Amino Acid and Biogenic Amine Profile Deviations in an Oral Glucose Tolerance Test: A Comparison between Healthy and Hyperlipidaemia Individuals Based on Targeted Metabolomics
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Subjects
2.2. Oral Glucose Tolerance Test (OGTT)
2.3. Biochemical Measurements
2.4. Serum Preparation for the Amino Acid Profiles
2.5. UPLC-TQ-MS Analysis
2.6. Statistical Analysis
3. Results
3.1. Demographic and Biochemical Characteristics
3.2. The Amino Acid and Biogenic Amine Profiles at Baseline
3.3. The Amino Acid and Biogenic Amine Profile Changes during the OGTT
3.4. Correlations between the Clinical Parameters and the Per Cent Changes from the 2-h Minus Fasting Metabolite Responses to the OGTT
3.5. Validation of the Significant Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Esteve, E.; Ricart, W.; Fernandez-Real, J.M. Dyslipidemia and inflammation: An evolutionary conserved mechanism. Clin. Nutr. 2005, 24, 16–31. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.H.; Bell, D.S. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am. J. Cardiol. 2007, 100, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Saydah, S.H.; Fradkin, J.; Cowie, C.C. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA 2004, 291, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Adiels, M.; Olofsson, S.O.; Taskinen, M.R.; Boren, J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Lindon, J.C.; Holmes, E. ‘Metabonomics’: Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 1999, 29, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Chen, H.; Zhang, X.; Yin, L.; Chen, D.Q.; Cheng, X.L.; Bai, X.; Wei, F. Urinary metabolomics on the biochemical profiles in diet-induced hyperlipidemia rat using ultraperformance liquid chromatography coupled with quadrupole time-of-flight synapt high-definition mass spectrometry. J. Anal. Methods Chem. 2014, 2014, 184162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, G.; Ma, B.; Dua, Y.; Zhu, L.; Wu, D. Metabonomic profiling of diet-induced hyperlipidaemia in a rat model. Biomarkers 2010, 15, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, C.; Wu, H.; Sheng, L.; Su, Y.; Luan, H.; Sun, G.; Sun, X.; Tian, Y.; Ji, Y.; et al. Anti-hyperlipidemic effects and potential mechanisms of action of the caffeoylquinic acid-rich pandanus tectorius fruit extract in hamsters fed a high fat-diet. PLoS ONE 2013, 8, e61922. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, G.J.; Ji-ye, A.; Wu, D.; Zhu, L.L.; Ma, B.; Du, Y. Application of GC/MS-based metabonomic profiling in studying the lipid-regulating effects of ginkgo biloba extract on diet-induced hyperlipidemia in rats. Acta Pharmacol. Sin. 2009, 30, 1674–1687. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Ding, L.L.; Li, J.M.; Xu, B.L.; Yang, L.; Bi, K.S.; Wang, Z.T. 1H-NMR and MS based metabolomics study of the intervention effect of curcumin on hyperlipidemia mice induced by high-fat diet. PLoS ONE 2015, 10, e0120950. [Google Scholar] [CrossRef] [PubMed]
- Shaham, O.; Wei, R.; Wang, T.J.; Ricciardi, C.; Lewis, G.D.; Vasan, R.S.; Carr, S.A.; Thadhani, R.; Gerszten, R.E.; Mootha, V.K. Metabolic profiling of the human response to a glucose challenge reveals distinct axes of insulin sensitivity. Mol. Syst. Biol. 2008, 4, 214. [Google Scholar] [CrossRef] [PubMed]
- Bentley-Lewis, R.; Xiong, G.; Lee, H.; Yang, A.; Huynh, J.; Kim, C. Metabolomic analysis reveals amino acid responses to an oral glucose tolerance test in women with prior history of gestational diabetes mellitus. J. Clin. Transl. Endocrinol. 2014, 1, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, C.; Zhu, K.; Feng, R.N.; Sun, C.H. Effects of multivitamin and mineral supplementation on adiposity, energy expenditure and lipid profiles in obese Chinese women. Int. J. Obes. (Lond.) 2010, 34, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Feng, R.; Guo, F.; Li, Y.; Jiao, J.; Sun, C. Targeted metabolomic analysis reveals the association between the postprandial change in palmitic acid, branched-chain amino acids and insulin resistance in young obese subjects. Diabetes Res. Clin. Pract. 2015, 108, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, X.; Wang, M.; Ning, H.; Lima, A.; Li, Y.; Sun, C. Lipoprotein lipase links vitamin D, insulin resistance, and type 2 diabetes: A cross-sectional epidemiological study. Cardiovasc. Diabetol. 2013, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, D.; Ceriello, A.; Paolisso, G. Oxidative stress and diabetic vascular complications. Diabetes Care 1996, 19, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Node, K.; Inoue, T. Postprandial hyperglycemia as an etiological factor in vascular failure. Cardiovasc. Diabetol. 2009, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Liu, L.; Sun, C. Effects of acute glucose loading on inflammation and oxidative stress in patients with simple hyperlipidemia. Acta Nutrimenta Sin. 2012, 34, 445–448. [Google Scholar]
- Gibbons, H.; McNulty, B.A.; Nugent, A.P.; Walton, J.; Flynn, A.; Gibney, M.J.; Brennan, L. A metabolomics approach to the identification of biomarkers of sugar-sweetened beverage intake. Am. J. Clin. Nutr. 2015, 101, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Holecek, M. The bcaa-bcka cycle: Its relation to alanine and glutamine synthesis and protein balance. Nutrition 2001, 17, 70. [Google Scholar] [CrossRef]
- Doi, M.; Yamaoka, I.; Nakayama, M.; Sugahara, K.; Yoshizawa, F. Hypoglycemic effect of isoleucine involves increased muscle glucose uptake and whole body glucose oxidation and decreased hepatic gluconeogenesis. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1683–E1693. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.J.; Gern, B.; Lloyd, C.; Hutson, S.M.; Eicher, R.; Vary, T.C. Leucine in food mediates some of the postprandial rise in plasma leptin concentrations. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E621–E630. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Tai, E.S.; Tan, M.L.; Stevens, R.D.; Low, Y.L.; Muehlbauer, M.J.; Goh, D.L.; Ilkayeva, O.R.; Wenner, B.R.; Bain, J.R.; Lee, J.J.; et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia 2010, 53, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Adhihetty, P.J.; Beal, M.F. Creatine and its potential therapeutic value for targeting cellular energy impairment in neurodegenerative diseases. Neuromol. Med. 2008, 10, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Y.; Li, S.; Pi, D.; Zhu, H.; Hou, Y.; Shi, H.; Leng, W. Asparagine attenuates intestinal injury, improves energy status and inhibits amp-activated protein kinase signalling pathways in weaned piglets challenged with escherichia coli lipopolysaccharide. Br. J. Nutr. 2015, 114, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lu, Y.; Shi, H.; Chen, F.; Zhu, H.; Hou, Y.; Ding, B.; Pi, A.; Leng, W. Effects of asparagine on growth performance, blood cell differential count and blood biochemical indices of weaned pigs challenged with lipopolysaccharide. Chin. J. Anim. Nutr. 2014, 4, 2450–2458. [Google Scholar]
- Thomas-Reetz, A.; Hell, J.W.; During, M.J.; Walch-Solimena, C.; Jahn, R.; De Camilli, P. A gamma-aminobutyric acid transporter driven by a proton pump is present in synaptic-like microvesicles of pancreatic beta cells. Proc. Natl. Acad. Sci. USA 1993, 90, 5317–5321. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Kumar, M.; Zhang, Y.; Gyulkhandanyan, A.; Xiang, Y.Y.; Ye, B.; Perrella, J.; Hyder, A.; Zhang, N.; Wheeler, M.; et al. Gamma-aminobutyric acid up- and downregulates insulin secretion from beta cells in concert with changes in glucose concentration. Diabetologia 2006, 49, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Liu, L.; Na, L.; Lu, H.; Li, S.; Li, Y.; Sun, C. Sterol regulatory element-binding protein-1c mediates increase of postprandial stearic acid, a potential target for improving insulin resistance, in hyperlipidemia. Diabetes 2013, 62, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Yokozawa, T.; Kim, H.J.; Shibahara, N. Protective effects of gamma-aminobutyric acid in rats with streptozotocin-induced diabetes. J. Nutr. Sci. Vitaminol. (Tokyo) 2005, 51, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F.; Hishikawa, K.; Katusic, Z.S.; Luscher, T.F. High glucose increases nitric oxide synthase expression and superoxide anion generation in human aortic endothelial cells. Circulation 1997, 96, 25–28. [Google Scholar] [CrossRef] [PubMed]
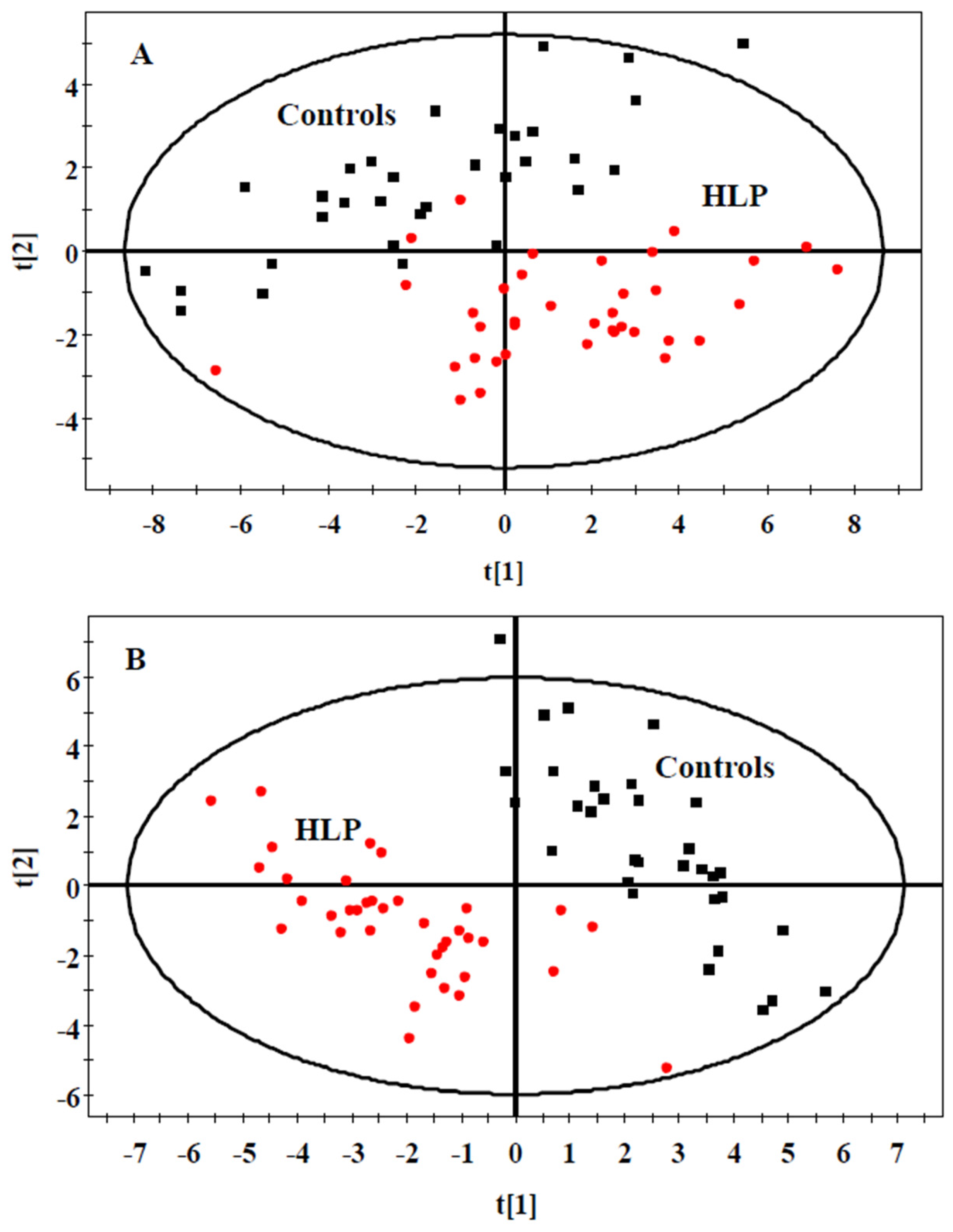


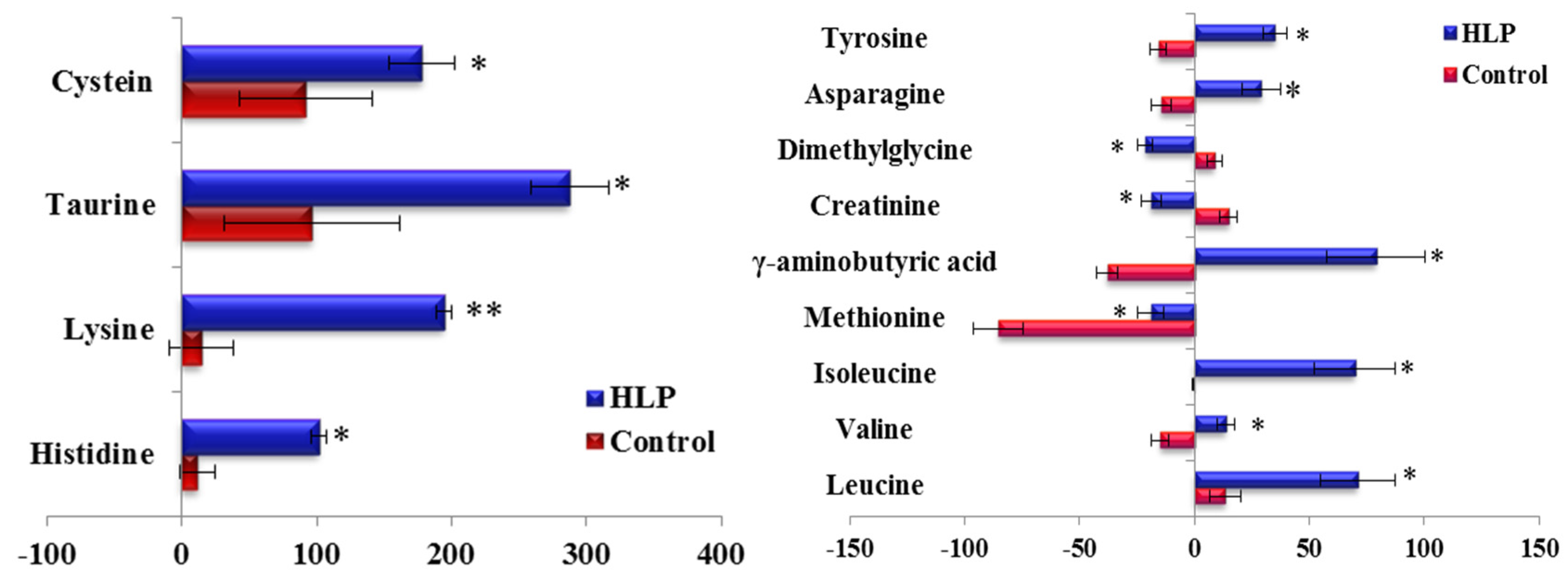
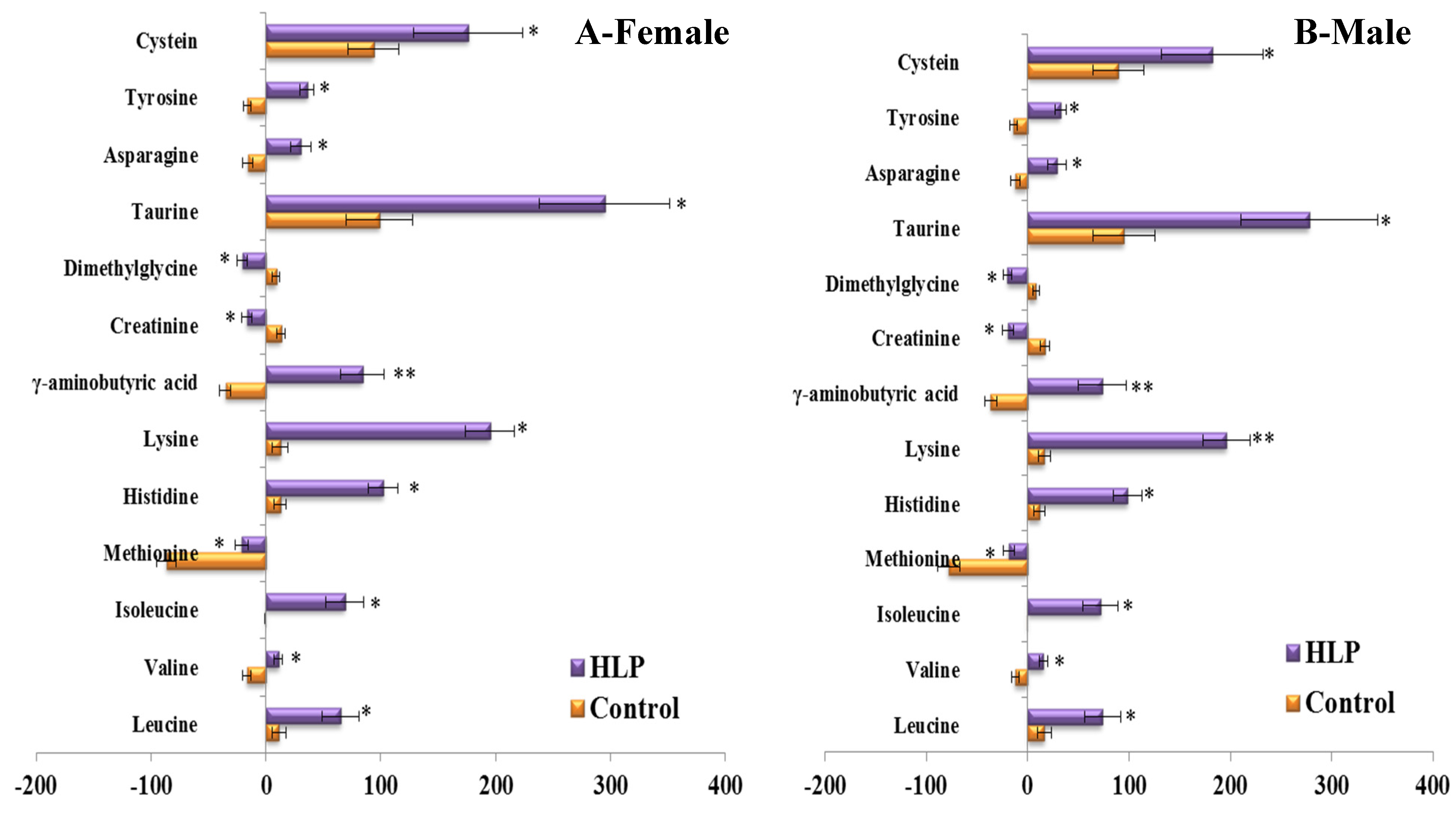


| Parameters | Control (n = 35) | HLP (n = 35) | p Value |
|---|---|---|---|
| Sex (female/male) | 17/18 | 16/19 | 0.12 |
| Age (years) | 47.54 ± 10.08 | 48.21 ± 8.24 | 0.11 |
| Smoker/non-smoker | 16/19 | 15/20 | 0.45 |
| Alcohol consumption (%) | 32.45 | 33.12 | 0.56 |
| Protein (g/day) | 61.34 ± 12.23 | 60.18 ± 14.31 | 0.67 |
| Fat (g/day) | 57.25 ± 10.04 | 56.94 ± 10.02 | 0.48 |
| Carbohydrate (g/day) | 302.45 ± 56.17 | 301.34 ± 58.28 | 0.51 |
| Physical activity level | 1.42 ± 0.34 | 1.37 ± 0.42 | 0.41 |
| BMI (kg/m2) | 22.30 ± 1.72 | 23.16 ± 1.68 | 0.32 |
| TC (mmmol/L) | 4.14 ± 0.53 | 6.41 ± 0.61 | <0.001 |
| TG (mmmol/L) | 0.94 ± 0.31 | 2.69 ± 0.83 | <0.001 |
| HDL-c (mmol/L) | 1.34 ± 0.18 | 1.00 ± 0.12 | <0.001 |
| LDL-c (mmol/L) | 2.51 ± 0.45 | 4.21 ± 0.76 | <0.001 |
| Fasting glucose (mmmol/L) | 4.08 ± 0.49 | 4.18 ± 0.47 | 0.08 |
| 2 h-glucose (mmmol/L) | 4.65 ± 0.94 | 5.01 ± 0.68 | 0.06 |
| SBP (mmHg) | 113.15 ± 6.31 | 115.68 ± 8.99 | 0.19 |
| DBP (mmHg) | 75.69 ± 6.23 | 79.15 ± 8.25 | 0.09 |
| Fasting insulin (mU/L) | 6.86 ± 3.07 | 13.91 ± 2.95 | <0.001 |
| 2h-insulin (mU/L) | 6.82 ± 2.14 | 36.72 ± 6.85 | <0.001 |
| HOMR-IR | 1.19 ± 0.36 | 2.82 ± 0.66 | <0.001 |
| Metabolites (μmol/L) | Control (n = 35) | HLP (n = 35) | p Value |
|---|---|---|---|
| Alanine | 522.80 ± 191.22 | 729.05 ± 164.80 | <0.001 |
| Arginine | 137.85 ± 65.43 | 268.08 ± 79.09 | <0.001 |
| Cystein | 39.42 ± 16.53 | 37.11 ± 20.01 | 0.607 |
| Glycine | 352.65 ± 123.97 | 365.16 ± 81.11 | 0.617 |
| Glutamic acid | 44.26 ± 15.47 | 54.73 ± 14.13 | 0.001 |
| Histidine | 128.31 ± 46.08 | 137.03 ± 48.55 | 0.449 |
| Isoleucine | 35.68 ± 10.84 | 45.58 ± 11.76 | 0.001 |
| Leucine | 21.39 ± 7.50 | 30.94 ± 8.51 | <0.001 |
| Lysine | 219.68 ± 79.02 | 226.50 ± 65.71 | <0.001 |
| Methionine | 9.98 ± 4.30 | 8.34 ± 3.44 | 0.077 |
| Phenylalanine | 119.44 ± 34.03 | 168.05 ± 34.36 | <0.001 |
| Proline | 208.95 ± 79.25 | 248.31 ± 62.49 | 0.024 |
| Serine | 170.18 ± 43.03 | 211.45 ± 42.34 | <0.001 |
| Tryptophan | 70.43 ± 16.72 | 82.66 ± 23.08 | 0.016 |
| Threonine | 164.72 ± 54.32 | 192.25 ± 39.57 | 0.018 |
| Tyrosine | 85.73 ± 29.39 | 118.24 ± 30.16 | <0.001 |
| Valine | 296.90 ± 90.61 | 370.06 ± 86.03 | 0.001 |
| Asparagine | 50.57 ± 15.10 | 48.45 ± 17.12 | 0.680 |
| Creatine | 13.31 ± 7.19 | 10.79 ± 4.62 | 0.084 |
| Creatinine | 84.39 ± 27.51 | 111.45 ± 28.19 | <0.001 |
| Cotinine | 0.039 ± 0.01 | 0.46 ± 0.24 | <0.001 |
| Dimethylglycine | 11.26 ± 5.06 | 9.71 ± 2.96 | 0.119 |
| Glutamine | 37.32 ± 11.52 | 23.93 ± 13.24 | 0.005 |
| Allantoin | 18.46 ± 9.74 | 18.16 ± 4.70 | 0.901 |
| 4-Hydroxy-l-proline | 15.53 ± 5.07 | 12.40 ± 6.37 | 0.202 |
| Niacinamide | 0.28 ± 0.09 | 0.42 ± 0.12 | <0.001 |
| Thyroxine | 0.021 ± 0.01 | 0.04 ± 0.01 | <0.001 |
| Trimethylamine-N-oxide | 0.36 ± 0.14 | 0.37 ± 0.20 | 0.859 |
| Aminobutyric acid | 11.83 ± 6.08 | 14.53 ± 6.23 | 0.075 |
| γ-aminobutyric acid | 264.56 ± 102.19 | 152.28 ± 35.40 | <0.001 |
| Taurine | 46.38 ± 16.46 | 34.50 ± 13.38 | 0.002 |
| l-α-Glycerophosphorylcholine | 12.98 ± 4.40 | 17.15 ± 4.79 | 0.004 |
| Metabolites | Control (n = 35) | ||||||
|---|---|---|---|---|---|---|---|
| TC | TG | HDL-c | LDL-c | Fasting Insulin | 2h-Insulin | HOMA-IR | |
| Valine | −0.345 (0.041) | −0.386 (0.032) | |||||
| Isoleucine | −0.347 (0.042) | ||||||
| Serine | 0.364 (0.034) | 0.351 (0.047) | |||||
| Histidine | −0.355 (0.039) | ||||||
| Creatine | 0.373 (0.035) | ||||||
| Lysine | −0.357 (0.045) | ||||||
| Creatinine | 0.379 (0.033) | ||||||
| Dimethylglycine | −0.408 (0.020) | ||||||
| Metabolites | HLP (n = 35) | ||||||
| TC | TG | HDL-c | LDL-c | Fasting insulin | 2h-insulin | HOMA-IR | |
| Valine | 0.398 (0.027) | 0.475 (0.006) | −0.358 (0.038) | 0.351 (0.041) | |||
| Isoleucine | 0.368 (0.038) | 0.387 (0.029) | −0.349 (0.042) | 0.371 (0.037) | 0.353 (0.047) | ||
| Serine | 0.352 (0.045) | 0.429 (0.014) | 0.352 (0.043) | ||||
| Histidine | −0.455 (0.009) | ||||||
| γ-aminobutyric acid | 0.374 (0.031) | ||||||
| Creatine | 0.473 (0.006) | ||||||
| Creatinine | −0.373 (0.03) | 0.409 (0.016) | |||||
| Dimethylglycine | −0.341 (0.048) | 0.475 (0.004) | |||||
| Asparagine | 0.441 (0.012) | 0.395 (0.025) | −0.338 (0.049) | ||||
| Tyrosine | 0.42 (0.017) | −0.383 (0.025) | 0.368 (0.032) | ||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Gu, W.; Ma, X.; Liu, Y.; Jiang, L.; Feng, R.; Liu, L. Amino Acid and Biogenic Amine Profile Deviations in an Oral Glucose Tolerance Test: A Comparison between Healthy and Hyperlipidaemia Individuals Based on Targeted Metabolomics. Nutrients 2016, 8, 379. https://doi.org/10.3390/nu8060379
Li Q, Gu W, Ma X, Liu Y, Jiang L, Feng R, Liu L. Amino Acid and Biogenic Amine Profile Deviations in an Oral Glucose Tolerance Test: A Comparison between Healthy and Hyperlipidaemia Individuals Based on Targeted Metabolomics. Nutrients. 2016; 8(6):379. https://doi.org/10.3390/nu8060379
Chicago/Turabian StyleLi, Qi, Wenbo Gu, Xuan Ma, Yuxin Liu, Lidan Jiang, Rennan Feng, and Liyan Liu. 2016. "Amino Acid and Biogenic Amine Profile Deviations in an Oral Glucose Tolerance Test: A Comparison between Healthy and Hyperlipidaemia Individuals Based on Targeted Metabolomics" Nutrients 8, no. 6: 379. https://doi.org/10.3390/nu8060379




