Control of Aflatoxin Production of Aspergillus flavus and Aspergillus parasiticus Using RNA Silencing Technology by Targeting aflD (nor-1) Gene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strain and Growth Conditions
2.2. Preparation of Protoplast
| siRNA Name | siRNA Sequence |
|---|---|
| Nor-Ia | Sense strand: CAUGUAUGCUCCCGUCCUAUU |
| Antisense strand : UAGGACGGGAGCAUACAUGUU | |
| Nor-Ib | Sense strand: GCAACAGGCCAAGUUUGUCUU |
| Antisense strand : GACAAACUUGGCCUGUUGCUU | |
| Nor-Ic | Sense strand: CAGGCCAAGUUUGUCUUGAUU |
| Antisense strand : UCAAGACAAACUUGGCCUGUU |
2.3. Delivery of siRNA to Protoplast
2.4. Aflatoxin Extraction and HPLC Analysis
2.5. Isolation of RNA from the Samples and RT-PCR
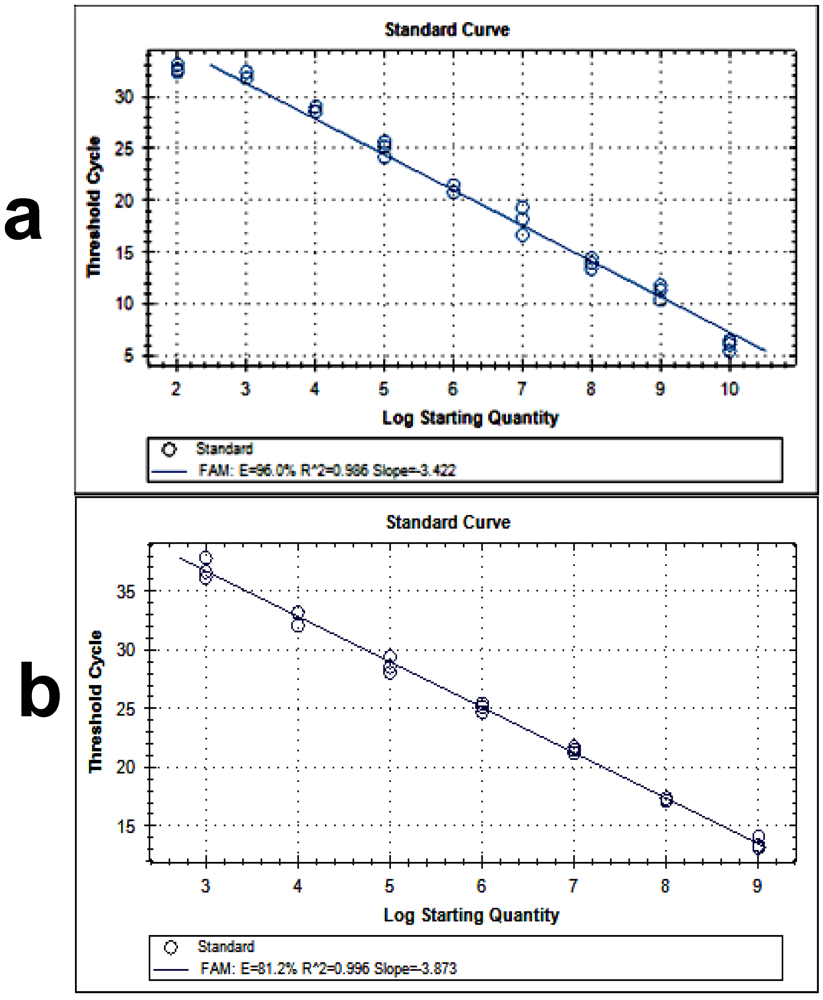
3. Results
3.1. Treatment of Aspergillus flavus NRRL with siRNA
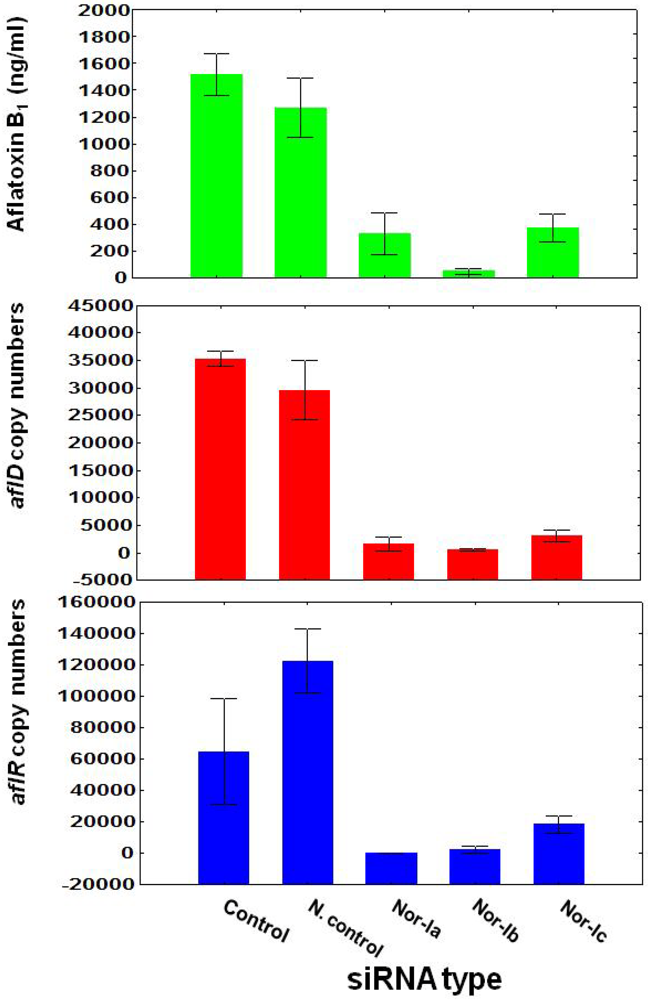
| DF | MS | F | P | |
|---|---|---|---|---|
| (a) | ||||
| Factor | ||||
| aflD copy numbers | 4 | 8.58 × 108 | 42.47 | 0.000003 |
| AFB1 | 4 | 1.24 × 108 | 18.74 | 0.0001 |
| aflR copy numbers | 4 | 8.16 × 109 | 8.63 | 0.0027 |
| (b) | ||||
| Factor | ||||
| log aflD | 5 | 1.87 | 10.95 | 0.0003 |
| log AFB1 | 5 | 0.41 | 199.13 | 0.00000 |
| log aflR | 5 | 2.4659 | 6.05 | 0.005 |
| Correlations | R Value | F | P |
|---|---|---|---|
| aflD andaflR | 0.82 | 28.41 | 0.0001 |
| aflD and AFB1 | 0.88 | 47.26 | 0.00001 |
| aflR and AFB1 | 0.66 | 10.039 | 0.0074 |
| log aflD and siRNA conc. | 0.86 | 46.31 | 0.000 |
| log AFB1 and siRNA conc. | 0.91 | 77.75 | 0.000 |
| log aflR and siRNA conc. | 0.45 | 4.07 | 0.06 |
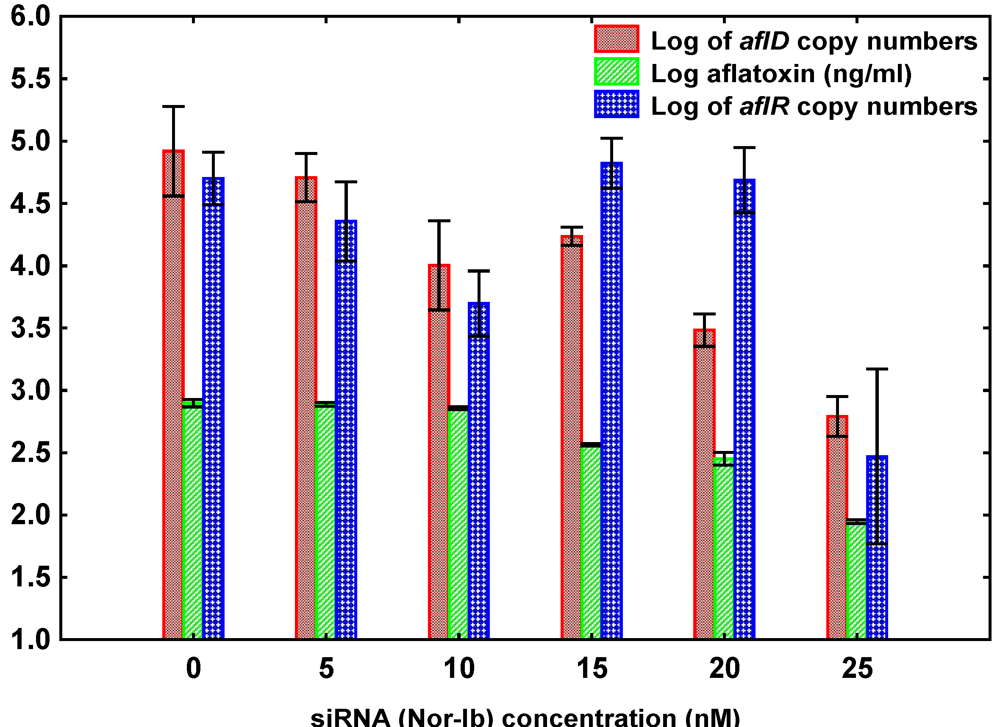
3.2. Effect of siRNA Concentrations on A. flavus NRRL3357
3.3. Treatment of A. flavus and A. parasiticus with siRNA
| Strains | AFB1 (µ/mL) | AFG1(µ/mL) | AflD Copy Numbers × 103 | AflR Copy Numbers × 103 | ||||
|---|---|---|---|---|---|---|---|---|
| Un-Treated | Treated | Un-Treated | Treated | Un-Treated | Treated | Un-Treated | Treated | |
| A. flavus EPG9 | 0.7 ± 0.05 | 0.088 ± 0.003 | 0 | 0 | 195.6 ± 56.9 | 0.69 ± 0.2 | 41 ± 10.7 | 6.8 ± 0.8 |
| A. parasiticus-NRRL13005 | 0.15 ± 0.04 | 0.05 ± 0.01 | 2.7 ± 0.5 | 0.6 ± 0.2 | 22.9 ± 4.7 | 2.4 ± 1.4 | 0.6 ± 0.2 | 0.05 ± 0.007 |
| A. parasiticus-SSWT2999 | 1.48 ± 0.37 | 1.1 ± 0.4 | 7.8 ± 1.9 | 5.5 ± 3.0 | 499.9 ± 85. | 43.6 ± 5.5 | 0.4 ± 0.1 | 0.06 ± 0.02 |
| DF | MS | F | P | |
|---|---|---|---|---|
| (a) | ||||
| A. flavus EPG9 Factor | ||||
| aflD copy numbers | 1 | 5.7 × 1010 | 11.71 | 0.026 * |
| AFB1 | 1 | 7.4 × 105 | 163.06 | 0.0002 * |
| (b) | ||||
| A. parasiticus NRRL13005 Factor | ||||
| aflD copy numbers | 1 | 6.3 × 108 | 17.07 | 0.01* |
| AFG1 | 1 | 6.9 × 106 | 12.34 | 0.02* |
| (c) | ||||
| A. parasiticus SSWT2999 Factor | ||||
| aflD copy numbers | 1 | 3.1 × 1011 | 28.68 | 0.005* |
| AFG1 | 1 | 8.3 × 106 | 0.42 | 0.54 |
4. Discussion
5. Conclusions
Acknowledgments
References
- Peraica, M.; Radic, B.; Lucic, A.; Pavlovic, M. Toxic effects of mycotoxins in humans. Bull. World Health Organ. 1999, 77, 754–766. [Google Scholar]
- Ketting, R.F.; Fischer, S.E.; Bernstein, E.; Sijen, T.; Hannon, G.J.; Plasterk, R.H. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev. 2001, 15, 2654–2659. [Google Scholar] [CrossRef] [PubMed]
- Bromley, M.; Gordon, C.; Rovira-Graells, N.; Oliver, J. The Aspergillus fumigatus cellobiohydrolase B (cbhB) promoter is tightly regulated and can be exploited for controlled protein expression and RNAi. FEMS Microbiol. Lett. 2006, 264, 246–254. [Google Scholar] [CrossRef] [PubMed]
- de Jong, J.F.; Deelstra, H.J.; Wosten, H.A.; Lugones, L.G. RNA mediated gene silencing in monokaryons and dikaryons of Schizophyllum commune. Appl. Environ. Microbiol. 2006, 72, 1267–1269. [Google Scholar] [CrossRef] [PubMed]
- Mouyna, I.; Henry, C.; Doering, T.L.; Latge, J.P. Gene silencing with RNA interference in the human pathogenic fungus Aspergillus fumigatus. FEMS Microbiol. Lett. 2004, 237, 317–324. [Google Scholar] [PubMed]
- Nakayashiki, H. RNA silencing in fungi: Mechanisms and applications. FEBS Lett. 2005, 579, 5950–5957. [Google Scholar]
- Liu, H.; Cottrell, T.R.; Pierini, L.M.; Goldman, W.E.; Doering, T.L. RNA interference in the pathogenic fungus Cryptococcus neoformans. Genetics 2002, 160, 463–470. [Google Scholar] [PubMed]
- Khatri, M.; Rajam, M.V. Targeting polyamines of Aspergillus nidulans by siRNA specific to fungal ornithine decarboxylase gene. Med. Mycol. 2007, 45, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Whisson, S.C.; Avrova, A.O.; West, P.V.; Jones, J.T. A method for double-stranded RNA mediated transient gene silencing in Phytophthora infestans. Mol. Plant Pathol. 2005, 6, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Trail, F.; Chang, P.K.; Cary, J.; Linz, J.E. Structural and functional analysis of the nor-1 gene involved in the biosynthesis of aflatoxins by Aspergillus parasiticus. Appl. Environ. Microbiol. 1994, 60, 4078–4085. [Google Scholar] [PubMed]
- Zhou, R.; Linz, J.E. Enzymatic function of the Nor-1 protein in aflatoxin biosynthesis in Aspergillus parasiticus. Appl. Environ. Microbiol. 1999, 65, 5639–5641. [Google Scholar] [PubMed]
- Cary, J.W.; Montalbano, B.G.; Ehrlich, K.C. Promoter elements involved in the expression of the Aspergillus parasiticus aflatoxin biosynthesis pathway gene avnA. Biochim. Biophys. Acta 2000, 1491, 7–12. [Google Scholar] [PubMed]
- Schmidt-Heydt, M.; Abdel-Hadi, A.; Magan, N.; Geisen, R. Complex regulation of the aflatoxin biosynthesis gene cluster of Aspergillus flavus in relation to various combinations of water activity and temperature. Int. J. Food Microbiol. 2009, 135, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hadi, A.; Carter, D.; Magan, N. Temporal monitoring of the nor-1 (aflD) gene of Aspergillus flavus in relation to aflatoxin B1 production during storage of peanuts under different environmental conditions. J. Appl. Microbiol. 2010, 109, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hadi, A.; Carter, D.; Magan, N. Discrimination between aflatoxigenic and non-aflatoxigenic strains of Aspergillus section flavi group contaminating Egyptian peanuts using molecular and analytical techniques. World Mycotoxin J. 2011, 4, 69–77. [Google Scholar] [CrossRef]
- Cary, J.W.; Ehrlich, K.C.; Kate, S.P.; Calvo, A.M.; Bhatnagar, D.; Cleveleand, D.E. Regulatory elements in aflatoxin biosynthesis. Mycotoxin Res. 2006, 22, 105–109. [Google Scholar]
- Natural Toxins, Official Methods of Analysis of AOAC International, 17t ed; Association of Official Analytical Chemists: Washington, DC, USA, 2000; Chapter 49.
- Mayer, Z.; Bagnara, A.; Färber, P.; Geisen, R. Quantification of the copy number of nor-1, a gene of the aflatoxin biosynthetic pathway by real-time PCR, and its correlation to the cfu of Aspergillus flavus in foods. Int. J. Food Microbiol. 2003, 82, 143–151. [Google Scholar] [PubMed]
- Geisen, R. Multiplex polymerase chain reaction for the detection of potential aflatoxin and sterigmatocystin producing fungi. Syst. Appl. Microbiol. 1996, 19, 388–392. [Google Scholar]
- Bustin, S.A. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J. Mol. Endocrinol. 2000, 25, 169–193. [Google Scholar] [PubMed]
- Fire, A.; Xu, S.; Montgomery, M.K. Potent and specific genetic interference by double-stranded RNA in C. elegans. Nature 1998, 391, 806–811. [Google Scholar] [PubMed]
- Zamore, P.D.; Tuschl, T.; Sharp, P.A.; Bartel, D.P. RNAi: Double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell 2000, 101, 25–33. [Google Scholar]
- Nakayashiki, H.; Nguyen, Q.B. RNA interference: Roles in fungal biology. Curr. Opin. Microbiol. 2008, 11, 494–502. [Google Scholar] [PubMed]
- Skory, C.D.; Chang, P.K.; Linz, J.E. Regulated expression of the nor-1 and ver-1 genes associated with aflatoxin biosynthesis. Appl. Environ. Microbiol. 1993, 59, 1642–1646. [Google Scholar] [PubMed]
- Yu, J.; Chang, P.K.; Ehrlich, K.C.; Cary, J.W.; Bhatnagar, D.; Cleveland, R.E.; Payne, G.A.; Linz, J.E.; Woloshuk, C.P.; Bennett, J.W. Clustered pathway genes in aflatoxin biosynthesis. Appl. Environ. Microbiol. 2004, 70, 1253–1262. [Google Scholar] [PubMed]
- Bennett, J.W. Loss of norsolorinic acid and aflatoxin production by a mutant of Aspergillus parasiticus. J. Gen. Microbiol. 1981, 124, 429–432. [Google Scholar]
- McDonald, T.; Brown, D.; Keller, N.P.; Hammond, T.M. RNA silencing of mycotoxin production in Aspergillus and Fusarium species. Mol. Plant-Microbe Interact. 2005, 18, 539–545. [Google Scholar] [PubMed]
- Hammond, T.M.; Keller, N.P. RNA silencing in Aspergillus nidulans is independent of RNA-dependent RNA polymerases. Genetics 2005, 169, 607–617. [Google Scholar] [PubMed]
- Butchko, R.A.E.; Adams, T.H.; Keller, N.P. Aspergillus nidulans mutants defective in stc gene cluster regulation. Genetics 1999, 153, 715–720. [Google Scholar] [PubMed]
- Trail, F.; Mahanti, N.; Rarick, M.; Mehigh, R.; Liang, S.H.; Zhou, R.; Linz, J.E. Physical and transcriptional map of an aflatoxin gene cluster in Aspergillus parasiticus and functional disruption of a gene involved early in the aflatoxin pathway. Appl. Environ. Microbiol. 1995, 61, 2665–2673. [Google Scholar] [PubMed]
- Yabe, K.; Nakamura, Y.; Nakajima, H.; Ando, Y.; Hamasaki, T. Enzymatic conversion of norsolorinic acid to averufin in aflatoxin biosynthesis. Appl. Environ. Microbiol. 1991, 57, 1340–1345. [Google Scholar] [PubMed]
- Jackson, A.L.; Bartz, S.R.; Schelter, J.; Kobayashi, S.V.; Burchard, J.; Mao, M.; Li, B.; Cavet, G.; Linsley, P.S. Expression profiling reveals off-target gene regulation by RNAi. Nat. Biotechnol. 2003, 21, 635–637. [Google Scholar] [PubMed]
- Wilkinson, J.R.; Yu, J.; Bland, J.M.; Nierman, W.C.; Bhatnagar, D.; Cleveland, T.E. Amino acid supplementation reveals differential regulation of aflatoxin biosynthesis in Aspergillus flavus NRRL 3357 and Aspergillus parasiticus SRRC 143. Appl. Microbiol. Biotechnol. 2007, 74, 1308–1319. [Google Scholar] [PubMed]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Abdel-Hadi, A.M.; Caley, D.P.; Carter, D.R.F.; Magan, N. Control of Aflatoxin Production of Aspergillus flavus and Aspergillus parasiticus Using RNA Silencing Technology by Targeting aflD (nor-1) Gene. Toxins 2011, 3, 647-659. https://doi.org/10.3390/toxins3060647
Abdel-Hadi AM, Caley DP, Carter DRF, Magan N. Control of Aflatoxin Production of Aspergillus flavus and Aspergillus parasiticus Using RNA Silencing Technology by Targeting aflD (nor-1) Gene. Toxins. 2011; 3(6):647-659. https://doi.org/10.3390/toxins3060647
Chicago/Turabian StyleAbdel-Hadi, Ahmed M., Daniel P. Caley, David R. F. Carter, and Naresh Magan. 2011. "Control of Aflatoxin Production of Aspergillus flavus and Aspergillus parasiticus Using RNA Silencing Technology by Targeting aflD (nor-1) Gene" Toxins 3, no. 6: 647-659. https://doi.org/10.3390/toxins3060647




