The Influence of the Support on the Activity of Mn–Fe Catalysts Used for the Selective Catalytic Reduction of NOx with Ammonia
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gao, F.; Tang, X.; Yi, H.; Zhao, S.; Li, C.; Li, J.; Shi, Y.; Meng, X. A Review on Selective Catalytic Reduction of NOx by NH3 over Mn–Based Catalysts at Low Temperatures: Catalysts, Mechanisms, Kinetics and DFT Calculations. Catalysts 2017, 7, 199. [Google Scholar] [CrossRef]
- Yu, J.J.; Cheng, J.; Ma, C.Y.; Wang, H.L.; Li, L.D.; Hao, Z.P.; Xu, Z.P. NO(x) decomposition, storage and reduction over novel mixed oxide catalysts derived from hydrotalcite-like compounds. J. Colloid Interface Sci. 2009, 333, 423–430. [Google Scholar] [CrossRef]
- Forzatti, P. Present status and perspectives in de-NOx SCR catalysis. Appl. Catal. A Gen. 2001, 222, 221–236. [Google Scholar] [CrossRef]
- Rutkowska, M.; Díaz, U.; Palomares, A.E.; Chmielarz, L. Cu and Fe modified derivatives of 2D MWW-type zeolites (MCM-22, ITQ-2 and MCM-36) as new catalysts for DeNO x process. Appl. Catal. B Environ. 2015, 168–169, 531–539. [Google Scholar] [CrossRef]
- Palomares, A.; Prato, J.; Imbert, F.; Corma, A. Catalysts based on tin and beta zeolite for the reduction of NOx under lean conditions in the presence of water. Appl. Catal. B Environ. 2007, 75, 88–94. [Google Scholar] [CrossRef]
- Palomares, A.E.; Franch, C.; Corma, A. Determining the characteristics of a Co-zeolite to be active for the selective catalytic reduction of NOx with hydrocarbons. Catal. Today 2011, 176, 239–241. [Google Scholar] [CrossRef]
- Palomares, A.E.; Prato, J.G.; Corma, A. Co-Exchanged IM5, a Stable Zeolite for the Selective Catalytic Reduction of NO in the Presence of Water and SO2. Ind. Eng. Chem. Res. 2003, 42, 1538–1542. [Google Scholar] [CrossRef]
- Wang, R.; Wu, X.; Zou, C.; Li, X.; Du, Y. NOx Removal by Selective Catalytic Reduction with Ammonia over a Hydrotalcite-Derived NiFe Mixed Oxide. Catalysts 2018, 8, 834. [Google Scholar] [CrossRef] [Green Version]
- Qi, G.; Yang, R.T. Low-temperature selective catalytic reduction of NO with NH3 over iron and manganese oxides supported on titania. Appl. Catal. B Environ. 2003, 44, 217–225. [Google Scholar] [CrossRef]
- Forzatti, P.; Nova, I.; Tronconi, E. Enhanced NH3 selective catalytic reduction for NOx abatement. Angew. Chem. Int. Ed. Engl. 2009, 48, 8366–8368. [Google Scholar] [CrossRef] [PubMed]
- Gillot, S.; Tricot, G.; Vezin, H.; Dacquin, J.-P.; Dujardin, C.; Granger, P. Induced effect of tungsten incorporation on the catalytic properties of CeVO4 systems for the selective reduction of NOx by ammonia. Appl. Catal. B Environ. 2018, 234, 318–328. [Google Scholar] [CrossRef]
- Krishnan, A.T.; Boehman, A.L. Selective catalytic reduction of nitric oxide with ammonia at low temperatures. Appl. Catal. B Environ. 1998, 18, 189–198. [Google Scholar] [CrossRef]
- Li, J.; Chang, H.; Ma, L.; Hao, J.; Yang, R.T. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts—A review. Catal. Today 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Boningari, T.; Smirniotis, P.G. Impact of nitrogen oxides on the environment and human health: Mn-based materials for the NOx abatement. Curr. Opin. Chem. Eng. 2016, 13, 133–141. [Google Scholar] [CrossRef]
- Putluru, S.S.R.; Schill, L.; Jensen, A.D.; Siret, B.; Tabaries, F.; Fehrmann, R. Mn/TiO2 and Mn–Fe/TiO2 catalysts synthesized by deposition precipitation—Promising for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B Environ. 2015, 165, 628–635. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kuśtrowski, P.; Dziembaj, R.; Cool, P.; Vansant, E.F. Catalytic performance of various mesoporous silicas modified with copper or iron oxides introduced by different ways in the selective reduction of NO by ammonia. Appl. Catal. B Environ. 2006, 62, 369–380. [Google Scholar] [CrossRef]
- Chmielarz, L.; Dziembaj, R.; Grzybek, T.; Klinik, J.; Łojewski, T.; Olszewska, D.; Papp, H. Pillared smectite modified with carbon and manganese as catalyst for SCR of NOx with NH3. Part I. General characterization and catalyst screening. Catal. Lett. 2000, 68, 95–100. [Google Scholar] [CrossRef]
- Gao, Y.; Luan, T.; Zhang, S.; Jiang, W.; Feng, W.; Jiang, H. Comprehensive Comparison between Nanocatalysts of Mn−Co/TiO2 and Mn−Fe/TiO2 for NO Catalytic Conversion: An Insight from Nanostructure, Performance, Kinetics, and Thermodynamics. Catalysts 2019, 9, 175. [Google Scholar] [CrossRef] [Green Version]
- Song, C.; Zhang, L.; Li, Z.; Lu, Y.; Li, K. Co-Exchange of Mn: A Simple Method to Improve Both the Hydrothermal Stability and Activity of Cu–SSZ-13 NH3–SCR Catalysts. Catalysts 2019, 9, 455. [Google Scholar] [CrossRef] [Green Version]
- Paolucci, C.; Di Iorio, J.R.; Ribeiro, F.H.; Gounder, R.; Schneider, W.F. Catalysis Science of NOx Selective Catalytic Reduction With Ammonia Over Cu-SSZ-13 and Cu-SAPO-34. In Advances in Catalysis; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–107. [Google Scholar] [CrossRef]
- Wu, Z.; Jiang, B.; Liu, Y. Effect of transition metals addition on the catalyst of manganese/titania for low-temperature selective catalytic reduction of nitric oxide with ammonia. Appl. Catal. B Environ. 2008, 79, 347–355. [Google Scholar] [CrossRef]
- Putluru, S.S.R.; Schill, L.; Jensen, A.D.; Fehrmann, R.S.N. Selective Catalytic Reduction of NOx with NH3 on Cu-, Fe-, and Mn-Zeolites Prepared by Impregnation: Comparison of Activity and Hydrothermal Stability. J. Chem. 2018, 2018, 8614747. [Google Scholar] [CrossRef] [Green Version]
- Thirupathi, B.; Smirniotis, P.G. Nickel-doped Mn/TiO2 as an efficient catalyst for the low-temperature SCR of NO with NH3: Catalytic evaluation and characterizations. J. Catal. 2012, 288, 74–83. [Google Scholar] [CrossRef]
- Peña, D.A.; Uphade, B.S.; Smirniotis, P.G. TiO2-supported metal oxide catalysts for low-temperature selective catalytic reduction of NO with NH3I. Evaluation and characterization of first row transition metals. J. Catal. 2004, 221, 421–431. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T.; Chang, R. MnOx-CeO2 mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B Environ. 2004, 51, 93–106. [Google Scholar] [CrossRef]
- Roy, S.B.V.; Hegde, M.S.; Madras, G. Low-Temperature Selective Catalytic Reduction of NO with NH3 over Ti0.9M0.1O2-δ (M ) Cr, Mn, Fe, Co, Cu). J. Phys. Chem. C 2008, 112, 6002–6012. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, Z.; Chen, M.; Zhang, Z.; Shangguan, W. Promotion effect of tungsten and iron co-addition on the catalytic performance of MnOx/TiO2 for NH3-SCR of NOx. Fuel 2017, 210, 783–789. [Google Scholar] [CrossRef]
- Husnain, N.; Wang, E.; Li, K.; Anwar, M.T.; Mehmood, A.; Gul, M.; Li, D.; Mao, J. Iron oxide-based catalysts for low-temperature selective catalytic reduction of NOx with NH3. Rev. Chem. Eng. 2019, 35, 239–264. [Google Scholar] [CrossRef]
- Wang, X.; Wu, S.; Zou, W.; Yu, S.; Gui, K.; Dong, L. Fe-Mn/Al2O3 catalysts for low temperature selective catalytic reduction of NO with NH3. Chin. J. Catal. 2016, 37, 1314–1323. [Google Scholar] [CrossRef]
- Thirupathi, B.; Smirniotis, P.G. Co-doping a metal (Cr, Fe, Co, Ni, Cu, Zn, Ce, and Zr) on Mn/TiO2 catalyst and its effect on the selective reduction of NO with NH3 at low-temperatures. Appl. Catal. B Environ. 2011, 110, 195–206. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kwon, H.J.; Heo, I.; Nam, I.-S.; Cho, B.K.; Choung, J.W.; Cha, M.-S.; Yeo, G.K. Mn–Fe/ZSM5 as a low-temperature SCR catalyst to remove NOx from diesel engine exhaust. Appl. Catal. B Environ. 2012, 126, 9–21. [Google Scholar] [CrossRef]
- Huang, J.; Tong, Z.; Huang, Y.; Zhang, J. Selective catalytic reduction of NO with NH3 at low temperatures over iron and manganese oxides supported on mesoporous silica. Appl. Catal. B Environ. 2008, 78, 309–314. [Google Scholar] [CrossRef]
- Li, J.; Yang, C.; Zhang, Q.; Li, Z.; Huang, W. Effects of Fe addition on the structure and catalytic performance of mesoporous Mn/Al–SBA-15 catalysts for the reduction of NO with ammonia. Catal. Commun. 2015, 62, 24–28. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, F.; Li, H.; Yang, Q.; Wang, L.; Li, X. Low-Temperature Selective Catalytic Reduction of NOx with NH3 over Fe-Mn Mixed-Oxide Catalysts Containing Fe3Mn3O8 Phase. Ind. Eng. Chem. Res. 2012, 51, 202–212. [Google Scholar] [CrossRef]
- Palomares, A.E.; Prato, J.G.; Corma, A. A new active zeolite structure for the selective catalytic reduction (SCR) of nitrogen oxides: ITQ7 zeolite the influence of NO2 on this reaction. Catal. Today 2002, 75, 367–371. [Google Scholar] [CrossRef]
- Jeong, N.C.; Lee, J.S.; Tae, E.L.; Lee, Y.J.; Yoon, K.B. Acidity scale for metal oxides and Sanderson’s electronegativities of lanthanide elements. Angew. Chem. Int. Ed. Engl. 2008, 47, 10128–10132. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Lan, B.; Yu, L.; Ye, F.; Song, W.; He, J.; Diao, G.; Zheng, Y. Manganese oxides with different crystalline structures: Facile hydrothermal synthesis and catalytic activities. Mater. Lett. 2012, 86, 18–20. [Google Scholar] [CrossRef]
- Deng, S.; Zhuang, K.; Xu, B.; Ding, Y.; Yu, L.; Fan, Y. Promotional effect of iron oxide on the catalytic properties of Fe–MnOx/TiO2 (anatase) catalysts for the SCR reaction at low temperatures. Catal. Sci. Technol. 2016, 6, 1772–1778. [Google Scholar] [CrossRef]
- Fang, N.; Guo, J.; Shu, S.; Luo, H.; Chu, Y.; Li, J. Enhancement of low-temperature activity and sulfur resistance of Fe 0.3 Mn 0.5 Zr 0.2 catalyst for NO removal by NH3-SCR. Chem. Eng. J. 2017, 325, 114–123. [Google Scholar] [CrossRef]
- Stobbe, E.R.; de Boer, B.A.; Geus, J.W. The reduction and oxidation behaviour of manganese oxides. Catal. Today 1999, 47, 161–167. [Google Scholar] [CrossRef]
- Fiorenza, R.; Spitaleri, L.; Gulino, A.; Scirè, S. Ru-Pd Bimetallic Catalysts Supported on CeO2-MnOx Oxides as Efficient Systems for H2 Purification through CO Preferential Oxidation. Catalysts 2018, 8, 203. [Google Scholar] [CrossRef] [Green Version]
- Tu, Y.; Luo, J.; Meng, M.; Wang, G.; He, J. Ultrasonic Ultrasonic-assisted synthesis of highly active catalyst Au/MnOx–CeO2 used for the preferential oxidation of CO in H2-rich stream. Int. J. Hydrog. Energy 2009, 34, 3743–3754. [Google Scholar] [CrossRef]
- Corma, A. From Microporous to Mesoporous Molecular Sieve Materials and Their Use in Catalysis. Chem. Rev. 1997, 97, 2373–2419. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.M.R.; Bisio, C.; Gatti, G.; Marchese, L.; Pastore, H.O. Physicochemical Characterization and Surface Acid Properties of Mesoporous [Al]-SBA-15 Obtained by Direct Synthesis. Langmuir 2010, 26, 5791–5800. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Han, Y.; Zou, Y.; Song, J.; Zhao, L.; Di, Y.; Liu, S.; Xiao, F. Synthesis of Heteroatom Substituted SBA-15 by the “pH-Adjusting” Method. Chem. Mater. 2004, 16, 486–492. [Google Scholar] [CrossRef]
- Chuah, G.K.; Liu, S.H.; Jaenicke, S.; Li, J. High surface area zirconia by digestion of zirconium propoxide at different pH. Micropor. Mesopor. Mat. 2000, 39, 381–392. [Google Scholar] [CrossRef]
- Anne Galarneau, M.N.; Guenneau, F.; di Renzo, F.; Gedeon, A. Understanding the Stability in Water of Mesoporous SBA-15 and MCM-41. J. Phys. Chem. C 2007, 111, 8268–8277. [Google Scholar] [CrossRef]
- Chen, C.; Li, H.; Davis, M.E. Studies on mesoporous materials I. Synthesis and characterization of MCM-41. Microp. Mater. 1993, 2, 17–26. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Q.; Yang, J.; Li, C. Synthesis of mesoporous aluminosilicates with low Si/Al ratios using a single-source molecular precursor under acidic conditions. J. Porous Mater. 2006, 13, 187–193. [Google Scholar] [CrossRef]

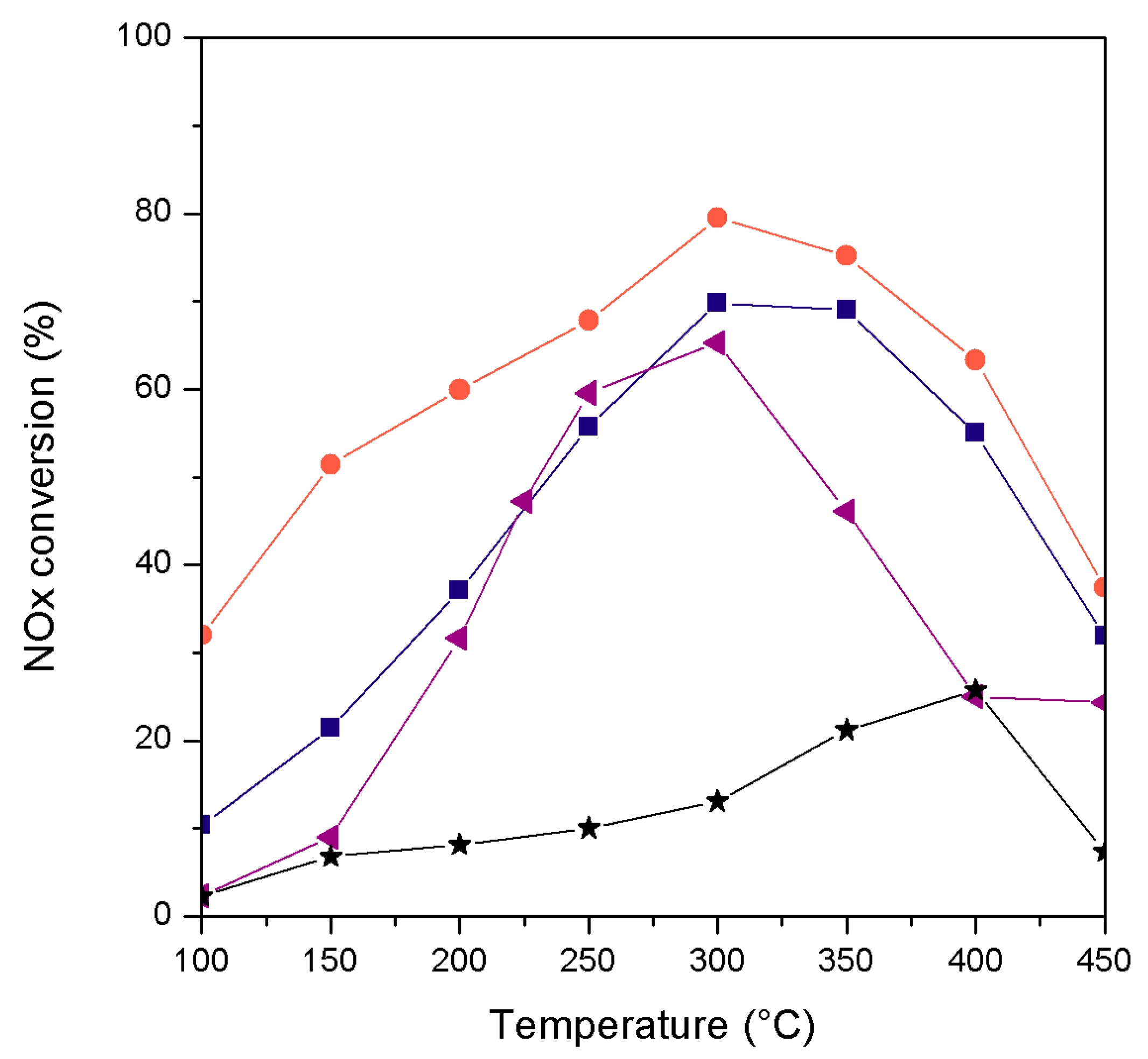


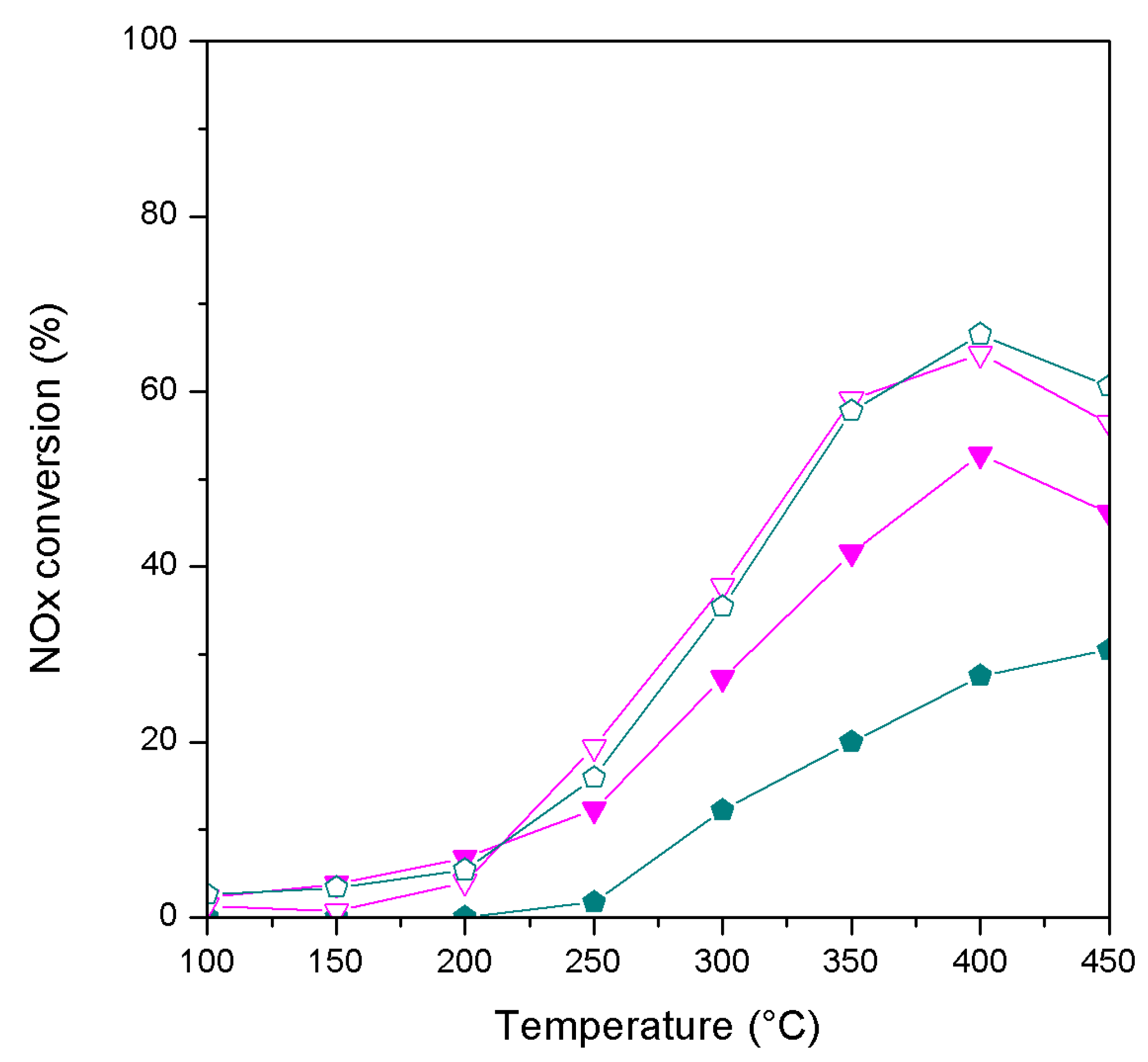
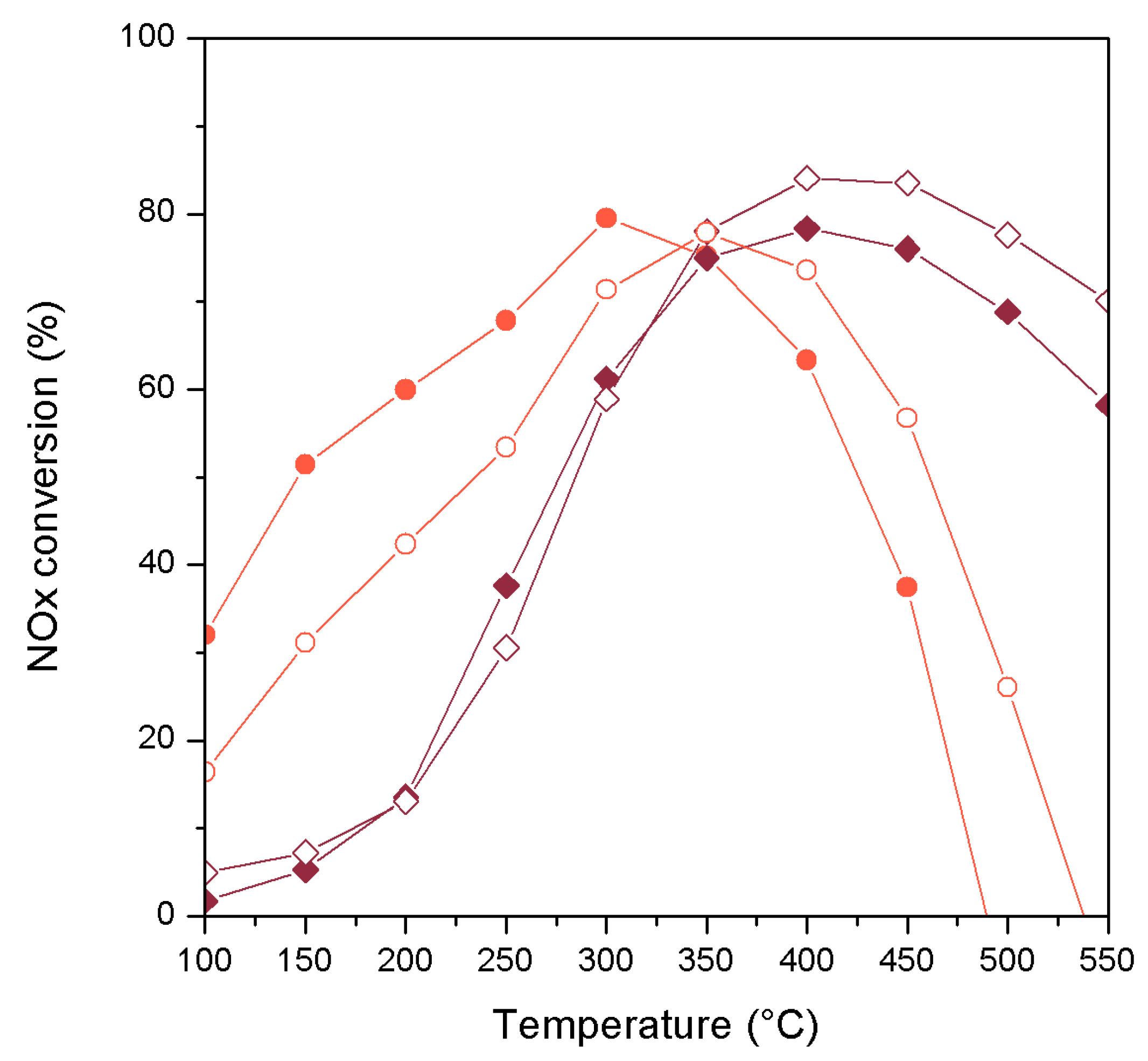
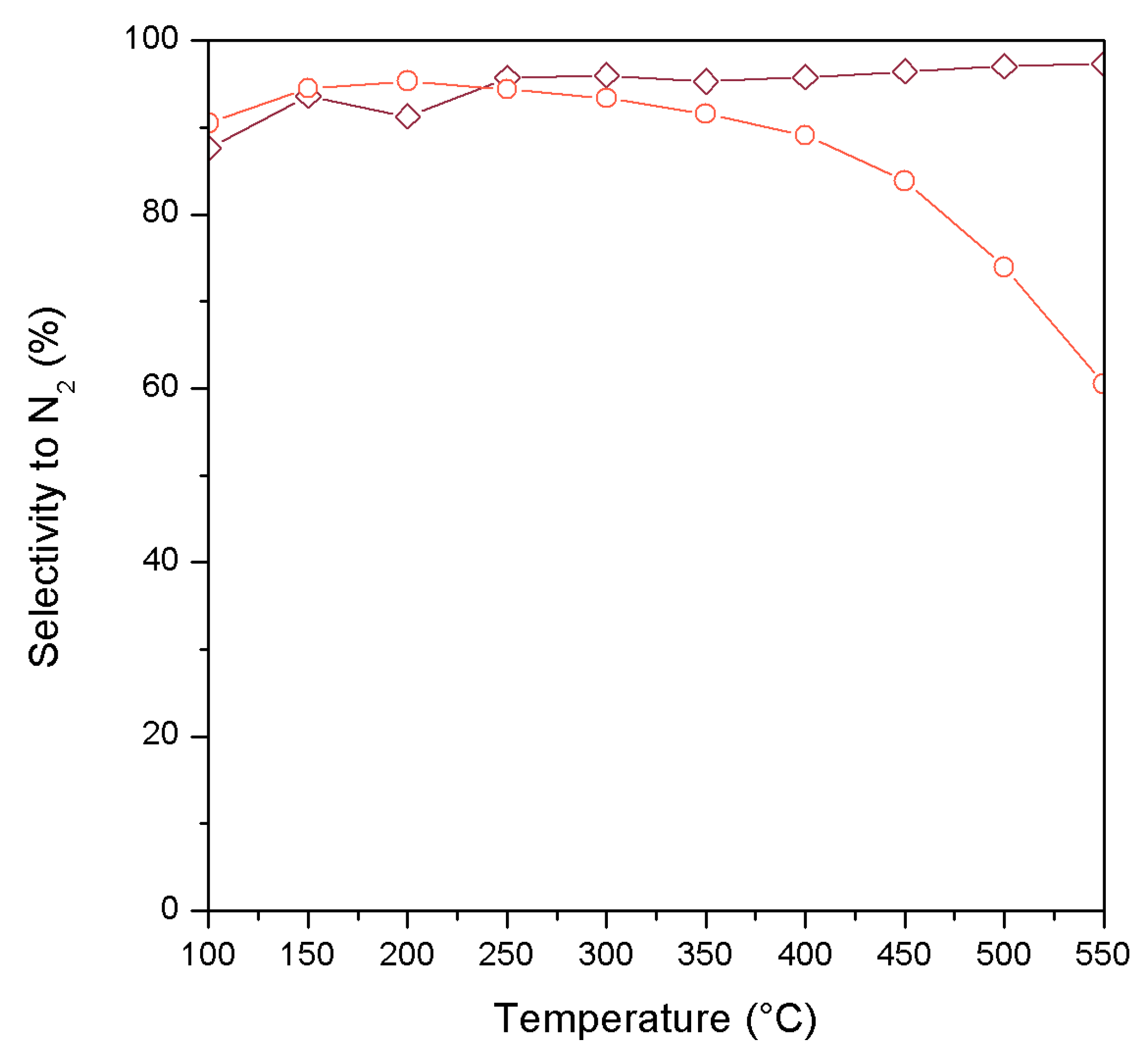
| Catalyst | wt. % Mn | wt. % Fe | Surface Area (m2/g) |
|---|---|---|---|
| TiO2 | -- | -- | 90.8 |
| Fe/TiO2 | -- | 1.79 | 87.6 |
| Mn/TiO2 | 3.75 | -- | 88.1 |
| Mn–Fe/TiO2 | 3.68 | 2.21 | 78.8 |
| ZrO2 | -- | -- | 96.2 |
| Mn–Fe/ZrO2 | 4.03 | 2.17 | 52.8 |
| Al2O3 | -- | -- | 212.8 |
| Mn–Fe/Al2O3 | 3.42 | 1.95 | 187.7 |
| MgO | -- | -- | 171.2 |
| Mn–Fe/MgO | 4.11 | 1.83 | 132.2 |
| MCM-41 | -- | -- | 924 |
| Mn–Fe/MCM-41 | 4.43 | 2.14 | 522.9 |
| Al-MCM-41 | -- | -- | 1020.2 |
| Mn–Fe/Al-MCM-41 | 4.80 | 1.74 | 989.4 |
| SBA-15 | -- | -- | 903.6 |
| Mn–Fe/SBA-15 | 4.85 | 2.03 | 475.9 |
| Al-SBA-15 | -- | -- | 967.3 |
| Mn–Fe/Al-SBA-15 | 3.89 | 2.08 | 712.4 |
| BEA | -- | -- | 581.2 |
| Mn–Fe/BEA | 4.56 | 2.03 | 521.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Hernández, I.; Mengual, J.; Palomares, A.E. The Influence of the Support on the Activity of Mn–Fe Catalysts Used for the Selective Catalytic Reduction of NOx with Ammonia. Catalysts 2020, 10, 63. https://doi.org/10.3390/catal10010063
López-Hernández I, Mengual J, Palomares AE. The Influence of the Support on the Activity of Mn–Fe Catalysts Used for the Selective Catalytic Reduction of NOx with Ammonia. Catalysts. 2020; 10(1):63. https://doi.org/10.3390/catal10010063
Chicago/Turabian StyleLópez-Hernández, Irene, Jesús Mengual, and Antonio Eduardo Palomares. 2020. "The Influence of the Support on the Activity of Mn–Fe Catalysts Used for the Selective Catalytic Reduction of NOx with Ammonia" Catalysts 10, no. 1: 63. https://doi.org/10.3390/catal10010063





