Catalytic Reactions on Model Gold Surfaces: Effect of Surface Steps and of Surface Doping
Abstract
: The adsorption energies and the activation energy barriers for a series of reactions catalyzed by gold surfaces and obtained theoretically through density functional theory (DFT) based calculations were considered to clarify the role of the low coordinated gold atoms and the role of doping in the catalytic activity of gold. The effect of the surface steps was introduced by comparison of the activation energy barriers and of the adsorption energies on flat gold surfaces such as the Au(111) surface with those on stepped surfaces such as the Au(321) or the Au(110) surfaces. It is concluded that the presence of low coordinated atoms on the latter surfaces increases the adsorption energies of the reactants and decreases the activation energy barriers. Furthermore, the increasing of the adsorption energy of the reaction products can lead to lower overall reaction rates in the presence of low gold coordinated atoms due to desorption limitations. On the other hand, the effect of doping gold surfaces with other transition metal atoms was analyzed using the dissociation reaction of molecular oxygen as a test case. The calculations showed that increasing the silver content in some gold surfaces was related to a considerable increment of the reactivity of bimetallic systems toward the oxygen dissociation. Importantly, that increment in the reactivity was enhanced by the presence of low coordinated atoms in the catalytic surface models considered.1. Introduction
Reactions on gold based catalysts have been intensely studied since the discovery at the end of the eighties that highly dispersed gold nanoparticles on a transition metal oxide support catalyze the oxidation of carbon monoxide, even at temperatures as low as −70 °C [1]. Due to the interesting practical applications of this reaction, a considerable number of studies focused in the study of metal based catalysts in the last years. For example, the improvements in the performance of the catalysts for this reaction can be used in the development of new CO sensors [2] or in the optimization of the direct methanol fuel cells (DMFC). In DMFCs employing a Pt-Ru/C substrate catalyst, CO appears as an intermediary that is strongly adsorbed on the platinum phase and, hence, it inhibits the oxidation of methanol. Very recently, CO elimination from the catalyst was successfully attained by the addition of gold nanoparticles [3,4]. On the other hand, it was found that gold cations supported on mordenite are inactive for the CO oxidation, being the reaction catalyzed at low temperature by gold clusters (diameter <2 nm) and at high temperature by gold nanoparticles [5]. Furthermore, unsupported gold may exhibit high catalytic activity for CO oxidation; this activity depends on its intrinsic structure (number and size of pores at nanometer scale), conferring a three-dimensional spongy morphology to the catalytic system [6–9].
In summary, the activity of gold based catalysts for CO oxidation seems to be related to the irregularities of the metal particles or films as suggested by Chen and Goodman [10]. Furthermore, at a nanoscale level, the difference between the regions presenting these irregularities and the regions with flat facets relies in the presence or absence of low coordinated atoms, respectively. In other words, the enhancement of the reactivity for the reaction of CO oxidation caused by this type of irregularities on gold based catalysts is very probably related with the presence and concentration of low coordinated metal atoms on the catalytic systems [11,12]. In fact, previous results show the importance of gold low coordinated atoms in the reaction of CO oxidation on gold surfaces [13]. It was found that the oxidation of CO by molecular oxygen on the stepped Au(321) surface occurs through the formation of OCOO 4-atoms species in the region of the surface step (low coordinated atoms) and their decomposition leads to the desorption of carbon dioxide. Interestingly, the formation of carbonates, surface species that were experimental detected [14,15], from co-adsorbed CO and O2 surface molecules was found to occur easily on the catalyst surface [13].
Another important reaction studied on gold-based catalysts is that of oxygen dissociation, a crucial step in many oxidation reactions including the oxidation of CO or methanol. In the oxidation of CO by O2, it is required the breaking of the O–O bond in the latter species and, according to this, several authors focused their works in the investigation of the oxygen deposition or in the investigation of the O2 dissociation on gold surfaces and gold nanoparticles [16–21]. In the case of the methanol oxidation on nanoporous gold [22], residual Ag on the catalyst was suggested to play an important role in the reaction performance, which was corroborated by theoretical work [23].
It is worth mentioning in this work the reactions of NOx oxidation or reduction. These types of reactions are vital in the elimination of NOx species from the automotive exhaust gas emissions due to the dramatic environmental problems caused by these gases [24]. This is of global concern and important efforts are being made to fulfill the pertinent normative implemented [25]. The catalytic reduction of these oxides to molecular nitrogen follows one of two generic schemes: Selective Catalytic Reduction (SCR) [26] or NOx Storage-Reduction (NSR). The SCR scheme consists of the direct reduction of NOx species on the catalyst and it is habitually employed in stationary NOx sources, needing a reducing agent: urea, ammonia or hydrocarbons (from the fuel). In the NSR method the NOx species are reduced along two cycles where the combustion in the engine runs alternately over long oxygen-rich and short fuel-rich periods; in the oxygen-rich regime, the NOx species are oxidized to be captured and stored. Then, in the fuel-rich cycle, the NOx species are reduced to N2 on pure noble metal or bimetallic alloys including a noble metal [27,28]. In order to understand and to improve these processes a series of research works have been recently carried out, several of them focused on the NO oxidation [29–33] or the NO reduction on gold based catalysts [34–39]. From the results of these works, it can be suggested that the cleaving of the N–O bond is a crucial step in the NOx reduction to N2, especially at low and moderate temperatures. It was found that the reaction of NO dissociation on a stepped gold surface would be easier if atomic hydrogen was available on the catalyst surface. Surface hydrogen adatoms were found to react quite easily with NO and the N–O bond dissociation was much more facile in the case of hydrogenated species [40]. The hydrogen dissociation on the stepped Au(321) surface was found to be limited by a moderate activation energy barrier. The activation barrier can be visibly reduced on other gold based catalysts such as gold nanoparticles [41] or supported gold nanoparticles, e.g., Au13/TiO2 [42] and Aux/TiC [43,44]. Very recently, the oxidation of alcohols to aldehydes was found to be affected by the surface roughness which was modeled by the consideration of flat and stepped surfaces, rod and Au38 nanoparticle as gold catalysts models [45].
In the following, some of the most recent results attained by computational work on some heterogeneous reactions catalyzed by gold surfaces are reviewed, paying special attention to the effect of the low coordinated and doping atoms in the catalytic reactions. The work is organized as follows: results are reported and discussed in Section 2 while computational methods are described in detail in Section 3. Finally, the most important conclusions are summarized in Section 4.
2. Results and Discussion
The CO oxidation on gold based catalysts was intensely studied in the last years due to the practical applications of this reaction [2–4]. From the literature, it seems clear that the low coordinated gold atoms have to have a central role in the catalysis as is indicated by the fact that high dispersed gold nanoparticles catalyze the CO oxidation or by the fact that nanoparticles with diameters <2 nm catalyze the CO oxidation at low temperature and also by the fact that the nanoporous gold activity for this reaction depends on the number and size of the pores in the gold catalysts [1,5–9]. Following these experimental evidences a series of theoretical works tried to clarify the role of the low coordinated gold atoms in the CO oxidation, i.e., they aimed to understand the reaction mechanism for the CO oxidation on gold. In the presence of only CO, O2 or O species, two different routes are possible, (i) reaction with molecular oxygen forming a four atoms (O2 + CO) transition state or (ii) direct reaction with atomic oxygen, which needs a source of O atoms that can be for example the dissociation reaction of molecular oxygen.
Liu et al. [46] studied these reaction mechanisms using DFT calculations, within the generalized-gradient approximation, GGA-PBE functional and ultrasoft pseudopotentials, and considered the Au(111), Au(211) and Au(221) surfaces as gold catalyst models. They found that the Au(221) surface is the most reactive for the CO oxidation, with electronic energy barriers of 0.25 eV for direct reaction with oxygen adatoms and of 0.59 eV for the reaction with molecular oxygen. The electronic energy barrier for molecular oxygen dissociation was calculated to be 1.16 eV on the same Au(221) surface, which can be compared with the calculated values of 0.93 eV on the Au(211) surface and of 2.23 eV on the Au(111) surface, clearly identifying the positive effects of the presence of steps in the catalyst models into the dissociation of molecular oxygen. Similar activation energy barriers to those calculated by Liu et al. [46] for the stepped surfaces were found by us but considering the Au(321) surface as the catalyst model, which possesses a large amount of low coordinated atoms. In the case of the CO oxidation [13] and of the O2 dissociation on Au(321) [20], the activation energy barriers for the most favorable reaction paths were calculated to be ∼0.6 eV in the case of the CO + O2 → CO2 + O reaction, 0 eV in the case of the CO + O → CO2 reaction and 1.0 eV in the case of the O2 → O + O reaction; in these three situations, the oxygen atom is adsorbed on the catalyst surface. Interestingly, the CO + O2 → CO2 + O reaction on the three stepped Au surfaces evolve through an OCOO four atoms compound. Activation energy barriers lower than 0.4 eV were obtained by Lopez and Nørskov [47], using the RPBE exchange-correlation functional and ultrasoft pseudopotentials, for the same reactions but on an isolated Au10 cluster that was considered as a model for a small gold nanoparticle. Lopez and Nørskov [47] concluded that the main factor for the enhanced activity of the gold particles when compared to the activity of extended gold surfaces was the increased number of low coordinated Au atoms associated with a large fraction of corner sites. Similar conclusions were extracted by Chen et al. [48] from their study of the CO oxidation on the larger Au29 nanoparticle. Therefore, collecting the information from all these research works we can conclude that the CO oxidation reaction occurs on gold based catalysts more easily in the presence of low coordinated gold atoms, which are available both at the steps of the infinite periodic surfaces or at the junction of the different facets in the gold nanoparticles. A similar picture can be drawn for the oxygen dissociation reaction or for the alcohols oxidation reactions [45].
In the case of the oxygen dissociation reaction, it is found that the low coordinated atoms considerably decrease the activation energy barrier for this reaction: the barrier of 2.23 eV calculated for the flat Au(111) surface [46] is strongly reduced to 1.16 eV on the Au(221) surface [46], to 0.93 eV on the Au(211) surface [46], to 1.00 eV on the Au(321) surface [20] and even to lower values if the reaction is considered on gold nanoparticles [21]. However, these activation energy barriers are quite high to confer a superior activity to some gold based catalytic systems as the nanoporous gold used in the methanol oxidation [22]; in fact, the authors attributed the high performance of this catalytic system to the presence of residual silver on the nanoporous gold. Following this experimental evidence we carried out a systematic work to check how the doping with silver atoms affects the catalytic activity of extended gold surfaces for the O2 dissociation [23]. We found that increasing the silver content of the gold surfaces significantly affects the catalyst activity for the O2 dissociation (please see Figure 1 which illustrates the effect of the Au(111) surface doping in the catalytic activity). The pure gold surfaces as the Au(111), Au(110) or Au(321) present activation energy barriers for the O2 dissociation of 1.93 eV [23], of 0.85 eV [23], and of 1.00 eV [20], respectively. The activation energy barrier decreases up to a value of 1.27 eV in the case of the flat Au(111) surface doping and even up to a value of 0.86 eV considering silver rows deposited on the Au(111) surface. In the case of the doped Au(110) surface, the activation energy barrier decreases up to a value of 0.59 eV which is very close to the calculated value for the activation energy barrier on the pure Ag(110) surface, e.g., 0.52 eV [23]. In the case of the Au(321) surface, possessing atoms in the step region with coordination numbers lower than those in the Au(110) surface, the doping with silver atoms decreases the activation energy barrier up to 0.89 eV [23]. Therefore, it seems that the catalytic activity for the O2 dissociation of the gold surfaces doped with silver atoms may be enhanced by the presence of low coordinated atoms leading to high reactivity catalytic systems toward this dissociation reaction.
The reactions involving the NOx species reactions on gold surface have also been considered in previous computational studies. The NOx oxidation is very important in the oxygen rich cycle of the NSR process [27,28] and the NOx reduction to N2 is very important in the SCR process [26] and in the fuel-rich cycle of the NSR process [27,28]. Experimental work employing molecular beam scattering techniques on the reaction of NO oxidation by atomic oxygen on the Au(111) surface suggested that the production of NO2 was limited by the NO lifetime on the catalytic surface and predicted an activation energy barrier of 0.21 ± 0.02 eV for the NO oxidation by atomic oxygen and an adsorption energy of 0.4 eV for the isolated NO surface species [33]. Easy formation of NO2 was suggested by the computational work of Torres et al. [31] who predicted that, on the Au(111) surface, the reaction of NO oxidation would occur without activation energy barrier and concluded also that discrepancies between theoretical and experimental work were due to the presence of defects on the surface. Zhang et al. [32] suggested a different picture, i.e., discrepancies between theoretical and experimental data had to be related with the surface coverage and not with the presence of surface defects. Another computational work devoted to the NO oxidation by molecular or atomic oxygen on the Au(111) surface by Fajín et al. is found in the literature [49]. The same authors also performed computational work for the same reaction but on the stepped Au(321) surface [50]. A direct comparison of the calculated data in the two latter studies can be done since they considered the same computational methodology. The main conclusion taken from these two works is that the NO oxidation by molecular oxygen has lower activation energy barriers on the Au(321) surface due to the presence of low gold coordinated atoms (Figure 2). Nevertheless, the overall reaction rate must be slower on the Au(321) surface due to the limitations in the NO2 desorption from this surface. Therefore, in some cases the presence of low gold coordinated atoms can be negative for the overall reaction rate due to the strong adsorption of some reaction products and concomitant limitations in their desorption.
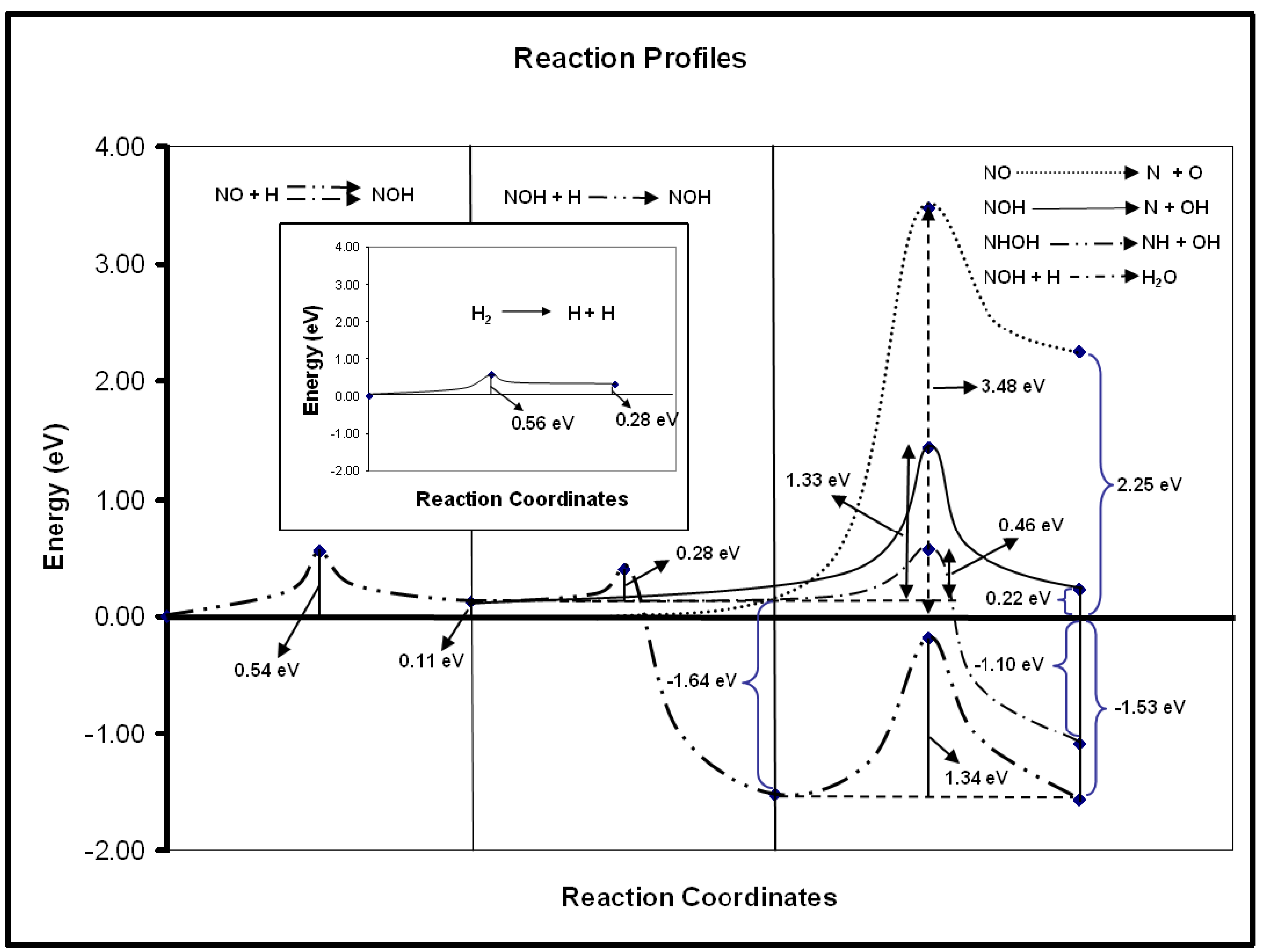
The cleavage of the N–O bond in the NO species is very relevant, for instance in the reactions involving the reduction of this species to gaseous N2. In the experimental study of NO reduction with CO on Au/TiO2 to yield N2, the NCO species was found to be an important intermediate at moderate temperatures, evidencing previous N–O bond break [34,35]. In fact, this step was also considered to be crucial for the same reaction on other catalytic systems based on gold, such as Au/α-Al2O3 [51] or as Au/NaY supported catalysts [52]. However, computational work shows that the N–O bond break is very difficult on gold surfaces, even on those presenting low-coordinated Au atoms such as the stepped Au(321) surface [40]. The calculations show that the cleaving of the N–O bond is blocked by a high activation energy barrier of ∼3.5 eV. Considering experimental works in which it was suggested that the inclusion of hydrogen in the catalytic system would activate the dissociation of the N–O bond [52,53], we have studied, by means of density functional theory, several possible reactions for the NO dissociation in the presence of hydrogen adatoms. It was found that the hydrogen was indeed able to promote the decrease of the activation energy barrier for the cleavage of the N–O bond. As can be seen in Figure 3, the reactions involving the hydrogenated species have moderate activation energy barriers. Therefore, N–O bond break seems to be feasible on gold surfaces. In these reactions, the surface H species can be formed by H2 dissociation, which has a moderate activation energy barrier (0.56 eV, cf. Figure 3) on the Au(321) surface [40] or lower barriers in the cases of gold nanoparticles or supported gold nanoparticles based catalysts [41–44]. This last example shows that in some cases, the presence of low gold coordinated atoms is not enough for catalysis but that gold can be active if adequate promoters able to considerably decrease the activation energy barriers are present on the catalyst surface.
3. Experimental Section
In all the calculations, the VASP 4.6.3 computer code [54–56] and the GGA-PW91 functional proposed by Perdew et al. [57] were used. The projected augmented-wave (PAW) method used by Bloch [58] and further implemented by Kresse and Joubert [59] was employed to describe the effect of core electrons on the valence shells together with a plane-wave basis set used to span the valence electronic states. The cutoff used to cut the plane wave expansion was set to 415 eV, value which was considered enough for a correct convergence of the calculations. Furthermore, the positions of the ions were relaxed using the conjugate-gradient algorithm and different Monkhorst-Pack k-points grids (5 × 5 × 1 or 7 × 7 × 1) were used to set the points where the electronic density is calculated. The electronic energetic barriers for the different reactions were obtained using the climbing-image nudged elastic band (cNEB) [60,61] or the dimer methods [62]. The computation of a single imaginary frequency ensured that the structures located with the cNEB or dimer approaches were true transition states.
The infinite gold surfaces and of their interactions with all the adsorbed species (reactants, intermediates, transition states and products) were modeled using a three-dimensional periodic-slab approach as usually done with calculations employing the VASP code. The positions of the gold atoms in the gold slabs used to simulate the infinite surfaces by its repetition, following the approach mentioned above, were obtained cutting the bulk along the Miller indices desired with the CRYSTAL 98 computer code [63]. Further, a vacuum region of 10 Å thick was introduced between repeated cells in the z direction in order to build the surfaces.
4. Conclusions
The role of the low coordinated gold atoms in the catalysis of reactions such as the CO oxidation, O2 dissociation, NO oxidation or NO dissociation was reviewed. This was done by a comparison of calculated adsorption energies and of activation energy barriers for these reactions on flat and on stepped gold surfaces. The experimental evidences that the presence of low coordinated gold atoms is crucial for the catalysis of several reactions by gold based catalysts were corroborated by computational works on several different gold surface models. These calculations were able to give an atomic level picture of the catalytic processes. The reactions of CO oxidation and of O2 dissociation are highly affected by the presence of low coordinated gold atoms. However, the activation of the O2 dissociation by the low coordinated gold atoms is not enough to explain the high activity of some gold catalytic systems such as the nanoporous gold for the methanol oxidation. Based on very recent computational work, the extraordinary activity of the nanoporous gold catalyst was found to be associated with a combination of two factors, namely, the presence of low Au coordinated atoms and the presence of silver atoms (doping). Interestingly, computational work showed also that the effect of low coordinated gold atoms in catalysis is not always evident as exemplified by the comparison of the reaction of NO oxidation on extended gold surfaces; in the case of the Au(321) surface, possessing low-coordinated atoms, the desorption of the product of reaction (NO2) is problematic due to its strong adsorption with the low coordinated atoms. Furthermore, in the case of the NO dissociation reaction, the presence of low coordinated gold atoms is not enough to decrease the activation energy barrier to the extent that the reaction becomes feasible but the presence of a promoter, e.g., H adatoms, is what is needed for the reaction to take place.
In summary, the importance of the low coordinated gold atoms in the catalysis of simple reactions on gold based catalysts was shown. In some cases this effect can be negative due to the strong adsorption of the reaction products on these low coordinated gold atoms which will eventually poison the reactions. In other situations, a combination of low-coordinated atoms and some other effects, e.g., presence of doping atoms or presence of promoters, is crucial for catalysis.


Acknowledgments
Thanks are due to Fundação para a Ciência e Tecnologia (FCT), Lisbon, Portugal and to FEDER for financial support to REQUIMTE and to CICECO, projects PEst-C/EQB/LA0006/2011 and PEst-C/CTM/LA0011/2011, respectively. Programa Ciência 2007 is also acknowledged. JLCF acknowledges FCT for the grant SFRH/BPD/64566/2009 co-financed by the Fundo Social Europeu (FSE).
References
- Haruta, M.; Yamada, N.; Kobayashi, T.; Iijima, S. Gold catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and carbon monoxide. J. Catal. 1989, 115, 301–309. [Google Scholar]
- Qian, L.H.; Wang, K.; Li, Y.; Fang, H.T.; Lu, Q.H.; Ma, X.L. CO sensor based on Au-decorated SnO2 nanobelt. Mater. Chem. Phys. 2006, 100, 82–84. [Google Scholar]
- Matsuoka, K.; Miyazaki, K.; Iriyama, Y.; Kikuchi, K.; Abe, T.; Ogumi, Z. Novel anode catalyst containing gold nanoparticles for use in direct methanol fuel cells. J. Phys. Chem. C 2007, 111, 3171–3174. [Google Scholar]
- Kim, H.-J.; Kim, D.-Y.; Han, H.; Shul, Y.-G. PtRu/C-Au/TiO2 electrocatalyst for a direct methanol fuel cell. J. Power Sources 2006, 159, 484–490. [Google Scholar]
- Pestryakov, A.N.; Bogdanchikova, N.; Simakov, A.; Tuzovskaya, I.; Jentoft, F.; Farias, M.; Díaz, A. Catalytically active gold clusters and nanoparticles for CO oxidation. Surf. Sci. 2007, 601, 3792–3795. [Google Scholar]
- Sanchez-Castillo, M.A.; Couto, C.; Kim, W.B.; Dumesic, J.A. Gold-nanotube membranes for the oxidation of CO at gas-water interfaces. Angew. Chem. Int. Ed. 2004, 43, 1140–1142. [Google Scholar]
- Xu, C.; Su, J.; Xu, X.; Liu, P.; Zhao, H.; Tian, F.; Ding, Y. Low temperature CO oxidation over unsupported nanoporous gold. J. Am. Chem. Soc. 2007, 129, 42–43. [Google Scholar]
- Zielasek, V.; Jurgens, B.; Schulz, C.; Biener, J.; Biener, M.M.; Hamza, A.V.; Bäumer, M. Gold catalysts: Nanoporous gold foams. Angew. Chem. Int. Ed. 2006, 45, 8241–8244. [Google Scholar]
- Xu, C.; Xu, X.; Su, J.; Ding, Y. Research on unsupported nanoporous gold catalyst for CO oxidation. J. Catal. 2007, 252, 243–248. [Google Scholar]
- Chen, M.; Goodman, D.W. Catalytically active gold: From nanoparticles to ultrathin films. Acc. Chem. Res. 2006, 39, 739–746. [Google Scholar]
- Lopez, N.; Janssens, T.V.W.; Clausen, B.S.; Xu, Y.; Mavrikakis, M.; Bligaard, T.; Nørskov, J.K. On the origin of the catalytic activity of gold nanoparticles for low-temperature CO oxidation. J. Catal. 2004, 223, 232–235. [Google Scholar]
- Remediakis, I.N.; López, N.; Nørskov, J.K. CO oxidation on gold nanoparticles: Theoretical studies. Appl. Catal. A Gen. 2005, 291, 13–20. [Google Scholar]
- Fajín, J.L.C.; Cordeiro, M.N.D.S.; Gomes, J.R.B. DFT study of the CO oxidation on the Au(321) surface. J. Phys. Chem. C 2008, 112, 17291–17302. [Google Scholar]
- Clark, J.C.; Dai, S.; Overbury, S.H. Operando studies of desorption, reaction and carbonate formation during CO oxidation by Au/TiO2 catalysts. Catal. Today 2007, 126, 135–142. [Google Scholar]
- Burch, R. Gold catalysts for pure hydrogen production in the water-gas shift reaction: Activity, structure and reaction mechanism. Phys. Chem. Chem. Phys. 2006, 8, 5483–5500. [Google Scholar]
- Lim, D.C.; Lopez-Salido, I.; Dietsche, R.; Bubek, M.; Kim, Y.D. Size-selectivity in the oxidation behaviors of Au nanoparticles. Angew. Chem. Int. Ed. 2006, 45, 2413–2415. [Google Scholar]
- Fajín, J.L.C.; Cordeiro, M.N.D.S.; Gomes, J.R.B. DFT study of the Au(321) surface reconstruction by consecutive deposition of oxygen atoms. Surf. Sci. 2008, 602, 424–435. [Google Scholar]
- Lim, D.C.; Lopez-Salido, I.; Dietsche, R.; Bubek, M.; Kim, Y.D. Oxidation of Au nanoparticles on HOPG using atomic oxygen. Surf. Sci. 2006, 600, 507–513. [Google Scholar]
- Min, B.K.; Alemozafar, A.R.; Pinnaduwage, D.; Deng, X.; Friend, C.M. Efficient CO oxidation at low temperature on Au(111). J. Phys. Chem. B 2006, 110, 19833–19838. [Google Scholar]
- Fajin, J.L.C.; Cordeiro, M.N.D.S.; Gomes, J.R.B. Adsorption of atomic and molecular oxygen on the Au(321) surface: DFT Study. J. Phys. Chem. C 2007, 111, 17311–17321. [Google Scholar]
- Roldán, A.; González, S.; Ricart, J.M.; Illas, F. Critical size for O2 dissociation by Au nanoparticles. Chem. Phys. Chem. 2009, 10, 348–351. [Google Scholar]
- Wittstock, A.A.; Zielasek, V.; Biener, J.; Friend, C.M.; Bäumer, M. Nanoporous gold catalysts for selective gas-phase oxidative coupling of methanol at low temperature. Science 2010, 327, 319–322. [Google Scholar]
- Fajin, J.L.C.; Cordeiro, M.N.D.S.; Gomes, J.R.B. On the theoretical understanding of the unexpected O2 activation by nanoporous gold. Chem. Commun. 2011, 47, 8403–8405. [Google Scholar]
- Inventory of U.S. Greenhouse Gas Emissions and Sinks; EPA 430-R-02-003 1990-2000, April 2002. Available online: http://www.epa.gov/globalwarming/publications/emissions (accessed on 15 November 2011).
- Proposal for a Regulation of the European Parliament and of the Council on Type Approval of Motor Vehicles with Respect to Emissions and on Access to Vehicle Repair Information; Amending Directive 72/306/EEC; European Commission: Brussels, Belgium, 2005.
- Chang, C.D.; Santiesteban, J.G.; Shihabi, D.S.; Stevenson, S.A. Selective catalytic reduction of nitrogen oxides. US Patent 5,401,478 1995. [Google Scholar]
- Miyoshi, N.; Matsumoto, S.; Katoh, K.; Tanaka, T.; Harada, J.; Takahashi, N.; Yokota, K.; Suguira, M.; Kasahara, K. Development of New Concept Three-Way Catalyst for Automotive Lean-Burn Engines; SAE International: Warrendale, PA, USA, 1995; p. 292. [Google Scholar]
- Matsumoto, S.; Ikeda, Y.; Suzuki, H.; Ogai, M.; Miyoshi, N. NOx storage-reduction catalyst for automotive exhaust with improved tolerance against sulfur poisoning. Appl. Catal. B Environ. 2000, 25, 115–124. [Google Scholar]
- Milsom, E.V.; Novak, J.; Oyama, M.; Marken, F. Electrocatalytic oxidation of nitric oxide at TiO2-Au nanocomposite film electrodes. Electrochem. Commun. 2007, 9, 436–442. [Google Scholar]
- Sobczak, I.; Kusior, A.; Ziolek, M. FTIR study of NO, C3H6 and O2 adsorption and interaction on gold modified MCM-41 materials. Catal. Today 2008, 137, 203–208. [Google Scholar]
- Torres, D.; González, S.; Neyman, K.M.; Illas, F. Adsorption and oxidation of NO on Au(111) surface: Density functional studies. Chem. Phys. Lett. 2006, 422, 412–416. [Google Scholar]
- Zhang, W.; Li, Z.; Luo, Y.; Yang, J. A first-principles study of NO adsorption and oxidation on Au(111) surface. J. Chem. Phys. 2008, 129, 134708:1–134708:5. [Google Scholar]
- McClure, S.M.; Kim, T.S.; Stiehl, J.D.; Tanaka, P.L.; Mullins, C.B. Adsorption and reaction of nitric oxide with atomic oxygen covered Au(111). J. Phys. Chem. B 2004, 108, 17952–17958. [Google Scholar]
- Solymosi, F.; Bánsági, T.; Zakar, T.S. Infrared study of the NO + CO interaction over Au/TiO2 catalyst. Catal. Lett. 2003, 87, 7–10. [Google Scholar]
- Solymosi, F.; Bánsági, T.; Zakar, T.S. Surface interaction and reaction of NO + CO on a supported Au catalyst. Phys. Chem. Chem. Phys. 2003, 5, 4724–4730. [Google Scholar]
- Debeila, M.A.; Coville, N.J.; Scurrell, M.S.; Hearne, G.R. DRIFTS studies of the interaction of nitric oxide and carbon monoxide on Au-TiO2. Catal. Today 2002, 72, 79–87. [Google Scholar]
- Debeila, M.A.; Coville, N.J.; Scurrell, M.S.; Hearne, G.R.; Witcomb, M.J. Effect of pretreatment variables on the reaction of nitric oxide (NO) with Au-TiO2: DRIFTS studies. J. Phys. Chem. B 2004, 108, 18254–18260. [Google Scholar]
- Debeila, M.A.; Coville, N.J.; Scurrell, M.S.; Hearne, G.R. The effect of calcination temperature on the adsorption of nitric oxide on Au-TiO2: Drifts studies. Appl. Catal. A Gen. 2005, 291, 98–115. [Google Scholar]
- Debeila, M.A.; Coville, N.J.; Scurrell, M.S.; Hearne, G.R. Direct observation of thermally activated NO adsorbate species on Au-TiO2: DRIFTS studies. J. Mol. Catal. A Chem. 2004, 219, 131–141. [Google Scholar]
- Fajín, J.L.C.; Cordeiro, M.N.D.S.; Gomes, J.R.B. The role of preadsorbed atomic hydrogen in the NO dissociation on a zigzag stepped gold surface: A DFT Study. J. Phys. Chem. C 2009, 113, 8864–8877. [Google Scholar]
- Corma, A.; Boronat, M.; González, S.; Illas, F. On the activation of molecular hydrogen by gold: A theoretical approximation to the nature of potential active sites. Chem. Commun. 2007, 43, 3371–3373. [Google Scholar]
- Boronat, M.; Corma, A. Origin of the different activity and selectivity toward hydrogenation of single metal Au and Pt on TiO2 and bimetallic Au-Pt/TiO2 catalysts. Langmuir 2010, 26, 16607–16614. [Google Scholar]
- Gómez, T.; Flórez, E.; Rodríguez, J.A.; Illas, F. Reactivity of transition metals (Pd, Pt, Cu, Ag, Au) toward molecular hydrogen dissociation: Extended surfaces versus particles supported on TiC(001) or small is not always better and large is not always bad. J. Phys. Chem. C 2011, 115, 11666–11672. [Google Scholar]
- Flórez, E.; Gómez, T.; Liu, P.; Rodríguez, J.A.; Illas, F. Hydrogenation reactions on Au/TiC(001): Effects of Au-C interactions on the dissociation of H2. ChemCatChem 2010, 2, 1219–1222. [Google Scholar]
- Boronat, M.; Corma, A.; Illas, F.; Radilla, J.; Ródenas, T.; Sabater, M.J. Mechanism of selective alcohol oxidation to aldehydes on gold catalysts: Influence of surface roughness on reactivity. J. Catal. 2011, 278, 50–58. [Google Scholar]
- Liu, Z.P.; Hu, P.; Alavi, A. Catalytic role of gold in gold-based catalysts: a density functional theory study on the CO oxidation on gold. J. Am. Chem. Soc. 2002, 124, 14770–14779. [Google Scholar]
- Lopez, N.; Nørskov, J.K. Catalytic CO oxidation by a gold nanoparticle: A density functional study. J. Am. Chem. Soc. 2002, 124, 11262–11263. [Google Scholar]
- Chen, H.-T.; Chang, J.-G.; Ju, S.-P.; Chen, H.-L. First-principle calculations on CO oxidation catalyzed by a gold nanoparticle. J. Comput. Chem. 2010, 31, 258–265. [Google Scholar]
- Fajín, J.L.C.; Cordeiro, M.N.D.S.; Gomes, J.R.B. DFT study on the NO oxidation on a flat gold surface model. Chem. Phys. Lett. 2011, 503, 129–133. [Google Scholar]
- Fajín, J.L.C.; Cordeiro, M.N.D.S.; Gomes, J.R.B. DFT study on the reaction of NO oxidation on a stepped gold surface. Appl. Catal. A Gen. 2010, 379, 111–120. [Google Scholar]
- Bera, P.; Patil, K.C.; Jayaram, V.; Hegde, M.S.; Subbannac, G.N. Combustion synthesis of nanometal particles supported on α-Al2O3: CO oxidation and NO reduction catalysts. J. Mater. Chem. 1999, 9, 1801–1805. [Google Scholar]
- Salama, T.M.; Ohnishi, R.; Ichikawa, M. Studies of the selective reduction of nitric oxide by carbon monoxide in the presence and absence of hydrogen over Au/NaY catalysts. J. Chem. Soc. Faraday Trans. 1996, 92, 301–306. [Google Scholar]
- Ilieva, L.; Pantaleo, G.; Sobczak, J.W.; Ivanov, I.; Venezia, A.M.; Andreeva, D. NO reduction by CO in the presence of water over gold supported catalysts on CeO2-Al2O3 mixed support, prepared by mechanochemical activation. Appl. Catal. B Environ. 2007, 76, 107–114. [Google Scholar]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar]
- Kresse, G.; Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab-initio total energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar]
- Jónsson, H.; Mills, G.; Jacobsen, K.W. Classical and Quantum Dynamics in Condensed Phase Simulations; Berne, B.J., Ciccotti, G., Coker, D.F., Eds.; World Scientific: Singapore, 1998; p. 385. [Google Scholar]
- Henkelman, G.; Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 2000, 113, 9978–9985. [Google Scholar]
- Henkelman, G.; Jónsson, H. A dimer method for finding saddle points on high dimensional potential surfaces using only first derivatives. J. Chem. Phys. 1999, 111, 7010–7022. [Google Scholar]
- Saunders, V.R.; Dovesi, R.; Roetti, C.; Causà, M.; Harrison, N.M.; Orlando, R.; Zicovich-Wilson, C.M. Computer Code CRYSTAL 98, User's Manual; University of Torino: Torino, Italy, 1998. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fajín, J.L.C.; Cordeiro, M.N.D.S.; Gomes, J.R.B. Catalytic Reactions on Model Gold Surfaces: Effect of Surface Steps and of Surface Doping. Catalysts 2011, 1, 40-51. https://doi.org/10.3390/catal1010040
Fajín JLC, Cordeiro MNDS, Gomes JRB. Catalytic Reactions on Model Gold Surfaces: Effect of Surface Steps and of Surface Doping. Catalysts. 2011; 1(1):40-51. https://doi.org/10.3390/catal1010040
Chicago/Turabian StyleFajín, José L. C., Maria Natália D. S. Cordeiro, and José R. B. Gomes. 2011. "Catalytic Reactions on Model Gold Surfaces: Effect of Surface Steps and of Surface Doping" Catalysts 1, no. 1: 40-51. https://doi.org/10.3390/catal1010040
APA StyleFajín, J. L. C., Cordeiro, M. N. D. S., & Gomes, J. R. B. (2011). Catalytic Reactions on Model Gold Surfaces: Effect of Surface Steps and of Surface Doping. Catalysts, 1(1), 40-51. https://doi.org/10.3390/catal1010040






