Discharge Enhancement Phenomenon and Streamer Control in Dielectric Barrier Discharge with Many Pores
Abstract
:1. Introduction
2. Simulation Model
2.1. Geometry and Physical Parameters
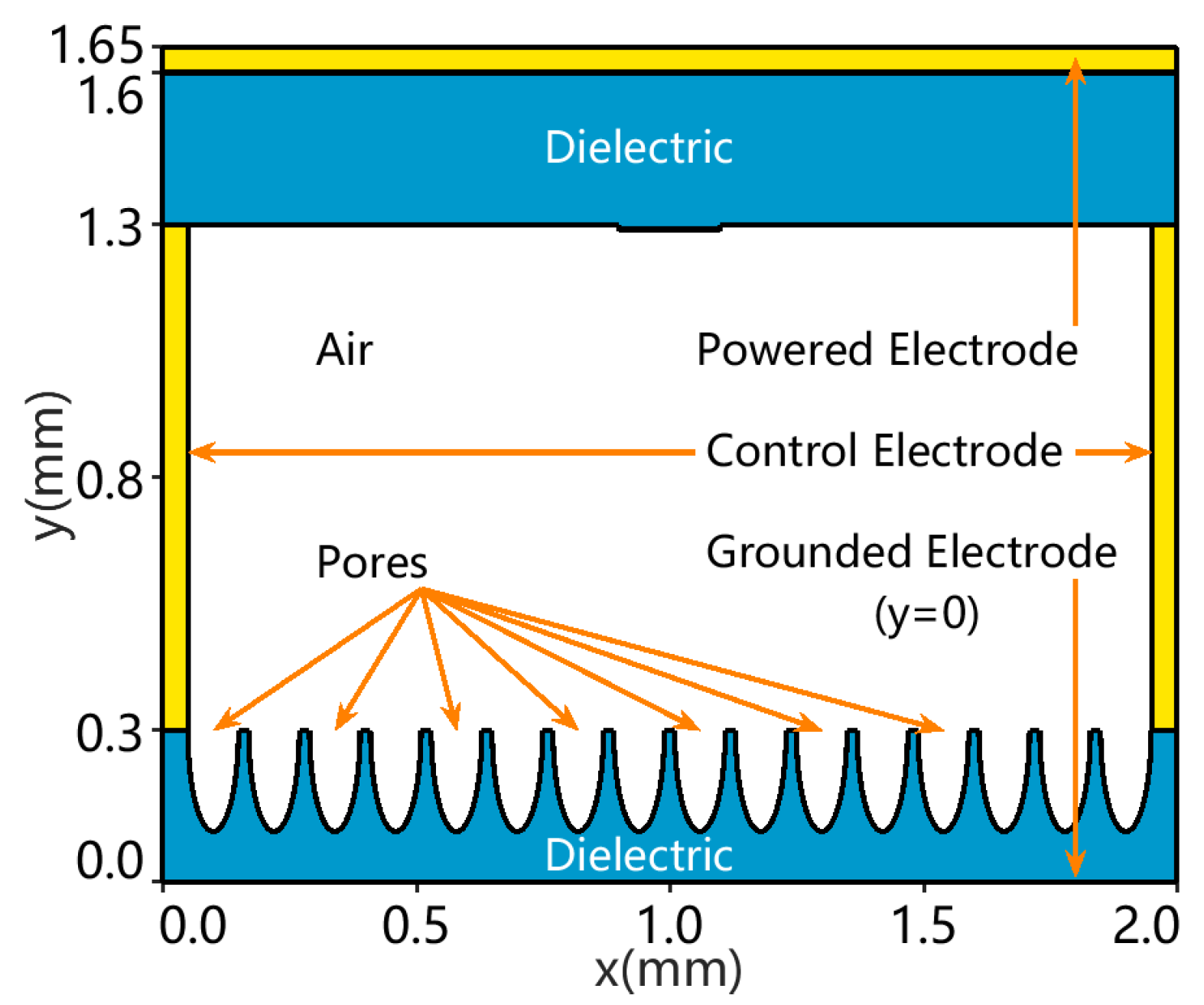
2.1.1. Geometry
2.1.2. Grid and Streamer
2.2. VSim
- (1)
- We need to obtain the charge-current density distribution and electromagnetic field distribution in the streamers to compute the particle movement paths.
- (2)
- Then, by summing the mean of all the particle paths, we can track the overall movement of the streamers.
2.3. Particle-In-Cell Algorithm
2.3.1. Particle Moving: The Newton Equations
2.3.2. Electric Field Solution: The Poisson Equation
2.4. Reactions between Species: The Monte Carlo Collisions
3. Results and Discussion
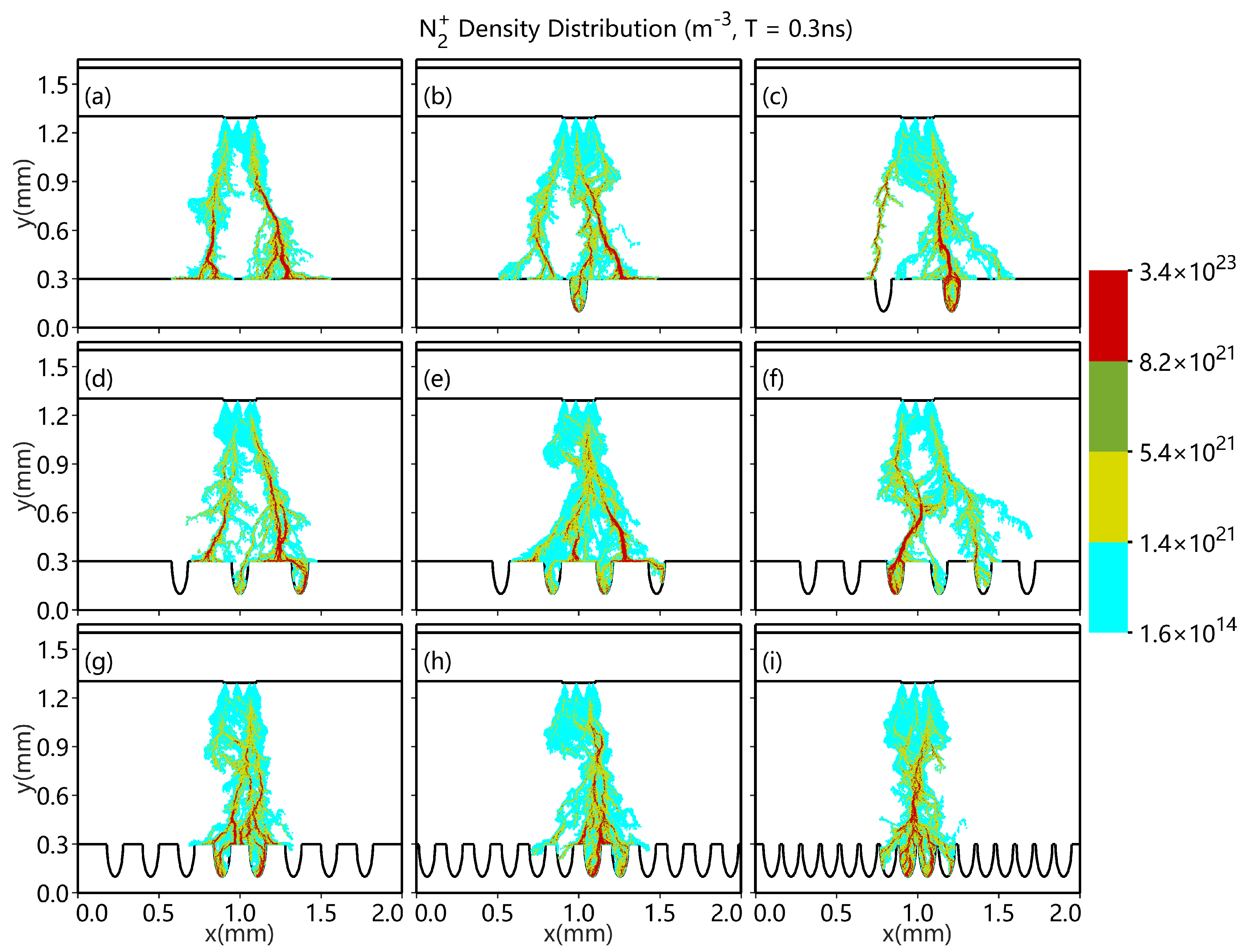
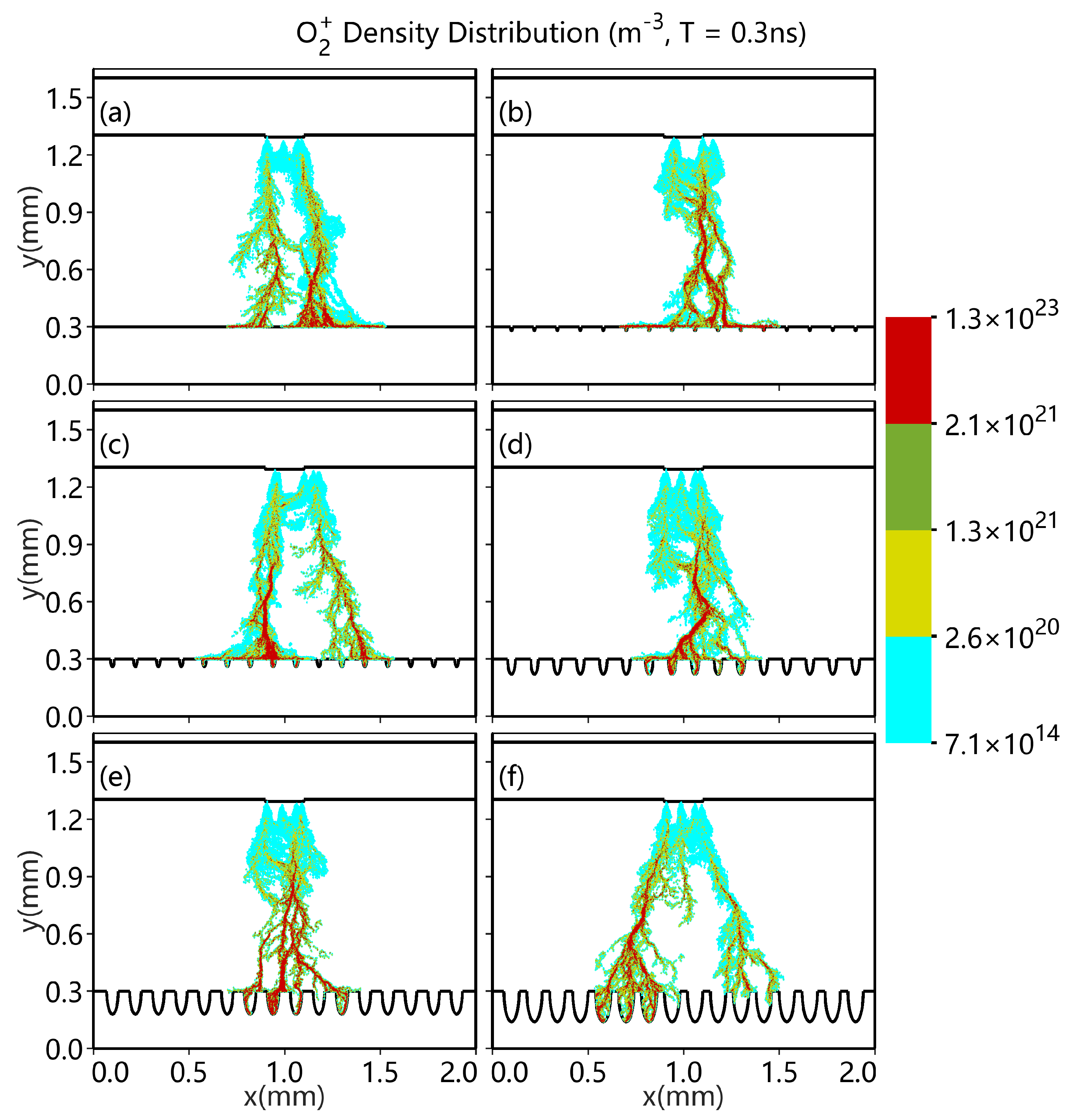
3.1. Different Shapes of Catalytic Pores
3.2. Different Numbers of Catalytic Pores
3.3. Different Catalytic Pore Sizes
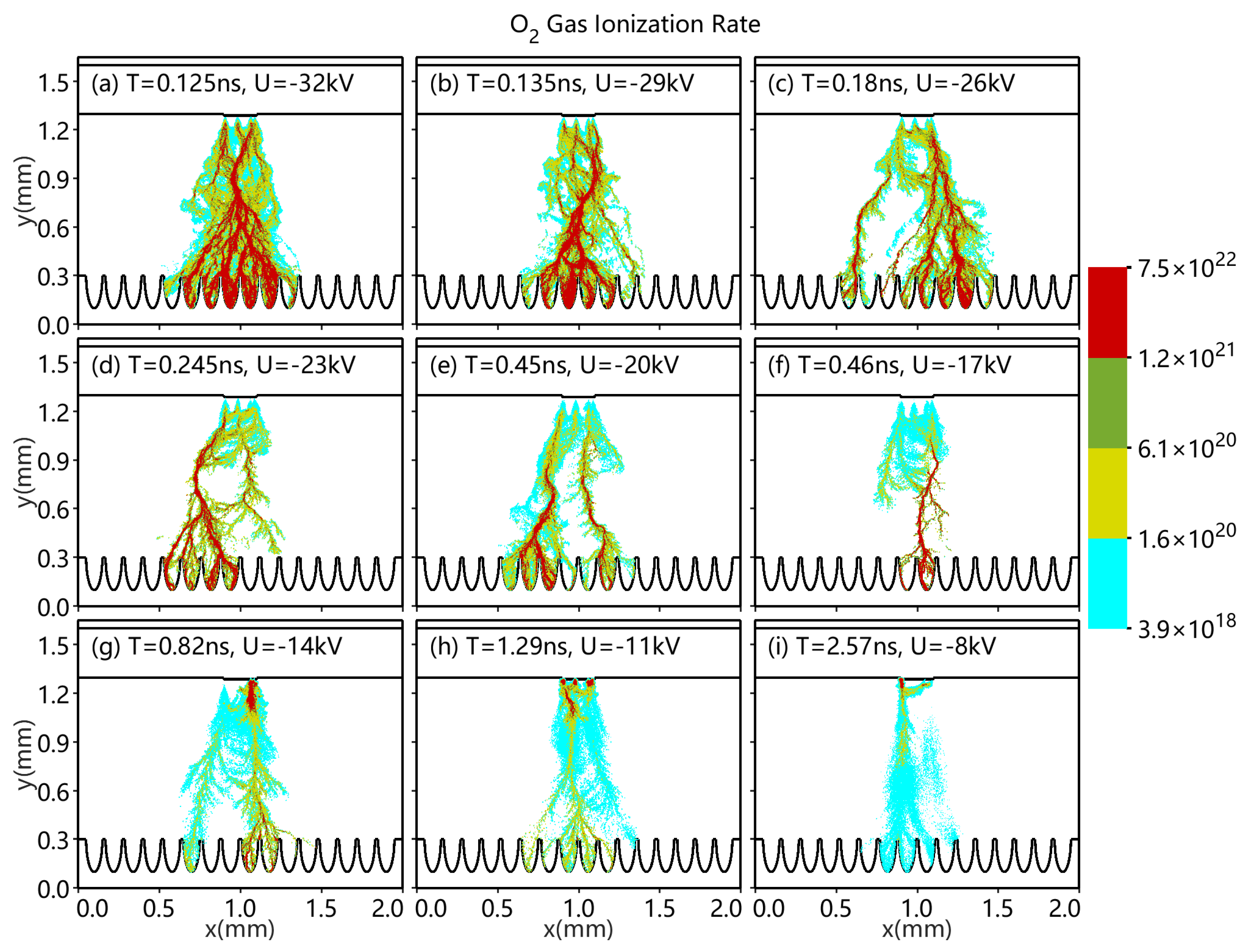
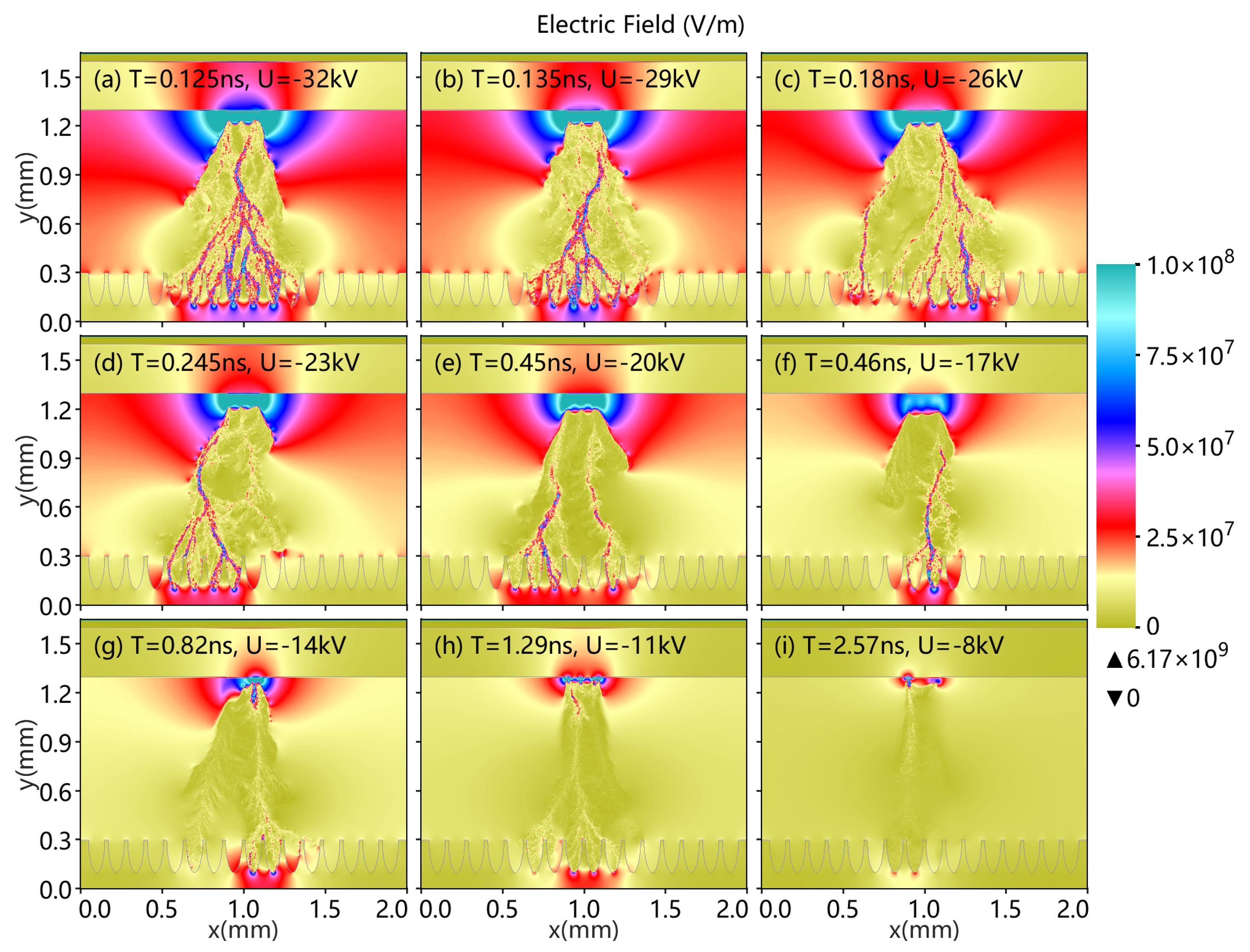
3.4. Different Applications of Top Voltage
3.5. Effect of Lateral Voltage
- (1)
- As the number of pores increases, the discharge enhancement phenomenon becomes increasingly distinct.
- (2)
- As the pore size increases from 0 × 0 mm to 0.08 × 0.16 mm, the surface and volume discharges are more easily distinguished.
- (3)
- As the top applied voltage increases from −8 to −32 kV, the surface and volume discharge enhancement phenomena become apparent.
- (4)
- When the top voltage is −20 kV, as the left and right voltages (of the same size) increase from −5 to −30 kV, streamer propagation is restricted within a narrow channel.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DBD | dielectric barrier discharge |
| PIC | particle-in-cell |
| MCC | Monte Carlo collision |
| VOC | volatile organic compound |
| AC | alternating current |
| ES | electrostatic |
| CFL | Courant-Friedrichs-Lewy |
References
- Neyts, E.C.; Bogaerts, A. Understanding Plasma Catalysis through Modelling and Simulation—A Review. J. Phys. D Appl. Phys. 2014, 47, 224010. [Google Scholar] [CrossRef]
- Van Durme, J.; Dewulf, J.; Leys, C.; Van Langenhove, H. Combining Non-Thermal Plasma with Heterogeneous Catalysis in Waste Gas Treatment: A Review. Appl. Catal. B Environ. 2008, 78, 324–333. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.L.; Lee, H.M.; Chen, S.H.; Chao, Y.; Chang, M.B. Review of Plasma Catalysis on Hydrocarbon Reforming for Hydrogen Production–Interaction, Integration, and Prospects. Appl. Catal. B Environ. 2008, 85, 1–9. [Google Scholar] [CrossRef]
- Neyts, E.C.; Ostrikov, K.K.; Sunkara, M.K.; Bogaerts, A. Plasma Catalysis: Synergistic Effects at the Nanoscale. Chem. Rev. 2015, 115, 13408–13446. [Google Scholar] [CrossRef]
- Holzer, F.; Roland, U.; Kopinke, F.D. Combination of Non-Thermal Plasma and Heterogeneous Catalysis for Oxidation of Volatile Organic Compounds: Part 1. Accessibility of the Intra-Particle Volume. Appl. Catal. B Environ. 2002, 38, 163–181. [Google Scholar] [CrossRef]
- Holzer, F.; Kopinke, F.D.; Roland, U. Influence of Ferroelectric Materials and Catalysts on the Performance of Non-Thermal Plasma (NTP) for the Removal of Air Pollutants. Plasma Chem. Plasma Process. 2005, 25, 595–611. [Google Scholar] [CrossRef]
- Whitehead, J.C. Plasma–Catalysis: The Known Knowns, the Known Unknowns and the Unknown Unknowns. J. Phys. D Appl. Phys. 2016, 49, 243001. [Google Scholar] [CrossRef]
- Zhang, Q.Z.; Wang, W.Z.; Bogaerts, A. Importance of Surface Charging during Plasma Streamer Propagation in Catalyst Pores. Plasma Sources Sci. Technol. 2018, 27, 065009. [Google Scholar] [CrossRef] [Green Version]
- Babaeva, N.Y.; Kushner, M.J. Self-Organization of Single Filaments and Diffusive Plasmas during a Single Pulse in Dielectric-Barrier Discharges. Plasma Sources Sci. Technol. 2014, 23, 065047. [Google Scholar] [CrossRef]
- Fan, W.L.; Sheng, Z.M.; Zhong, X.X.; Wang, W.M.; Li, Y.T.; Zhang, J. Particle Simulation of Filamentary Structure Formation in Dielectric Barrier Discharge. Appl. Phys. Lett. 2013, 102, 094103. [Google Scholar] [CrossRef]
- Fan, W.L.; Sheng, Z.M.; Liu, F.C.; Zhong, X.X.; Dong, L.F. Mechanisms of Fine Structure Formation in Dielectric Barrier Discharges. Phys. Plasmas 2018, 25, 023502. [Google Scholar] [CrossRef] [Green Version]
- Kushner, M.J. Modeling of Microdischarge Devices: Pyramidal Structures. J. Appl. Phys. 2004, 95, 846–859. [Google Scholar] [CrossRef] [Green Version]
- Bruggeman, P.; Brandenburg, R. Atmospheric Pressure Discharge Filaments and Microplasmas: Physics, Chemistry and Diagnostics. J. Phys. D Appl. Phys. 2013, 46, 464001. [Google Scholar] [CrossRef]
- Nikandrov, D.; Tsendin, L.D. Low-Frequency Dielectric-Barrier Discharge in the Townsend Mode. Tech. Phys. 2005, 50, 1284–1294. [Google Scholar] [CrossRef]
- Luque, A.; Ebert, U. Density Models for Streamer Discharges: Beyond Cylindrical Symmetry and Homogeneous Media. J. Comput. Phys. 2012, 231, 904–918. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, J.C. Plasma Catalysis: A Solution for Environmental Problems. Pure Appl. Chem. 2010, 82, 1329–1336. [Google Scholar] [CrossRef]
- Kim, H.H.; Ogata, A.; Futamura, S. Oxygen Partial Pressure-Dependent Behavior of Various Catalysts for the Total Oxidation of VOCs Using Cycled System of Adsorption and Oxygen Plasma. Appl. Catal. B Environ. 2008, 79, 356–367. [Google Scholar] [CrossRef]
- Morent, R.; Leys, C.; Dewulf, J.; Neirynck, D.; Durme, J.; Van Langenhove, H. DC-Excited Non-Thermal Plasmas for VOC Abatement. J. Adv. Oxid. Technol. 2007, 10, 127–136. [Google Scholar]
- Kogelschatz, U. Collective Phenomena in Volume and Surface Barrier Discharges. J. Phys. Conf. Ser. 2010, 257, 012015. [Google Scholar] [CrossRef] [Green Version]
- Narengerile; Watanabe, T. Acetone Decomposition by Water Plasmas at Atmospheric Pressure. Chem. Eng. Sci. 2012, 69, 296–303. [Google Scholar] [CrossRef]
- Van Durme, J.; Dewulf, J.; Sysmans, W.; Leys, C.; Van Langenhove, H. Efficient Toluene Abatement in Indoor Air by a Plasma Catalytic Hybrid System. Appl. Catal. B Environ. 2007, 74, 161–169. [Google Scholar] [CrossRef]
- Koo, I.G.; Choi, M.Y.; Kim, J.H.; Cho, J.H.; Lee, W.M. Microdischarge in Porous Ceramics with Atmospheric Pressure High Temperature H2O/SO2 Gas Mixture and Its Application for Hydrogen Production. Jpn. J. Appl. Phys. 2008, 47, 4705–4709. [Google Scholar] [CrossRef]
- Roland, U.; Holzer, F.; Kopinke, F.D. Combination of Non-Thermal Plasma and Heterogeneous Catalysis for Oxidation of Volatile Organic Compounds: Part 2. Ozone decomposition and deactivation of λ-Al2O3. Appl. Catal. B Environ. 2005, 58, 217–226. [Google Scholar] [CrossRef]
- Hensel, K.; Matsui, Y.; Katsura, S.; Mizuno, A. Generation of Microdischarges in Porous Materials. Czechoslov. J. Phys. 2004, 54, C683. [Google Scholar] [CrossRef]
- Hensel, K.; Katsura, S.; Mizuno, A. DC Microdischarges inside Porous Ceramics. IEEE Trans. Plasma Sci. 2005, 33, 574–575. [Google Scholar] [CrossRef]
- Hensel, K.; Martišovitš, V.; Machala, Z.; Janda, M.; Leštinský, M.; Tardiveau, P.; Mizuno, A. Electrical and Optical Properties of AC Microdischarges in Porous Ceramics. Plasma Process. Polym. 2007, 4, 682–693. [Google Scholar] [CrossRef]
- Hensel, K. Microdischarges in Ceramic Foams and Honeycombs. Eur. Phys. J. D 2009, 54, 141–148. [Google Scholar] [CrossRef]
- Trinh, H.Q.; Mok, Y.S. Plasma-Catalytic Oxidation of Acetone in Annular Porous Monolithic Ceramic- Supported Catalysts. Chem. Eng. J. 2014, 251, 199–206. [Google Scholar] [CrossRef]
- Kameshima, S.; Mizukami, R.; Yamazaki, T.; Prananto, L.A.; Nozaki, T. Interfacial Reactions between DBD and Porous Catalyst in Dry Methane Reforming. J. Phys. D Appl. Phys. 2018, 51, 114006. [Google Scholar] [CrossRef]
- Wang, M.C.; Kunhardt, E.E. Streamer Dynamics. Phys. Rev. A 1990, 42, 2366. [Google Scholar] [CrossRef]
- Kulikovsky, A.A. Two-Dimensional Simulation of the Positive Streamer in N2 between Parallel-Plate Electrodes. J. Phys. D Appl. Phys. 1995, 28, 2483–2493. [Google Scholar] [CrossRef]
- Kang, W.S.; Park, J.M.; Kim, Y.; Hong, S.H. Numerical Study on Influences of Barrier Arrangements on Dielectric Barrier Discharge Characteristics. IEEE Trans. Plasma Sci. 2003, 31, 504–510. [Google Scholar] [CrossRef]
- Montijn, C.; Hundsdorfer, W.; Ebert, U. An Adaptive Grid Refinement Strategy for the Simulation of Negative Streamers. J. Comput. Phys. 2006, 219, 801–835. [Google Scholar] [CrossRef] [Green Version]
- Chanrion, O.; Neubert, T. A PIC-MCC Code for Simulation of Streamer Propagation in Air. J. Comput. Phys. 2008, 227, 7222–7245. [Google Scholar] [CrossRef]
- Luque, A.; Ratushnaya, V.; Ebert, U. Positive and Negative Streamers in Ambient Air: Modelling Evolution and Velocities. J. Phys. D Appl. Phys. 2008, 41, 234005. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.R.; Van Laer, K.; Neyts, E.C.; Bogaerts, A. Can Plasma Be Formed in Catalyst Pores? A Modeling Investigation. Appl. Catal. B Environ. 2016, 185, 56–67. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Neyts, E.C.; Bogaerts, A. Influence of the Material Dielectric Constant on Plasma Generation inside Catalyst Pores. J. Phys. Chem. C 2016, 120, 25923–25934. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Neyts, E.C.; Bogaerts, A. Enhancement of Plasma Generation in Catalyst Pores with Different Shapes. Plasma Sources Sci. Technol. 2018, 27, 055008. [Google Scholar] [CrossRef]
- Li, C.; Ebert, U.; Hundsdorfer, W. 3D Hybrid Computations for Streamer Discharges and Production of Run-Away Electrons. J. Phys. D Appl. Phys. 2009, 42, 202003. [Google Scholar] [CrossRef] [Green Version]
- Soria-Hoyo, C.; Pontiga, F.; Castellanos, A. Two Dimensional Numerical Simulation of Gas Discharges: Comparison between Particle-in-Cell and FCT Techniques. J. Phys. D Appl. Phys. 2008, 41, 205206. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, J.L.; Yi, L.; Wang, H.Y.; Jiang, W. Electron Energy Probability Function Modulation with External Electron Beam in Capacitive Coupled Radio Frequency Discharges. Plasma Process. Polym. 2018, 15, 1700169. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.Y.; Zhang, Y.R.; Bogaerts, A. Formation of Microdischarges inside a Mesoporous Catalyst in Dielectric Barrier Discharge Plasmas. Plasma Sources Sci. Technol. 2017, 26, 054002. [Google Scholar] [CrossRef]
- Gu, J.G.; Zhang, Y.; Gao, M.X.; Wang, H.Y.; Zhang, Q.Z.; Yi, L.; Jiang, W. Enhancement of Surface Discharge in Catalyst Pores in Dielectric Barrier Discharges. J. Appl. Phys. 2019, 125, 153303. [Google Scholar] [CrossRef]
- Zhang, Q.Z.; Bogaerts, A. Propagation of a Plasma Streamer in Catalyst Pores. Plasma Sources Sci. Technol. 2018, 27, 035009. [Google Scholar] [CrossRef]
- Matyash, K.; Schneider, R.; Taccogna, F.; Hatayama, A.; Longo, S.; Capitelli, M.; Tskhakaya, D.; Bronold, F.X. Particle in Cell Simulation of Low Temperature Laboratory Plasmas. Contrib. Plasma Phys. 2007, 47, 595–634. [Google Scholar] [CrossRef]
- Wang, H.Y.; Jiang, W.; Sun, P.; Kong, L.B. On the Energy Conservation Electrostatic Particle-in-Cell/Monte Carlo Simulation: Benchmark and Application to the Radio Frequency Discharges. Chin. Phys. B 2014, 23, 035204. [Google Scholar] [CrossRef] [Green Version]
- Nieter, C.; Cary, J.R. VORPAL: A Versatile Plasma Simulation Code. J. Comput. Phys. 2004, 196, 448–473. [Google Scholar] [CrossRef]
- Tech-X Corporation. VSim Reference Manual. 2018. Available online: www.txcorp.com (accessed on 30 January 2018).
- Pearlman, M.; Browning, J. Simulation of a Distributed Cathode in a Linear-Format Crossed-Field Amplifier. IEEE Trans. Plasma Sci. 2018, 46, 2497–2504. [Google Scholar] [CrossRef]
- Lécz, Z.; Andreev, A.; Konoplev, I.; Seryi, A.; Smith, J. Trains of Electron Micro-Bunches in Plasma Wake-Field Acceleration. Plasma Phys. Control. Fusion 2018, 60, 075012. [Google Scholar] [CrossRef]
- Zhang, H.; Konoplev, I.V.; Doucas, G.; Smith, J. Concept of a Tunable Source of Coherent THz Radiation Driven by a Plasma Modulated Electron Beam. Phys. Plasmas 2018, 25, 043111. [Google Scholar] [CrossRef]
- Likhanskii, A. Particle-in-Cell Modeling of the Pulsed DBD Plasma Actuator. In Proceedings of the 40th Fluid Dynamics Conference and Exhibit, Chicago, IL, USA, 28 June–1 July 2010. [Google Scholar] [CrossRef]
- Pancheshnyi, S.; Biagi, S.; Bordage, M.C.; Hagelaar, G.J.M.; Morgan, W.L.; Phelps, A.V.; Pitchford, L.C. The LXCat Project: Electron Scattering Cross Sections and Swarm Parameters for Low Temperature Plasma Modeling. Chem. Phys. 2012, 398, 148–153. [Google Scholar] [CrossRef]
- Plasma Data Exchange Project. Itikawa Database, Morgan Database. Retrieved on 11 June 2015. Available online: www.lxcat.net (accessed on 11 June 2015).
- Zhang, Y.; Wang, H.Y.; Jiang, W.; Bogaerts, A. Two-Dimensional Particle-in Cell/Monte Carlo Simulations of a Packed-Bed Dielectric Barrier Discharge in Air at Atmospheric Pressure. New J. Phys. 2015, 17, 083056. [Google Scholar] [CrossRef] [Green Version]
- Levko, D.; Pachuilo, M.; Raja, L.L. Particle-in-cell modeling of streamer branching in CO2 gas. J. Phys. D Appl. Phys. 2014, 50, 354004. [Google Scholar] [CrossRef]
- Ayrault, C.; Barrault, J.; Blin-Simiand, N.; Jorand, F.; Pasquiers, S.; Rousseau, A.; Tatibouët, J.M. Oxidation of 2-Heptanone in Air by a DBD-Type Plasma Generated within a Honeycomb Monolith Supported Pt-Based Catalyst. Catal. Today 2004, 89, 75–81. [Google Scholar] [CrossRef]
- Veerapandian, S.K.P.; Leys, C.; De Geyter, N.; Morent, R. Abatement of VOCs Using Packed Bed Non-Thermal Plasma Reactors: A Review. Catalysts 2017, 7, 113. [Google Scholar] [CrossRef]
- Sultana, S.; Vandenbroucke, A.M.; Leys, C.; De Geyter, N.; Morent, R. Abatement of VOCs with Alternate Adsorption and Plasma-Assisted Regeneration: A Review. Catalysts 2015, 5, 718–746. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, Y.; Wang, H.; Guo, B.; Zhang, Q.; Bogaerts, A. Mode Transition of Filaments in Packed-Bed Dielectric Barrier Discharges. Catalysts 2018, 8, 248. [Google Scholar] [CrossRef] [Green Version]
- Thoma, C.; Welch, D.R.; Rose, D.V.; Zimmerman, W.R.; Miller, C.L.; Clark, R.E.; Schwarz, J.; Rambo, P.; Savage, M.E.; Woodworth, J.R. Fully Kinetic Particle-in-Cell Simulations of Triggered Three-Electrode Gas Switches. In Proceedings of the 2013 19th IEEE Pulsed Power Conference (PPC), San Francisco, CA, USA, 16–21 June 2013; pp. 1–6. [Google Scholar] [CrossRef]



| Reaction | Threshold (eV) |
|---|---|
| Electron-impact ionization | |
| e + → e + + e | 12.06 |
| e + → e + + e | 15.58 |
| Electron-impact excitation | |
| e + → e + | 0.98 |
| e + → e + | 1.63 |
| e + → e + O+O | 6.0 |
| e + → e + O+ | 8.4 |
| e + → e + + | 10.0 |
| e + → e + | 6.169 |
| e + → e + | 7.353 |
| e + → e + → e + | 7.362 |
| e + → e + → e + | 8.165 |
| e + → e + | 8.399 |
| e + → e + → e + | 8.549 |
| e + → e + → e + | 8.89 |
| e + → e + N + N | 9.7537 |
| e + → e + | 11.032 |
| Elastic collision | |
| e + → e+ | |
| e + → e+ | |
| Attachment | |
| e + → |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.-G.; Zhao, P.; Zhang, Y.; Wang, H.-Y.; Jiang, W. Discharge Enhancement Phenomenon and Streamer Control in Dielectric Barrier Discharge with Many Pores. Catalysts 2020, 10, 68. https://doi.org/10.3390/catal10010068
Gu J-G, Zhao P, Zhang Y, Wang H-Y, Jiang W. Discharge Enhancement Phenomenon and Streamer Control in Dielectric Barrier Discharge with Many Pores. Catalysts. 2020; 10(1):68. https://doi.org/10.3390/catal10010068
Chicago/Turabian StyleGu, Jian-Guo, Pan Zhao, Ya Zhang, Hong-Yu Wang, and Wei Jiang. 2020. "Discharge Enhancement Phenomenon and Streamer Control in Dielectric Barrier Discharge with Many Pores" Catalysts 10, no. 1: 68. https://doi.org/10.3390/catal10010068





