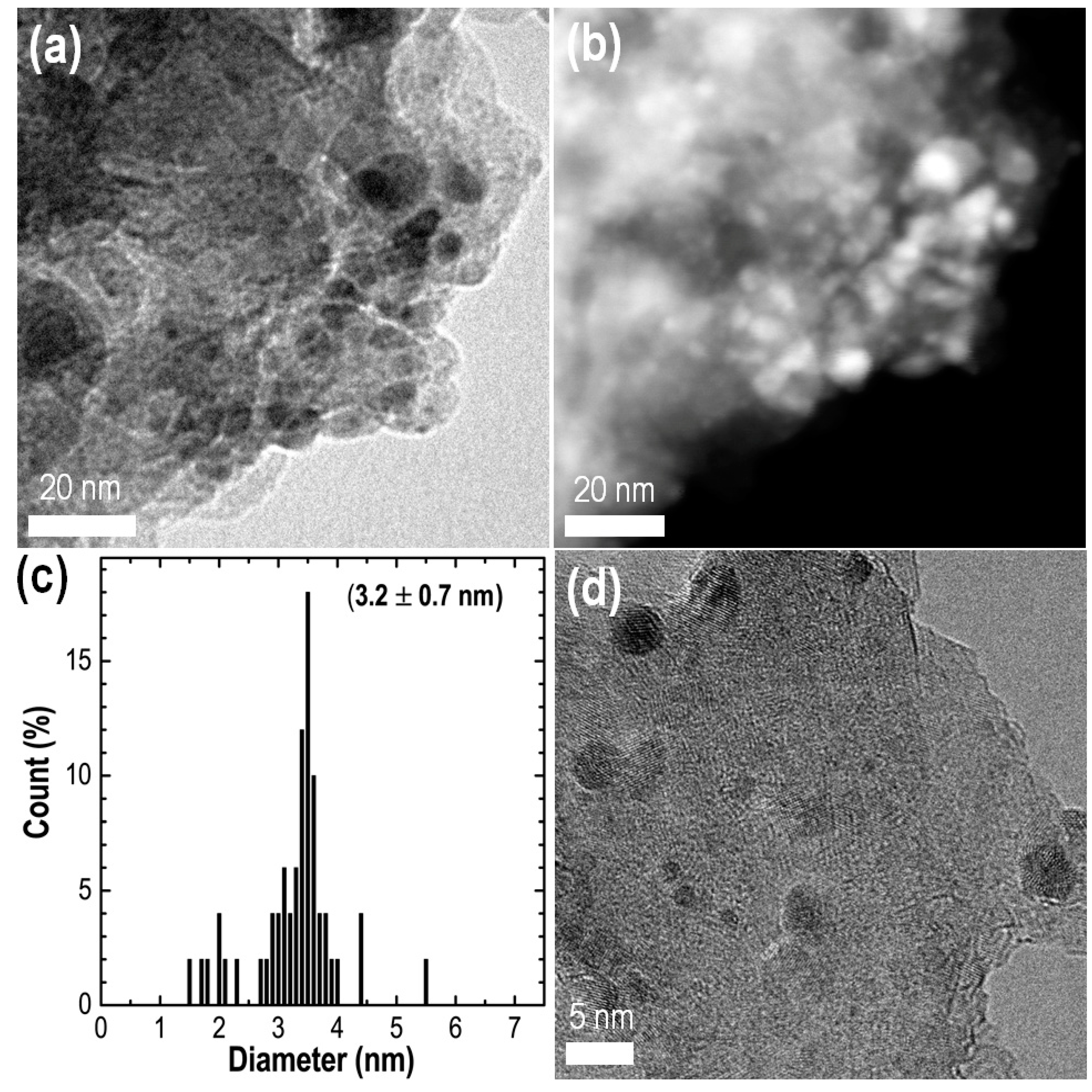
2.1. Synthesis of Catalyst
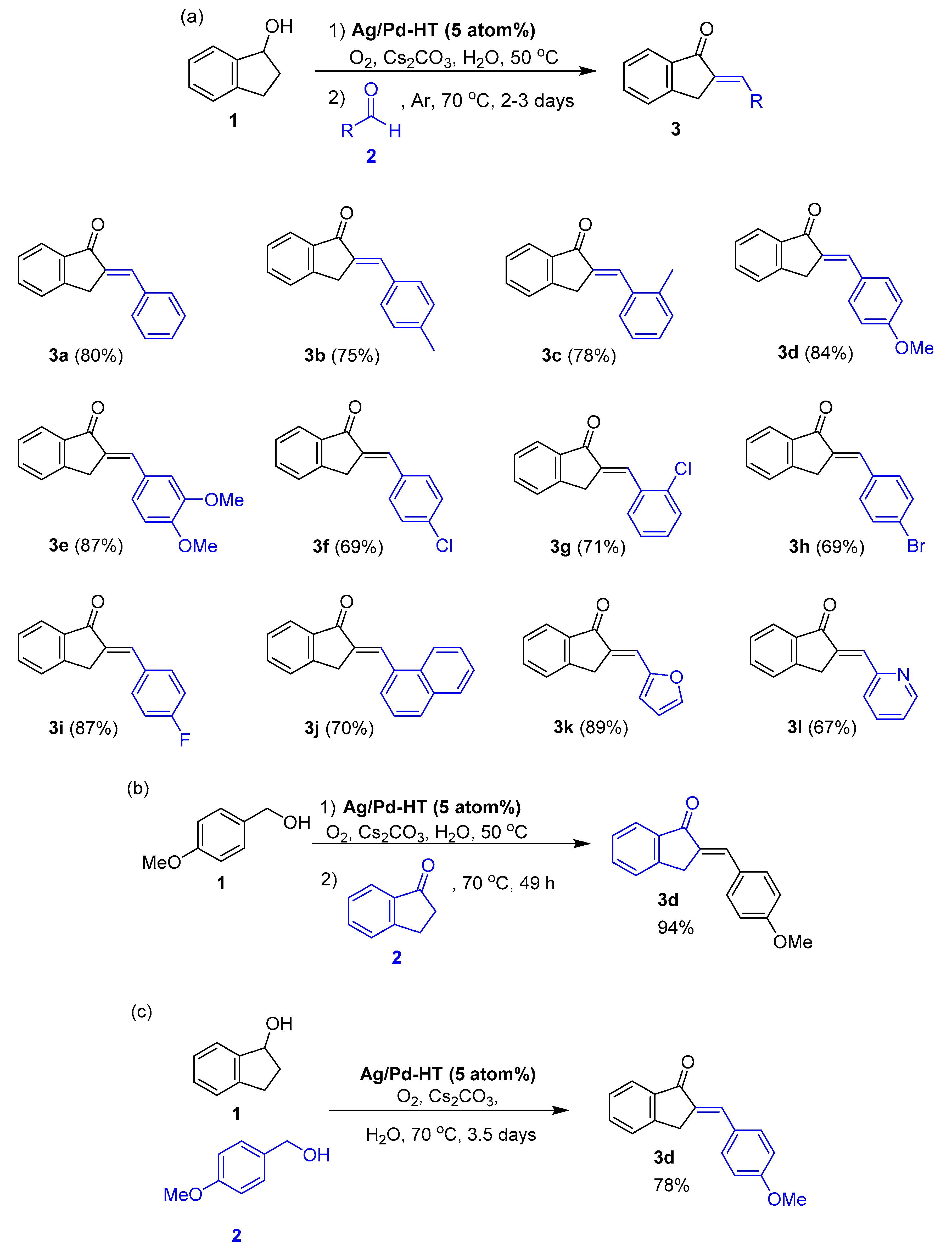
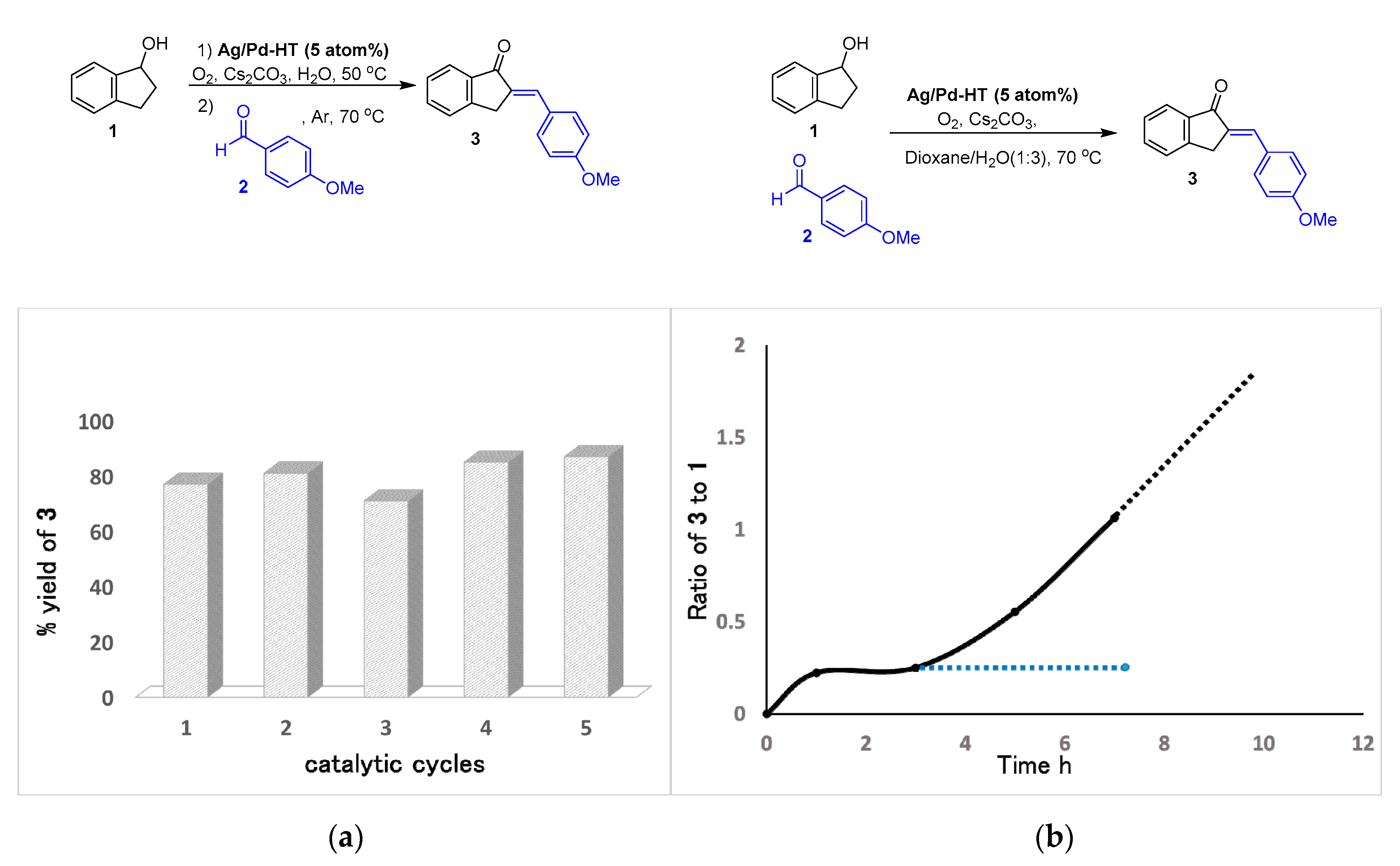
2.2. Catalytic Performance
3. Materials and Methods
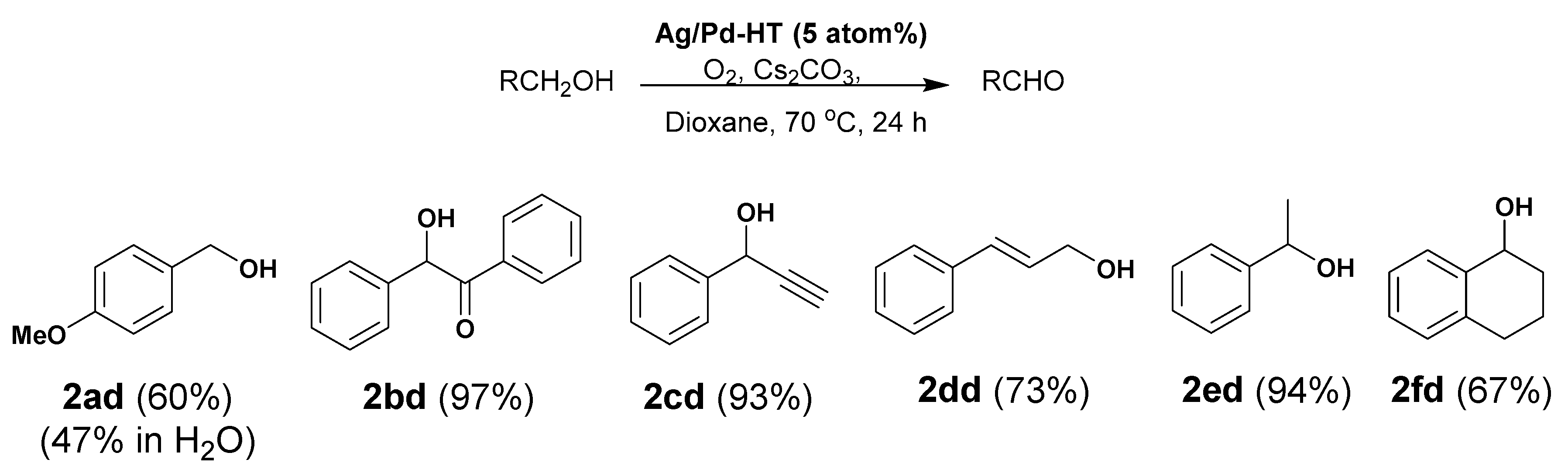
3.1. General Procedure for Oxidation
3.2. General Procedure for Oxidation and One-pot Aldol Reaction in Water
3.2.1. Method A
3.2.2. Method B
3.2.3. Method C
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nel, M.S.; Petzer, A.; Petzer, J.P.; Legoabe, L.J. 2-Heteroarylidene-1-indanone derivatives as inhibitors of monoamine oxidase. Bioorgan. Chem. 2016, 69, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Varma, R.S.; Kabalka, G.W.; Evans, L.T.; Pagni, R.M. Aldol condensations on basic alumina: The facile syntheses of chalcones and enones in a solvent-free medium. Synth. Commun. 1985, 15, 279–284. [Google Scholar] [CrossRef]
- Guida, A.; Lhouty, M.H.; Tichit, D.; Figueras, F.; Geneste, P. Hydrotalcites as base catalysts. Kinetics of Claisen-Schmidt condensation, intramolecular condensation of acetonylacetone and synthesis of chalcone. Appl. Catal. 1997, 164, 251–264. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S.; Primo, J. Base catalysis for fine chemicals production: Claisen-Schmidt condensation on zeolites and hydrotalcites for the production of chalcones and flavanones of pharmaceutical interest. J. Catal. 1995, 151, 60–66. [Google Scholar] [CrossRef]
- Solhy, A.; Tahir, R.; Sebti, S.; Skouta, R.; Bousmina, M.; Zahouily, M.; Larzek, M. Efficient synthesis of chalcone derivatives catalyzed by re-usable hydroxyapatite. Appl. Catal. A Gen. 2010, 374, 189–193. [Google Scholar] [CrossRef]
- Gawali, S.S.; Pandia, B.K.; Gunanathan, C. Manganese(I)-catalyzed α-Alkenylation of Ketones Using Primary Alcohols. Org. Lett. 2019, 21, 3842–3847. [Google Scholar] [CrossRef]
- Kwon, M.S.; Kim, N.; Seo, S.H.; Park, I.S.; Cheedrala, R.K.; Park, J. Recyclable Palladium Catalyst for Highly Selective α-Alkylation of Ketones with Alcohols. Angew. Chem. 2005, 117, 7073–7075. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Wang, M.; Lu, J.; Zhang, C.; Li, L.; Jianga, J.; Wang, F. The cascade synthesis of α,β-unsaturated ketones via oxidative C–C coupling of ketones and primary alcohols over a ceria catalyst. Catal. Sci. Technol. 2016, 6, 1693–1700. [Google Scholar] [CrossRef]
- Kim, S.; Bae, S.W.; Lee, J.S.; Park, J. Recyclable gold nanoparticle catalyst for the aerobic alcohol oxidation and C–C bond forming reaction between primary alcohols and ketones under ambient conditions. Tetrahedron 2009, 65, 1461–1466. [Google Scholar] [CrossRef]
- Kakiuchi, N.; Maeda, Y.; Nishimura, T.; Uemura, S. Pd(II)−Hydrotalcite-catalyzed oxidation of alcohols to aldehydes and ketones using atmospheric pressure of air. J. Org. Chem. 2001, 66, 6620–6625. [Google Scholar] [CrossRef]
- Karimi, B.; Abedi, S.; Clark, J.H.; Budarin, V. Highly efficient aerobic oxidation of alcohols using a recoverable catalyst: The role of mesoporous channels of SBA-15 in stabilizing palladium nanoparticle. Angew. Chem. Int. Ed. 2006, 45, 4776–4779. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.S.; Kim, N.; Park, C.M.; Lee, J.S.; Kang, K.Y.; Park, J. Palladium nanoparticles entrapped in aluminum hydroxide: Dual catalyst for alkene hydrogenation and aerobic alcohol oxidation. Org. Lett. 2005, 7, 1077–1079. [Google Scholar] [CrossRef] [PubMed]
- Uozumi, Y.; Nakao, R.; Rhee, H. Development of an amphiphilic resin-dispersion of nanopalladium catalyst: Design, preparation, and its use in aquacatalytic hydrodechlorination and aerobic oxidation. J. Organomet. Chem. 2007, 692, 420–427. [Google Scholar] [CrossRef]
- Biffis, A.; Minati, L. Efficient aerobic oxidation of alcohols in water catalysed by microgel-stabilised metal nanoclusters. J. Catal. 2005, 236, 405–409. [Google Scholar] [CrossRef]
- Lucchesi, C.; Inasaki, T.; Miyamura, H.; Matsubara, R.; Kobayashi, S. Aerobic oxidation of alcohols under mild conditions catalyzed by novel polymer-incarcerated, carbon-stabilized gold nanoclusters. Adv. Synth. Catal. 2008, 350, 1996–2000. [Google Scholar] [CrossRef]
- Sun, F.; Zhou, J.; Zhou, W.; Pan, J.; Qian, J.; He, M.; Chena, Q. Efficient aerobic oxidation of alcohols catalyzed by NiGa hydrotalcites in the absence of any additives. New J. Chem. 2018, 42, 4029–4035. [Google Scholar] [CrossRef]
- Hou, W.; Dehm, N.A.; Scott, R.W.J. Alcohol oxidations in aqueous solutions using Au, Pd, and bimetallic AuPd nanoparticle catalysts. J. Catal. 2008, 253, 22–27. [Google Scholar] [CrossRef]
- Abad, A.; Almela, C.; Corma, A.; Garcia, H. Efficient chemoselective alcohol oxidation using oxygen as oxidant. Superior performance of gold over palladium catalysts. Tetrahedron 2006, 62, 6666–6672. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Deng, Q.; Xu, J.; Han, P.; Pan, L.; Wang, L.; Zhang, X.; Zou, J.-J. Efficient synthesis of high-density aviation biofuel via solvent-free aldol condensation of cyclic ketones and furanic aldehydes. Fuel Process. Technol. 2016, 148, 361–366. [Google Scholar] [CrossRef]
- West, R.M.; Liu, Z.Y.; Peter, M.; Gärtner, C.A.; Dumesic, J.A. Carbon–carbon bond formation for biomass-derived furfurals and ketones by aldol condensation in a biphasic system. J. Mol. Catal. A Chem. 2008, 296, 18–27. [Google Scholar] [CrossRef]
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals. Angew. Chem. Int. Ed. 2007, 46, 7164–7183. [Google Scholar] [CrossRef]
- Karanjit, S.; Kashihara, M.; Nakayama, A.; Shrestha, L.K.; Ariga, K.; Namba, K. Highly active and reusable hydrotalcite-supported Pd(0) catalyst for Suzuki coupling reactions of aryl bromides and chlorides. Tetrahedron 2018, 74, 948–954. [Google Scholar] [CrossRef]
- Choudary, B.M.; Madhi, S.; Chowdari, N.S.; Kantam, M.L.; Sreedhar, B. Layered double hydroxide supported nanopalladium catalyst for Heck-, Suzuki-, Sonogashira-, and Stille-type coupling reactions of chloroarenes. J. Am. Chem. Soc. 2002, 124, 14127–14136. [Google Scholar] [CrossRef] [PubMed]
- Aramendıa, M.A.; Aviles, Y.; Borau, V.; Luque, J.M.; Marinas, J.M.; Ruiz, J.R.; Urbano, F.J. Thermal decomposition of Mg/Al and Mg/Ga layered-double hydroxides: A spectroscopic study. J. Mater. Chem. 1999, 9, 1603–1607. [Google Scholar] [CrossRef]
- Gracia, T.; Agouram, S.; Dejoz, A.; Sanchez-Royo, J.F.; Torrente-Murciano, L.; Solsona, B. Enhanced H2O2 production over Au-rich bimetallic Au–Pd nanoparticles on ordered mesoporous carbons. Catal. Today 2015, 248, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Long, J.; Liu, H.; Wu, S.; Liao, S.; Li, Y. Selective oxidation of saturated hydrocarbons using Au–Pd alloy nanoparticles supported on metal–organic frameworks. ACS Catal. 2013, 3, 647–654. [Google Scholar] [CrossRef]
- Enache, D.I.; Edwards, J.K.; Landon, P.; Solsona-Espriu, B.; Carley, A.F.; Herzing, A.A.; Watanabe, M.; Kiely, C.J.; Knight, D.W.; Hutchings, G.J. Solvent-free oxidation of primary alcohols to aldehydes using Au-Pd/TiO2 catalysts. Science 2006, 311, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Brett, G.L.; He, Q.; Hammond, C.; Miedziak, P.J.; Dimitratos, N.; Sankar, M.; Herzing, A.A.; Conte, M.; Lopez-Sanchez, J.A.; Kiely, C.J.; et al. Selective oxidation of glycerol by highly active bimetallic catalysts at ambient temperature under base-free conditions. Angew. Chem. Int. Ed. 2011, 50, 10136–10139. [Google Scholar] [CrossRef]
- Dhital, R.N.; Kamonsatikul, C.; Somsook, E.; Bobuatong, K.; Ehara, M.; Karanjit, S.; Sakurai, H. Low-temperature carbon–chlorine bond activation by bimetallic gold/palladium alloy nanoclusters: An application to Ullmann coupling. J. Am. Chem. Soc. 2012, 134, 20250–20253. [Google Scholar] [CrossRef]
- Karanjit, S.; Jinasan, A.; Samsook, E.; Dhital, R.N.; Motomiya, K.; Sato, Y.; Tohji, K.; Sakurai, H. Significant stabilization of palladium by gold in the bimetallic nanocatalyst leading to an enhanced activity in the hydrodechlorination of aryl chlorides. Chem. Commun. 2015, 51, 12724–12727. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Entry | Catalyst (Atom%) | Solvent | Base | Temperature (°C) | Yield (%) b |
|---|---|---|---|---|---|
| 1 | Ag-HT (5) | dioxane | Cs2CO3 | 70 | 60 |
| 2 | Pd-HT (5) | dioxane | Cs2CO3 | 70 | 38 |
| 3 | Ag/Pd-HT(1:1) (5) | dioxane | Cs2CO3 | 70 | 93 |
| 4 | Ag/Pd-HT(3:7) (5) | dioxane | Cs2CO3 | 70 | 28 |
| 5 | Ag/Pd-HT(7:3) (5) | dioxane | Cs2CO3 | 70 | 51 |
| 6 | Ag/Pd-HT(1:1) (3) | dioxane | Cs2CO3 | 50 | 92 |
| 7 | Ag/Pd-HT(1:1) (3) | H2O | Cs2CO3 | 50 | 87 |
| 8 c | Ag/Pd-HT(1:1) (3) | H2O | Cs2CO3 | 50 | 30 |
| 9 | HT | H2O | Cs2CO3 | 50−70 | ― |
| 10 | No catalyst | H2O | Cs2CO3 | 50−70 | ― |
| 11 | Ag/Pd-HT(1:1) (3) | H2O | NaOH | 50 | 90 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karanjit, S.; Tamura, A.; Kashihara, M.; Ushiyama, K.; Shrestha, L.K.; Ariga, K.; Nakayama, A.; Namba, K. Hydrotalcite-Supported Ag/Pd Bimetallic Nanoclusters Catalyzed Oxidation and One-Pot Aldol Reaction in Water. Catalysts 2020, 10, 1120. https://doi.org/10.3390/catal10101120
Karanjit S, Tamura A, Kashihara M, Ushiyama K, Shrestha LK, Ariga K, Nakayama A, Namba K. Hydrotalcite-Supported Ag/Pd Bimetallic Nanoclusters Catalyzed Oxidation and One-Pot Aldol Reaction in Water. Catalysts. 2020; 10(10):1120. https://doi.org/10.3390/catal10101120
Chicago/Turabian StyleKaranjit, Sangita, Ayumu Tamura, Masaya Kashihara, Kazuki Ushiyama, Lok Kumar Shrestha, Katsuhiko Ariga, Atsushi Nakayama, and Kosuke Namba. 2020. "Hydrotalcite-Supported Ag/Pd Bimetallic Nanoclusters Catalyzed Oxidation and One-Pot Aldol Reaction in Water" Catalysts 10, no. 10: 1120. https://doi.org/10.3390/catal10101120