Protective Effects of Intratracheally-Administered Bee Venom Phospholipase A2 on Ovalbumin-Induced Allergic Asthma in Mice
Abstract
:1. Introduction
2. Results
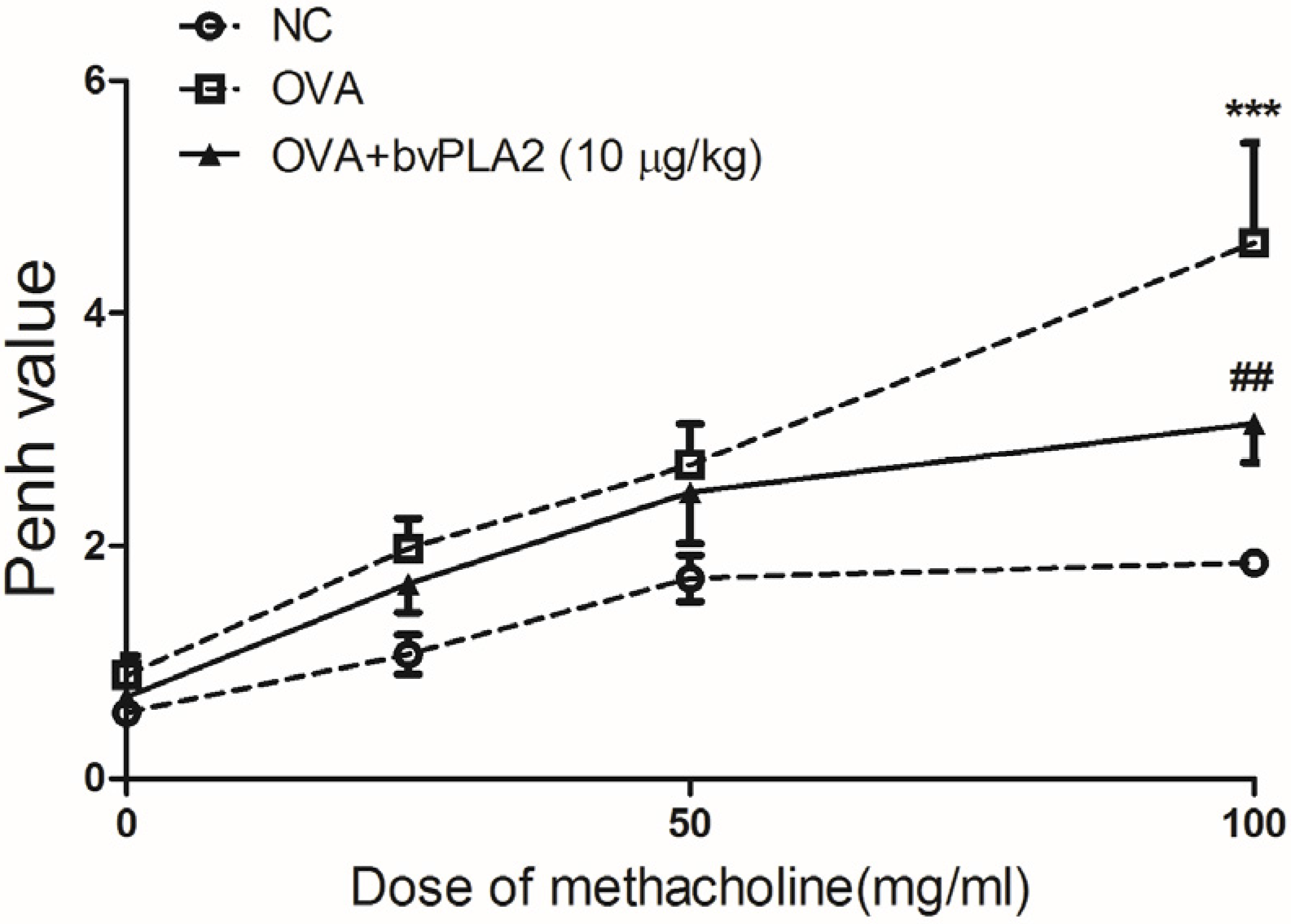
2.1. Intratracheal Administration of bvPLA2 Alleviates Airway Hyperresponsiveness (AHR) to Methacholine Induced by OVA
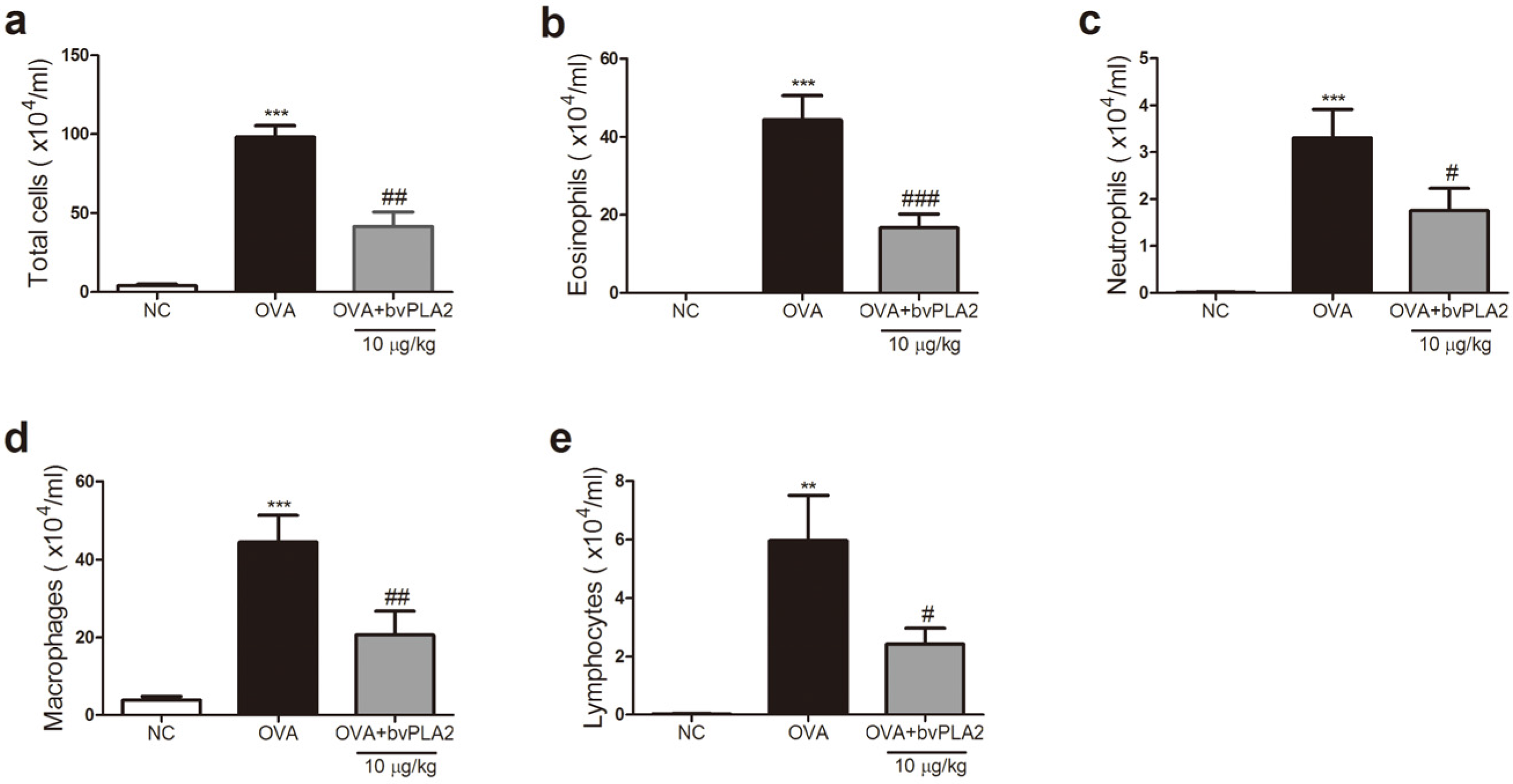
2.2. bvPAL2 Inhibits the Recruitment of Inflammatory Cells in BAL Fluid Induced by OVA
2.3. bvPLA2 Inhibits Th2 Cytokine Secretion in the Lungs Induced by OVA
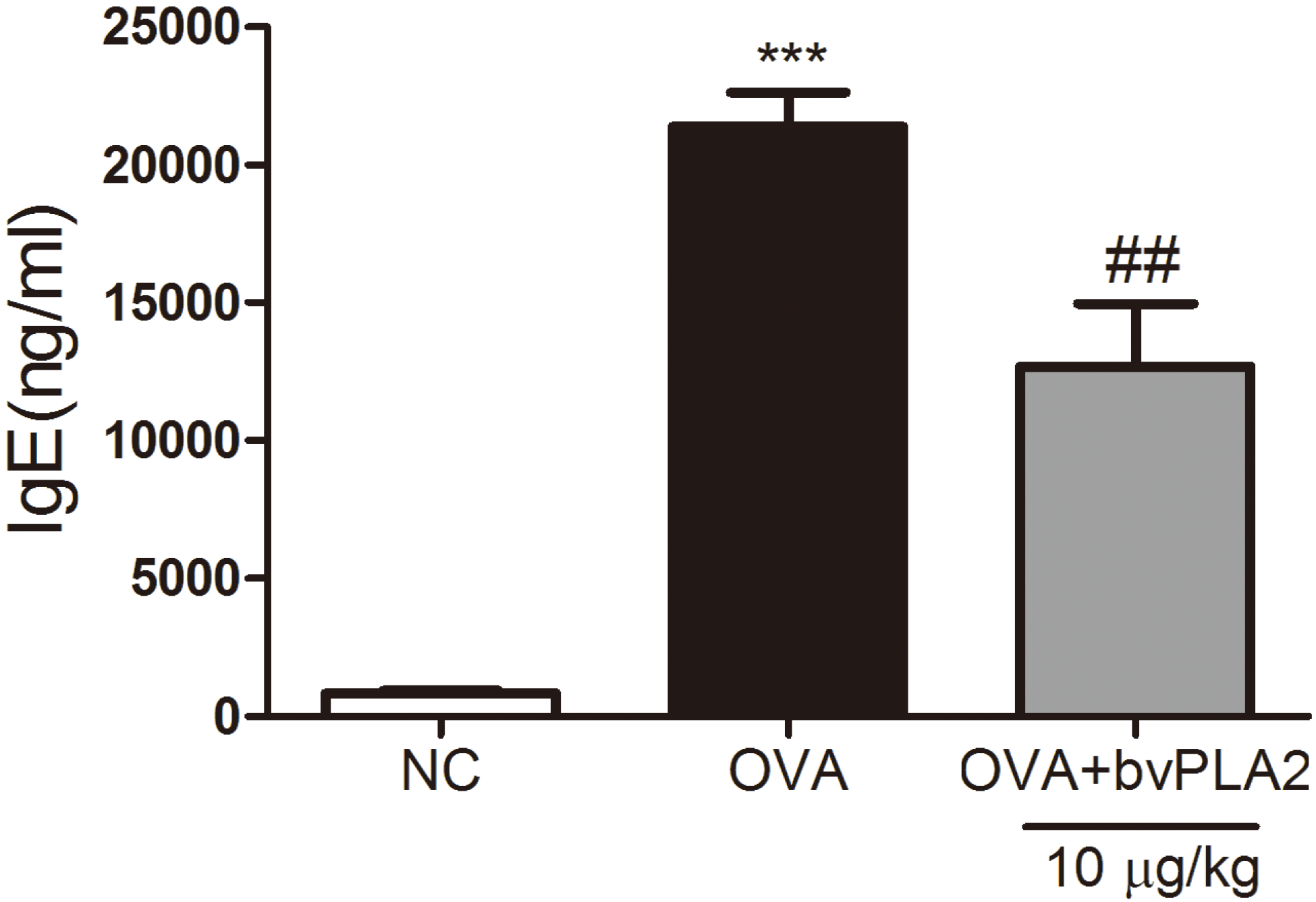
2.4. bvPAL2 Suppresses the Secretion of Total IgE in Serum Induced by OVA
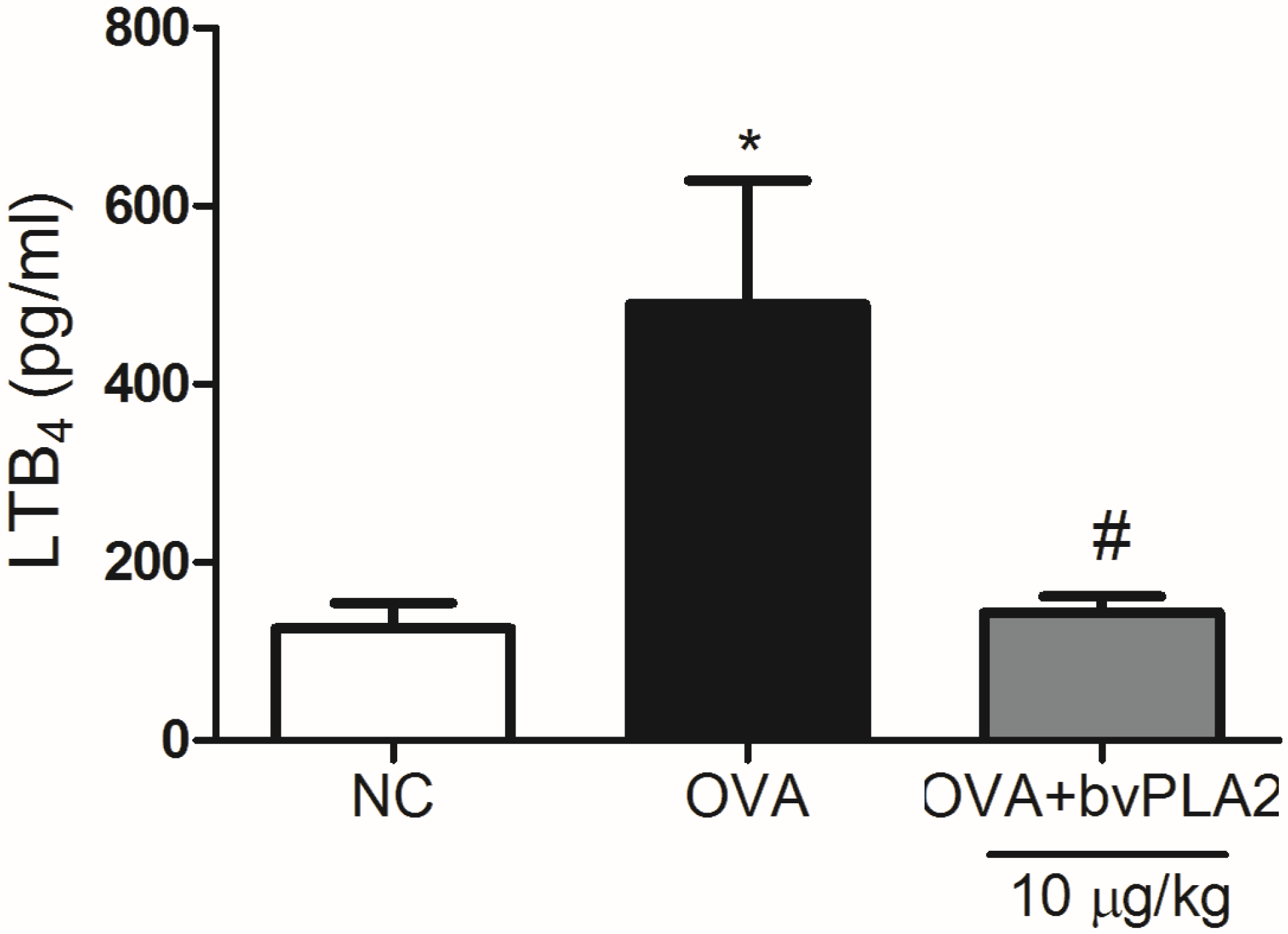
2.5. bvPLA2 Suppresses LTB4 Production in the Lungs Induced by OVA
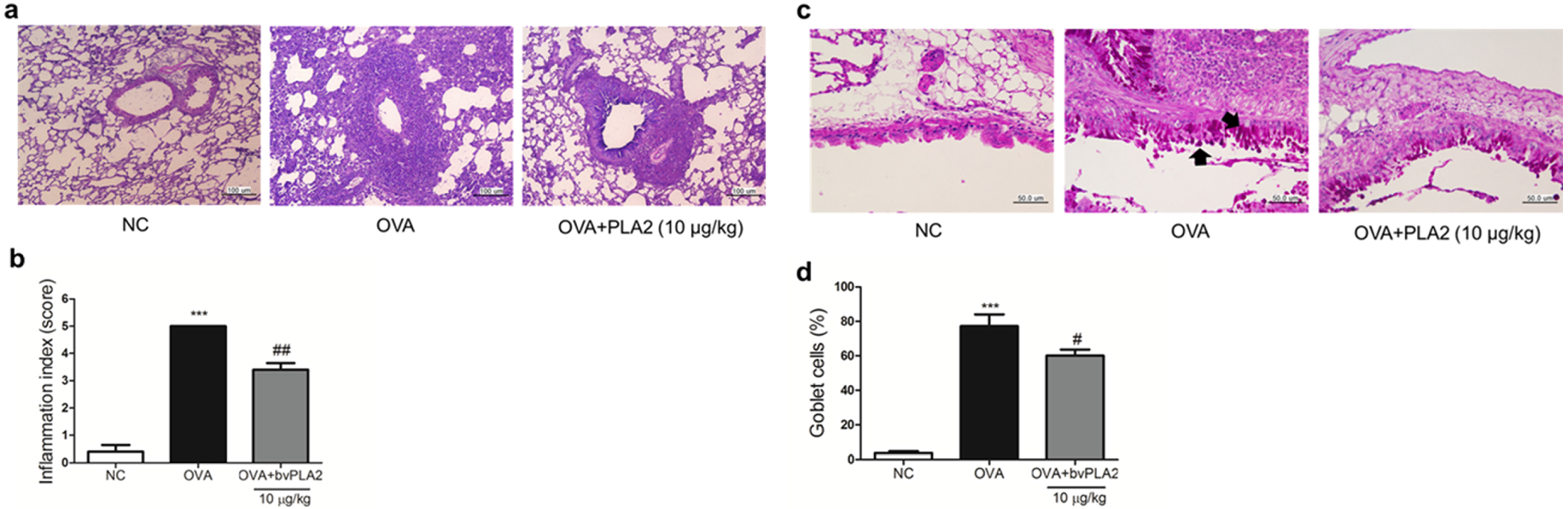
2.6. bvPLA2 Alleviates Histological Change in the Lung Tissues of an OVA-Induced Airway Inflammation Model
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Reagents
4.3. Experimental Procedures
4.4. Assessment of Airway Hyperresponsiveness (AHR)
4.5. Analysis of Bronchoalveolar Lavage (BAL) Fluid
4.6. Immunoglobulin Assay
4.7. Th2 Cytokines Assays
4.8. Leukotriene B4 Assay
4.9. Histological Analysis
4.10. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pedersen, S.E.; Hurd, S.S.; Lemanske, R.F., Jr.; Becker, A.; Zar, H.J.; Sly, P.D.; Soto-Quiroz, M.; Wong, G.; Bateman, E.D. Global strategy for the diagnosis and management of asthma in children 5 years and younger. Pediatr. Pulmonol. 2011, 46, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, E.A.; Lee, N.A.; Lee, J.J. Re-defining the unique roles for eosinophils in allergic respiratory inflammation. Clin. Exp. Allergy 2014, 44, 1119–1136. [Google Scholar] [CrossRef] [PubMed]
- Tamaoki, J.; Kondo, M.; Sakai, N.; Aoshiba, K.; Tagaya, E.; Nakata, J.; Isono, K.; Nagai, A. Effect of suplatast tosilate, a Th2 cytokine inhibitor, on steroid-dependent asthma: A double-blind randomised study. Lancet 2000, 356, 273–278. [Google Scholar] [CrossRef]
- Kelly, H.W. Inhaled corticosteroid dosing: Double for nothing? J. Allergy Clin. Immunol. 2011, 128, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Weers, J. Inhaled antimicrobial therapy—Barriers to effective treatment. Adv. Drug Deliv. Rev. 2015, 85, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Castro, H.J.; Mendez-Lnocencio, J.I.; Omidvar, B.; Omidvar, J.; Santilli, J.; Nielsen, H.S., Jr.; Pavot, A.P.; Richert, J.R.; Bellanti, J.A. A phase I study of the safety of honeybee venom extract as a possible treatment for patients with progressive forms of multiple sclerosis. Allergy Asthma Proc. 2005, 26, 470–476. [Google Scholar] [PubMed]
- Chen, J.; Lariviere, W.R. The nociceptive and anti-nociceptive effects of bee venom injection and therapy: A double-edged sword. Prog. Neurobiol. 2010, 92, 151–183. [Google Scholar] [CrossRef] [PubMed]
- Palm, N.W.; Rosenstein, R.K.; Yu, S.; Schenten, D.D.; Florsheim, E.; Medzhitov, R. Bee venom phospholipase A2 induces a primary type 2 response that is dependent on the receptor ST2 and confers protective immunity. Immunity 2013, 39, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.S.; Lee, G.; Lee, C.; Ye, M.; Chung, H.S.; Kim, H.; Bae, S.J.; Hwang, D.S.; Bae, H. Bee venom phospholipase A2, a novel FOXP3+ regulatory T cell inducer, protects dopaminergic neurons by modulating neuroinflammatory responses in a mouse model of parkinson’s disease. J. Immunol. 2015, 195, 4853–4860. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, H.; Lee, G.; Jang, H.; Kim, S.S.; Yoon, H.; Kang, G.H.; Hwang, D.S.; Kim, S.K.; Chung, H.S.; et al. Phospholipase A2 inhibits cisplatin-induced acute kidney injury by modulating regulatory T cells by the CD206 mannose receptor. Kidney Int. 2015, 88, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Baek, H.; Jung, K.H.; Lee, G.; Lee, H.; Kang, G.H.; Lee, G.; Bae, H. Bee venom phospholipase A2 suppresses allergic airway inflammation in an ovalbumin-induced asthma model through the induction of regulatory T cells. Immun. Inflamm. Dis. 2015, 3, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Chen, R.F.; Kuo, H.C. Different implications of paternal and maternal atopy for perinatal IgE production and asthma development. Clin. Dev. Immunol. 2012, 2012, 132142. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.L.; Riley, J.P.; Banie, H.; Xue, X.; Sun, B.; Crawford, S.; Lundeen, K.A.; Yu, F.; Karlsson, L.; Fourie, A.M.; et al. Leukotriene A4 hydrolase inhibition attenuates allergic airway inflammation and hyperresponsiveness. Am. J. Respir. Crit. Care Med. 2010, 181, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Fireman, P. Understanding asthma pathophysiology. Allergy Asthma Proc. 2003, 24, 79–83. [Google Scholar] [PubMed]
- Durham, S.R. The significance of late responses in asthma. Clin. Exp. Allergy 1991, 21, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, R. The relationship between airway inflammation and bronchial hyperresponsiveness. Clin. Exp. Allergy 1989, 19, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Chanez, P.; Lacoste, J.Y.; Barneon, G.; Ghavanian, N.; Enander, I.; Venge, P.; Ahlstedt, S.; Simony-Lafontaine, J.; Godard, P.; et al. Eosinophilic inflammation in asthma. N. Engl. J. Med. 1990, 323, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- De Monchy, J.G.; Kauffman, H.F.; Venge, P.; Koeter, G.H.; Jansen, H.M.; Sluiter, H.J.; De Vries, K. Bronchoalveolar eosinophilia during allergen-induced late asthmatic reactions. Am. Rev. Respir. Dis. 1985, 131, 373–376. [Google Scholar] [PubMed]
- Corry, D.B.; Folkesson, H.G.; Warnock, M.L.; Erle, D.J.; Matthay, M.A.; Wiener-Kronish, J.P.; Locksley, R.M. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J. Exp. Med. 1996, 183, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Grunig, G.; Warnock, M.; Wakil, A.E.; Venkayya, R.; Brombacher, F.; Rennick, D.M.; Sheppard, D.; Mohrs, M.; Donaldson, D.D.; Locksley, R.M.; et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 1998, 282, 2261–2263. [Google Scholar] [PubMed]
- Aikawa, T.; Shimura, S.; Sasaki, H.; Ebina, M.; Takishima, T. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest 1992, 101, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Ebina, M.; Takahashi, T.; Chiba, T.; Motomiya, M. Cellular hypertrophy and hyperplasia of airway smooth muscles underlying bronchial asthma: A 3-D morphometric study. Am. Rev. Respir. Dis. 1993, 148, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Pittler, M.H.; Shin, B.C.; Kong, J.C.; Ernst, E. Bee venom acupuncture for musculoskeletal pain: A review. J. Pain 2008, 9, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Son, D.J.; Lee, J.W.; Lee, Y.H.; Song, H.S.; Lee, C.K.; Hong, J.T. Therapeutic application of anti-arthritis, pain-releasing, and anti-cancer effects of bee venom and its constituent compounds. Pharmacol. Ther. 2007, 115, 246–270. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, E.A.; Subramaniam, S.; Cheng, T.Y.; De Jong, A.; Layre, E.; Ly, D.; Salimi, M.; Legaspi, A.; Modlin, R.L.; Salio, M.; et al. Bee venom processes human skin lipids for presentation by CD1A. J. Exp. Med. 2015, 212, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.S.; Kim, H.; Lee, G.; Park, S.; Kim, H.; Bae, H. Neuro-protective effects of bee venom by suppression of neuroinflammatory responses in a mouse model of parkinson’s disease: Role of regulatory T cells. Brain Behav. Immun. 2012, 26, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Bae, H. Bee venom phospholipase A2: Yesterday’s enemy becomes today’s friend. Toxins 2016, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Montuschi, P.; Peters-Golden, M.L. Leukotriene modifiers for asthma treatment. Clin. Exp. Allergy 2010, 40, 1732–1741. [Google Scholar] [CrossRef] [PubMed]
- Cifone, M.G.; Botti, D.; Festuccia, C.; Napolitano, T.; Delgrosso, E.; Cavallo, G.; Chessa, M.A.; Santoni, A. Involvement of phospholipase-A2 activation and arachidonic-acid metabolism in the cytotoxic functions of rat NK cells. Cell Immunol. 1993, 148, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Bauldry, S.A.; Wooten, R.E. Leukotriene B4 and platelet activating factor production in permeabilized human neutrophils: Role of cytosolic PLA2 in LTB4 and PAF generation. BBA Lipids Lipid Metab. 1996, 1303, 63–73. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, K.P.; Han, S.K.; Munoz, N.M.; Zhu, X.D.; Sano, H.; Leff, A.R.; Cho, W.H. Group V phospholipase A2 induces leukotriene biosynthesis in human neutrophils through the activation of group IVA phospholipase A2. J. Biol. Chem. 2002, 277, 36479–36488. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, K.-H.; Baek, H.; Shin, D.; Lee, G.; Park, S.; Lee, S.; Choi, D.; Kim, W.; Bae, H. Protective Effects of Intratracheally-Administered Bee Venom Phospholipase A2 on Ovalbumin-Induced Allergic Asthma in Mice. Toxins 2016, 8, 269. https://doi.org/10.3390/toxins8100269
Jung K-H, Baek H, Shin D, Lee G, Park S, Lee S, Choi D, Kim W, Bae H. Protective Effects of Intratracheally-Administered Bee Venom Phospholipase A2 on Ovalbumin-Induced Allergic Asthma in Mice. Toxins. 2016; 8(10):269. https://doi.org/10.3390/toxins8100269
Chicago/Turabian StyleJung, Kyung-Hwa, Hyunjung Baek, Dasom Shin, Gihyun Lee, Sangwon Park, Sujin Lee, Dabin Choi, Woojin Kim, and Hyunsu Bae. 2016. "Protective Effects of Intratracheally-Administered Bee Venom Phospholipase A2 on Ovalbumin-Induced Allergic Asthma in Mice" Toxins 8, no. 10: 269. https://doi.org/10.3390/toxins8100269





