The Essentiality of Arachidonic Acid in Infant Development
Abstract
:1. Introduction
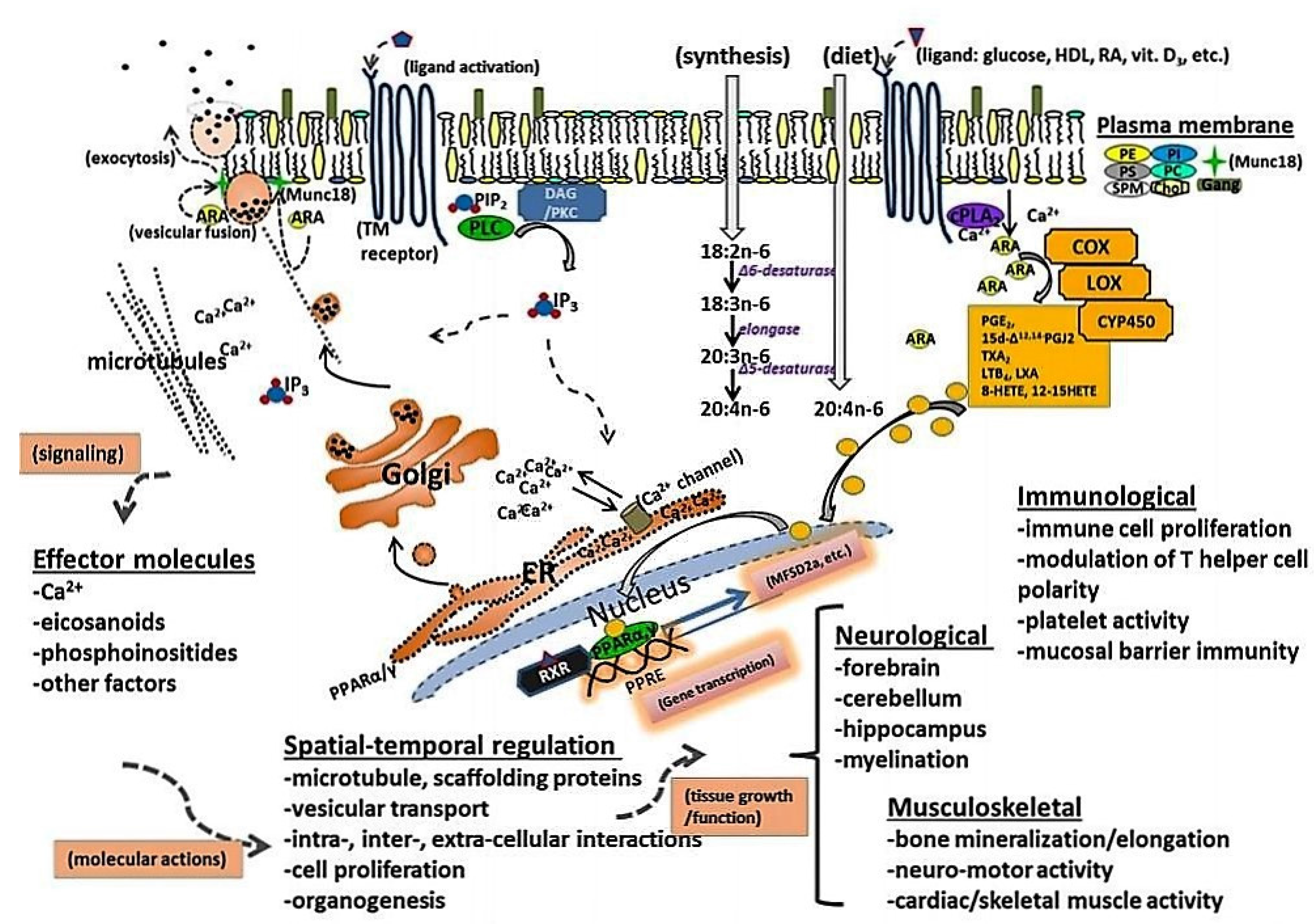
2. ARA Accumulation and Function in Brain and Tissue
3. Levels of ARA in Human Milk, Brain, and Tissues
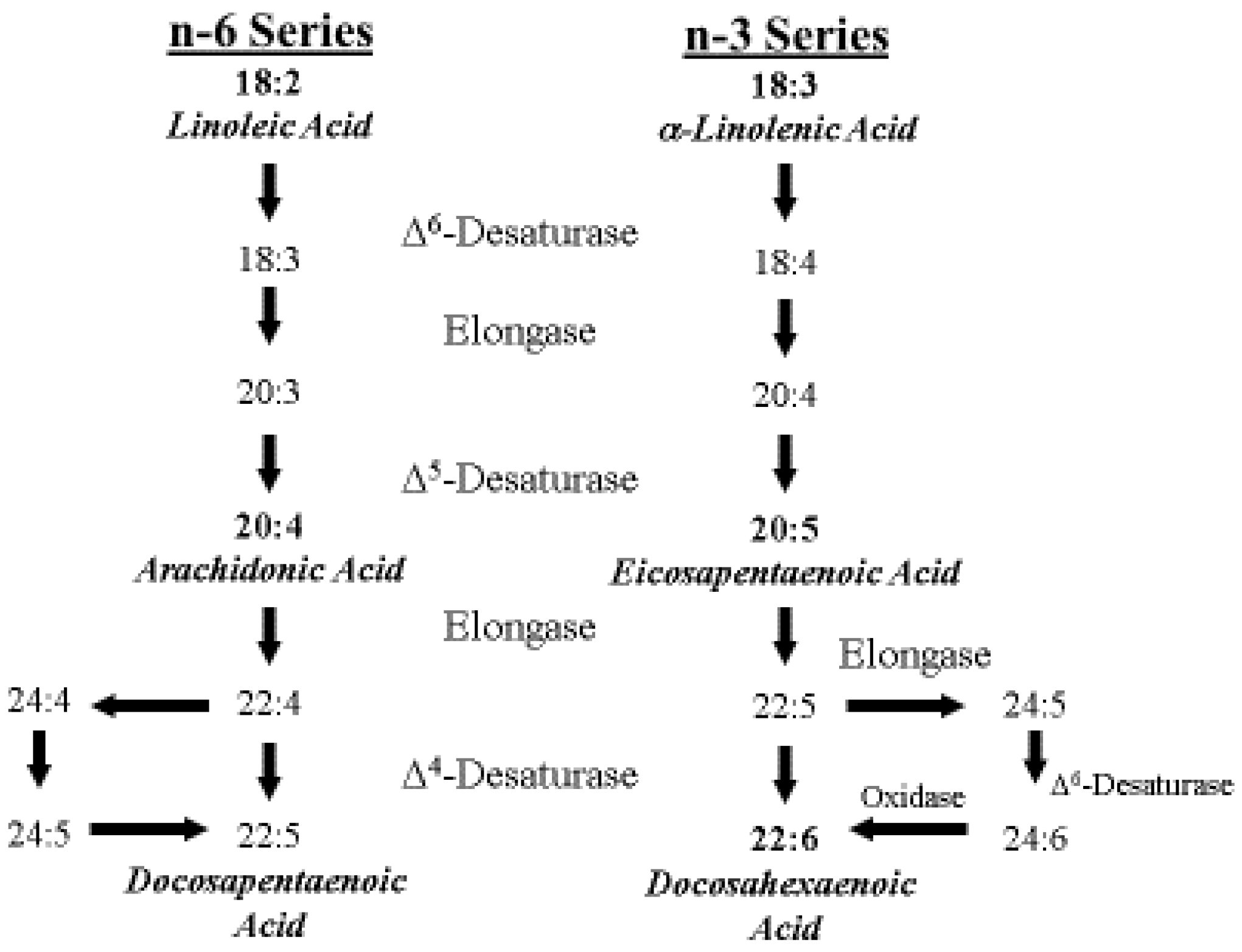
4. ARA Biosynthesis and Metabolism
5. Global Intake of ARA in Early Life
5.1. ARA Intake from Human Milk
5.2. ARA Intake from Infant Formula
5.3. ARA Intake from Weaning Foods
6. ARA and Its Role in Immune System Development and Function
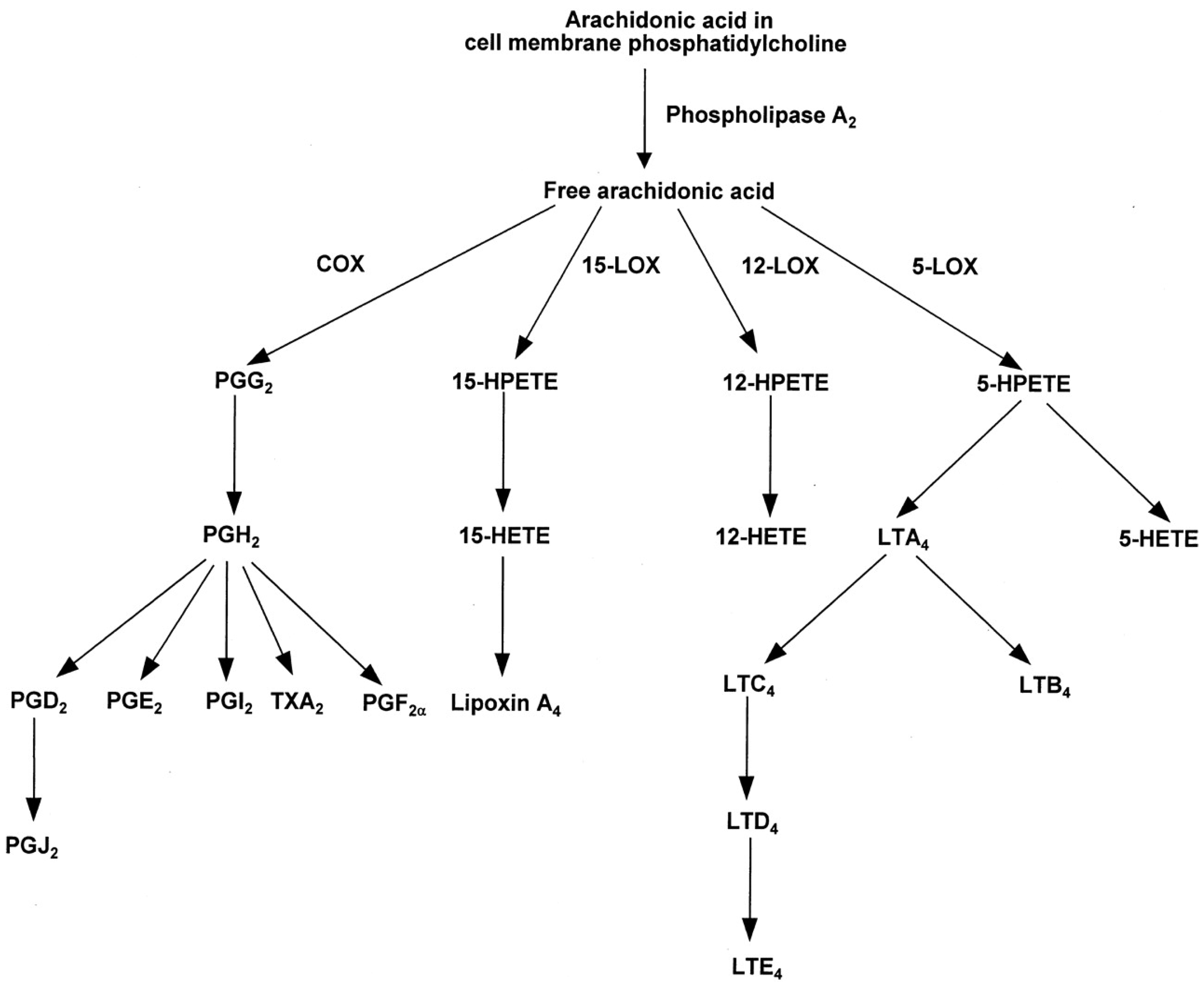
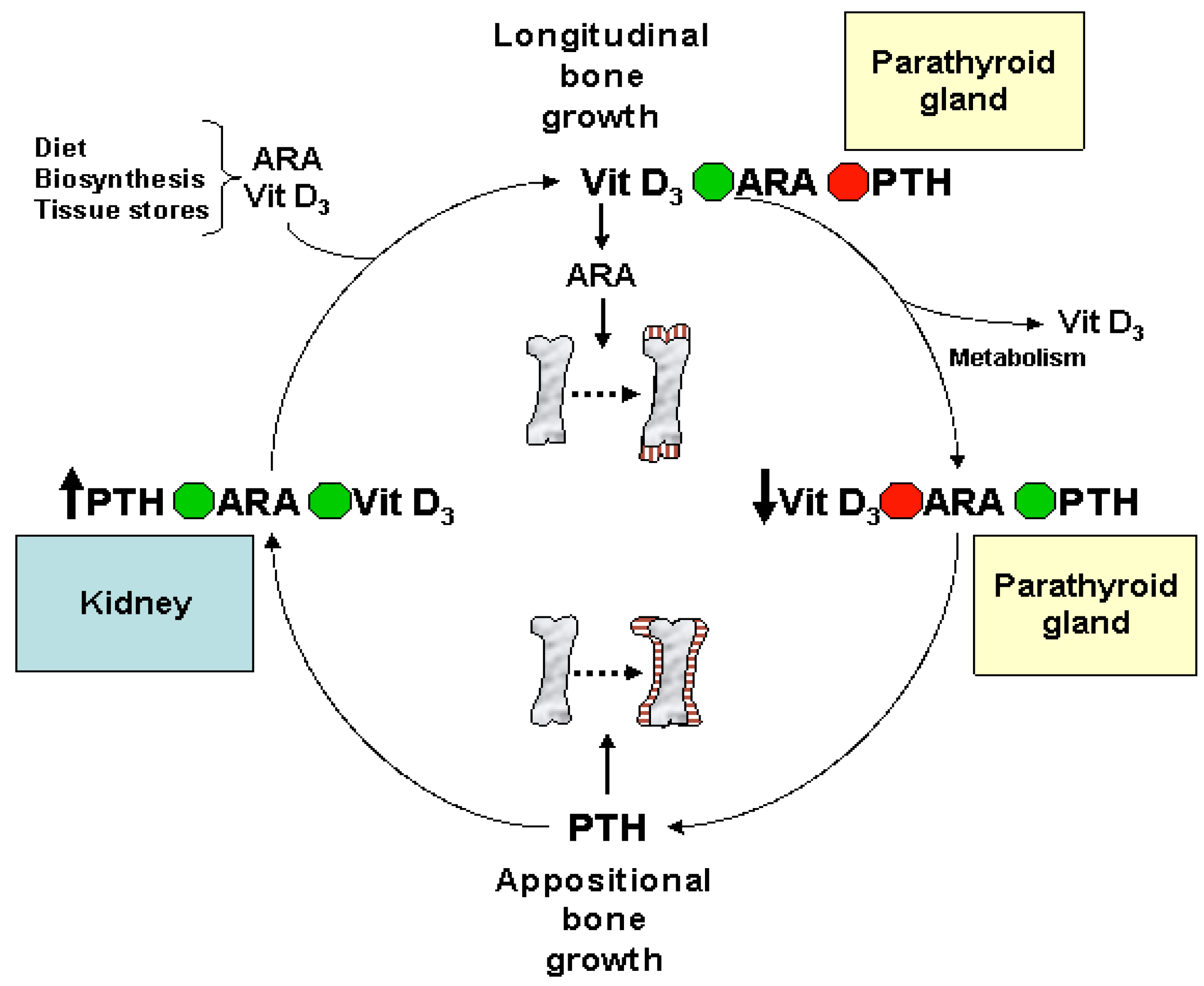
Eicosanoids and Their Effects on Hormones and Bone Formation
7. ARA in Skeletal and Cardiac Muscle
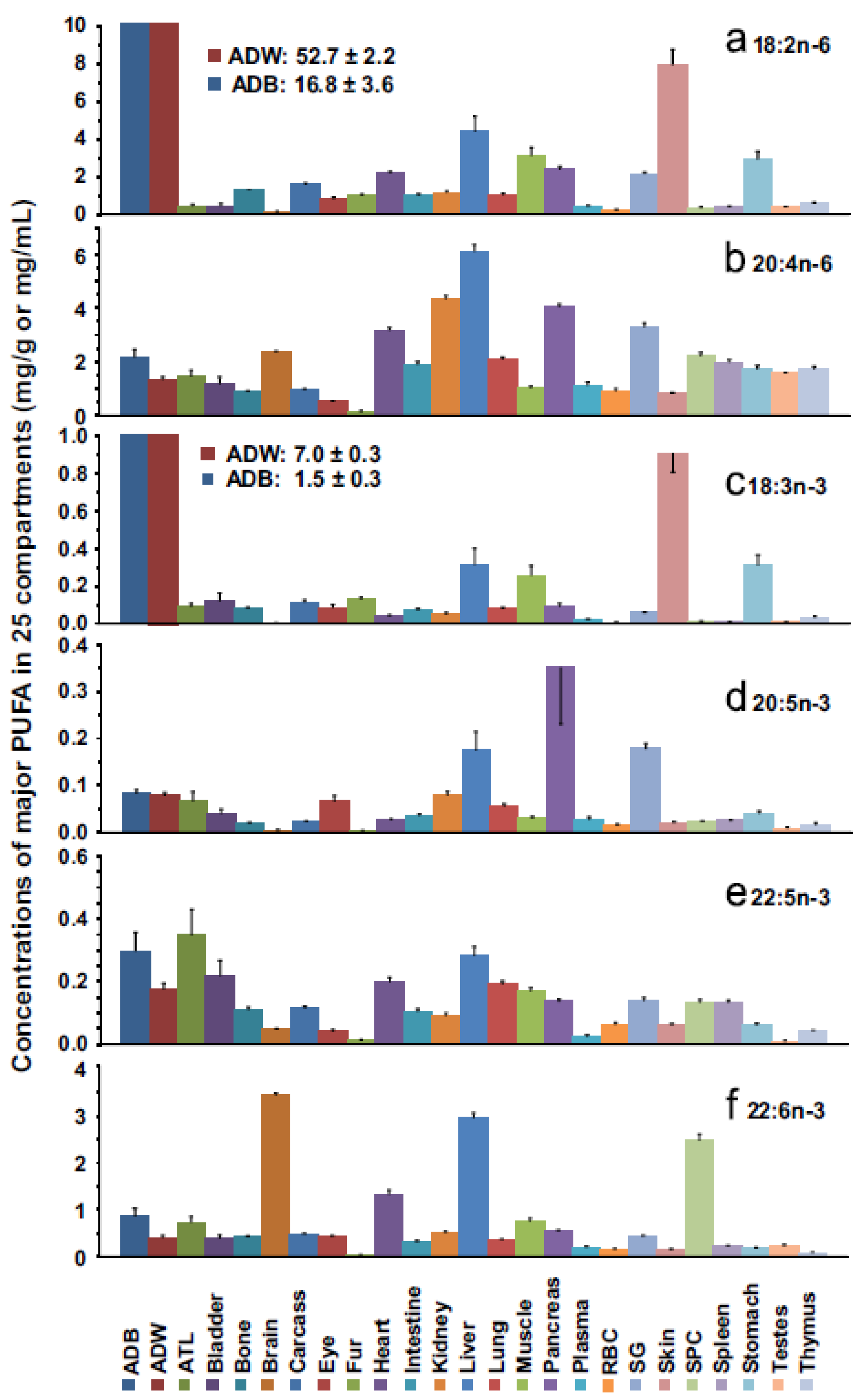
8. Biomagnification and Accretion of ARA in Infants
9. Consequences of ARA Deficiency
10. Animal Studies of ARA Supplementation
10.1. Immunomodulatory Effects of ARA and DHA Supplementation
10.2. Retinal and Neurodevelopmental Effects of ARA and DHA Supplementation
11. Introduction of DHA and ARA in Infant Formulas
12. The Benefits of ARA for Infant Health
13. The Regulatory Requirements for ARA and DHA in Infant Formulas
14. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Martinez, M. Tissue levels of polyunsaturated fatty acids during early human development. J. Pediatr. 1992, 120, S129–S138. [Google Scholar] [CrossRef]
- Koletzko, B.; Carlson, S.E.; van Goudoever, J.B. Should infant formula provide both omega-3 DHA and omega-6 arachidonic acid? Ann. Nutr. Metab. 2015, 66, 137–138. [Google Scholar] [CrossRef] [PubMed]
- Bolling, K. Infant Feeding Survey, 2005. Available online: http://www.hscic.gov.uk/pubs/ifs2005 (accessed on 17 August 2015).
- Centers for Disease Control and Prevention, Division of Nutrition, Physical Activity, and Obesity. Breastfeeding Report Card. 2014. Available online: http://www.cdc.gov/breastfeeding/data/reportcard.htm (accessed on 18 August 2015). [Google Scholar]
- Brenna, J.T.; Varamini, B.; Jensen, R.G.; Diersen-Schade, D.A.; Boettcher, J.A.; Arterburn, L.M. Docosahexaenoic and arachidonic acid concentrations in human milk worldwide. Am. J. Clin. Nutr. 2007, 85, 1457–1464. [Google Scholar] [PubMed]
- British Nutrition Foundation. Unsaturated Fatty Acids: Nutritional and Physiological Significance; Chapman & Hall: London, UK, 1992; pp. 152–163. [Google Scholar]
- Food and Agricultural Organization of the United Nations/World Health Organization Joint Expert Consultation. Lipids in early development. In Fats and Oils in Human Nutrition; FAO Food and Nutrition Papers; FAO: Rome, Italy, 1994; Volume 57, pp. 49–55. [Google Scholar]
- Simopoulos, A.P.; Leaf, A.; Salem, N., Jr. Workshop on the essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids. J. Am. Coll. Nutr. 1999, 18, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Baker, S.; Cleghorn, G.; Neto, U.F.; Gropalan, S.; Hernell, O.; Hock, Q.S.; Jirapinyo, P.; Lonnerdal, B.; Pencharz, P.; et al. Global standard for the composition of infant formula: Recommendations of an ESPAGHAN coordinated international expert group. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 584–599. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, L.; Hensen, H.S.; Jorgensen, M.H.; Michaelsen, K.F. The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog. Lipid Res. 2001, 40, 1–94. [Google Scholar] [CrossRef]
- Katsuki, H.; Okuda, S. Arachidonic acid as a neurotoxic and neurotrophic substance. Prog. Neurobiol. 1995, 46, 607–636. [Google Scholar] [CrossRef]
- Crawford, M.A.; Broadhurst, C.L. The role of docosahexaenoic and the marine food web as determinants of evolution and hominid brain development: The challenges for human sustainability. Nutr. Health 2012, 21, 17–39. [Google Scholar] [CrossRef] [PubMed]
- Bazan, N.G.; Reddy, T.S.; Bazan, H.E.P.; Birkle, D.L. Metabolism of arachidonic acid and docosahexaenoic acid in the retina. Prog. Lipid Res. 1886, 25, 595–606. [Google Scholar] [CrossRef]
- Crawford, M.A.; Sinclair, A.J. Nutritional influences in the evolution of mammalian brain. In Lipids, Malnutrition & the Developing Brain; A Ciba Foundation Symposium: Amsterdam, The Netherlands, 1971; pp. 267–292. [Google Scholar]
- Martinez, M. Polyunsaturated fatty acids in the developing human brain, red cells and plasma: Influence of nutrition and peroxisomal disease. In Fatty Acids and Lipids: Biological Aspects; Galli, C., Simopoulos, A.P., Tremoli, E., Eds.; Karger: Basel, Switzerland, 1994; Volume 75, pp. 70–78. [Google Scholar]
- Dobbing, J.; Sands, J. Comparative aspects of the brain growth spurt. Early Hum. Dev. 1979, 3, 79–83. [Google Scholar] [CrossRef]
- Su, H.-M.; Corso, T.N.; Nathanielsz, P.W.; Brenna, J.T. Linoleic acid kinetics and conversion to arachidonic acid in the pregnant and fetal baboon. J. Lipid Res. 1999, 40, 1304–1311. [Google Scholar] [PubMed]
- Sanchez-Mejia, R.O.; Newman, J.W.; Toh, S.; Yu, G.; Zhou, G.Q.; Halabisky, B.; Cissé, M.; Scearce-Levie, K.; Cheng, I.H.; Gan, L.; et al. Phospholipase A2 reduction ameliorates cognitive deficits in a mouse model of Alzheimer’s disease. Nat. Neurosci. 2008, 11, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghaven, S.; Huang, B.; Blumenthal, E.M.; Berg, D.L. Arachidonic acid as a possible negative feedback inhibitor of nicotinic acetylcholine receptors on neurons. J. Neurosci. 1995, 15, 3679–3687. [Google Scholar]
- Williams, J.H.; Errington, M.L.; Lynch, M.A.; Bliss, T.V. Arachidonic acid induces a long term activity-dependent enhancement of synaptic transmission in the hippocampus. Nature 1989, 341, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Fukaya, T.; Gondaira, T.; Kashiyae, Y.; Kotani, S.; Ishikura, Y.; Fujikawa, S.; Kiso, Y.; Sakakibara, M. Arachidonic acid preserves hippocampal neuron membrane fluidity in senescent rats. Neurobiol. Aging 2007, 28, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-J.; Liang, C.-L.; Li, G.-M.; Yu, C.-Y.; Yin, M. Neuroprotective effects of arachidonic acid against oxidative stress on rat hippocampal slices. Chem. Biol. Interact. 2006, 163, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Wijendran, V.; Lawrence, P.; Diau, G.-Y.; Boehm, G.; Nathanielsz, P.W.; Brenna, J.T. Significant utilization of dietary arachidonic acid is for brain adrenic acid in baboon neonates. J. Lipid Res. 2002, 43, 762–767. [Google Scholar] [PubMed]
- Hsieh, A.T.; Anthony, J.C.; Diersen-Schade, D.A.; Rumsey, S.C.; Lawrence, P.; Li, C.; Nathanielsz, P.W.; Brenna, J.T. The influence of moderate and high dietary long chain polyunsaturated fatty acids (LCPUFA) on baboon neonate tissue fatty acids. Pediatr. Res. 2007, 61, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-J.; Sugiura, Y.; Ikegami, K.; Konishi, Y.; Setou, M. Axonal gradient of arachidonic acid-containing phosphatidylcholine and its dependence on actin dynamics. J. Biol. Chem. 2012, 287, 5290–5300. [Google Scholar] [CrossRef] [PubMed]
- Bazan, N.G. The neuromessenger platelet-activation factor in plasticity and neurodegeneration. Prog. Brain Res. 1998, 118, 281–291. [Google Scholar] [PubMed]
- Hattori, M.H.; Adachi, H.; Tsujimoto, M.; Arai, H.; Inoue, K. Miller-Dieker lissencephaly gene encodes a subunit of brain platelet-activation factor acetylhydrolase. Nature 1994, 370, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Darios, F.; Davletov, B. Omega-3 and omega-6 fatty acids stimulate cell membrane expansion by acting on syntaxin 3. Nature 2006, 440, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Darios, F.; Ruiperez, V.; Lopez, I.; Villanueva, J.; Gutierrez, L.M.; Davletov, B. α-synuclein sequesters arachidonic acid to modulate SNARE-mediated exocytosis. EMBO Rep. 2010, 11, 528–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smart, E.J.; Graf, G.A.; McNiven, M.A.; Sessa, W.C.; Engelman, J.A.; Scherer, P.E.; Okamoto, T.; Lisanti, M.P. Caveolins, liquid-ordered domains, and signal transduction. Mol. Cell. Biol. 1999, 19, 7289–7304. [Google Scholar] [CrossRef] [PubMed]
- Pike, L.J.; Han, X.; Chung, K.-N.; Gross, R.W. Lipid rafts are enriched in arachidonic acid and plasmmenylethanolamine and their composition is independent of caveolin-1 expression: A quantitative electrospray ionization/mass spectrometric analysis. Biochemistry 2002, 41, 2075–2088. [Google Scholar] [CrossRef] [PubMed]
- Pike, L. Lipid rafts: Bringing order to chaos. J. Lipid Res. 2003, 44, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-C.; Sasaki, J.; Kubo, T.; Matsuda, S.; Nakasaki, Y.; Hattori, M.; Tanaka, F.; Udagawa, O.; Kono, N.; Itoh, T.; et al. LPIAT1 regulates arachidonic acid content in phosphatidylinositol and is required for cortical lamination in mice. Mol. Biol. Cell 2012, 23, 4689–4697. [Google Scholar] [CrossRef] [PubMed]
- Wolf, B.A.; Turk, J.; Sherman, W.R.; McDaniel, M.L. Intracellular Ca2+ mobilization of arachidonic acid. J. Biol. Chem. 1986, 261, 3501–3511. [Google Scholar] [PubMed]
- Cao, Y.; Pearman, A.T.; Zimmermann, G.A.; McIntyre, T.M.; Prescott, S.M. Intracellular unesterified arachidonic acid signals apoptosis. PNAS 2000, 97, 11280–11285. [Google Scholar] [CrossRef] [PubMed]
- Hicks, A.M.; DeLong, C.J.; Thomas, M.J.; Samuel, M.; Cui, Z. Unique molecular signatures of glycerophospholipid species in different rat tissues analyzed by tandem mass spectrometry. Biochim. Biophys. Acta 2006, 71, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Iwawaki, D.; Sakamoto, M.; Takai, Y.; Morishige, J.-I.; Murakami, K.; Satouchi, K. Mechanisms of accumulation of aracidonate in phoshatidylinositol in yellowtail. Eur. J. Biochem. 2003, 270, 1466–1473. [Google Scholar] [CrossRef] [PubMed]
- Jungalwala, F.B.; Evans, J.E.; McCluer, R.H. Compositional and molecular species analysis of phospholipids by high performance liquid chromatography couples with chemical ionization mass spectrometry. J. Lipid Res. 1984, 25, 738–749. [Google Scholar] [PubMed]
- Szentpetery, Z.; Varnai, P.; Balla, T. Acute manipulation of Golgi phosphoinositides to assess their importance in cellular trafficking and signaling. PNAS 2010, 107, 8225–8230. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, G.; De Camilli, P. Phosphoinositides in cell regulation and membrane dynamics. Nature 2006, 44, 12. [Google Scholar] [CrossRef] [PubMed]
- Malaiyandi, L.M.; Honick, A.S.; Rintoul, G.L.; Wang, Q.L.; Reynolds, I.J. Zn2+ inhibits mitochondrial movement in neurons by phosphatidylinositol 3-kinase activation. J. Neurosci. 2005, 25, 9507–9514. [Google Scholar] [CrossRef] [PubMed]
- De Vos, K.J.; Sable, J.; Miller, K.E.; Sheetz, M.P. Expression of phosphatidylinositol (4,5) bisphosphate-specific pleckstrin homology domains alters direction but not the level of axonal transport of mitochondria. Mol. Biol. Cell 2002, 14, 3636–3649. [Google Scholar] [CrossRef] [PubMed]
- Caroni, P. New EMBO members’ review: Actin cytoskeleton regulation through modulation of PI(4,5)P(2) rafts. EMBO J. 2001, 20, 4332–4336. [Google Scholar] [CrossRef] [PubMed]
- Del Prado, M.; Villalpando, S.; Elizondo, A.; Rodriguez, M.; Demmelmair, H.; Koletzko, B. Contribution of dietary and newly formed arachidonic acid to human milk lipids in women eating a low-fat diet. Am. J. Clin. Nutr. 2001, 74, 242–247. [Google Scholar] [PubMed]
- Crawford, M.A.; Golfetto, I.; Ghebremeskel, K.; Min, Y.; Moodley, T.; Poston, L.; Phylactos, A.; Cunnane, S.; Schmidt, W. The potential role for arachidonic and docosahexaenoic acids in protection against some central nervous system injuries in preterm infants. Lipids 2003, 38, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Larque, E.; Ruiz-Palacios, M.; Koletzko, B. Placental regulation of fetal nutrient supply. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Agostini, C.; Bergmann, R.; Ritzenthaler, K.; Shamir, R. Physiological aspects of human milk lipids and implications for infant feeding: A workshop report. Acta Paediatr. 2011, 100, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.J.; Crawford, M.A. The accumulation of arachidonate and docosahexaenoate in the developing rat brain. J. Neurochem. 1972, 19, 1753–1758. [Google Scholar] [CrossRef] [PubMed]
- Makrides, M.; Neumann, M.; Byard, R.W.; Simmer, K.; Gibson, R.A. Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am. J. Clin. Nutr. 1994, 60, 189–194. [Google Scholar] [PubMed]
- Kuipers, R.S.; Luxwolda, M.F.; Offringa, P.J.; Boersma, E.R.; Dijck-Brouwer, D.A.; Muskiet, F.A.J. Fetal intrauterine whole body linoleic, arachidonic, and docosahexaenoic acid contents and accretion rates. Prostaglandins Leukot. Essent. Fat. Acids 2011, 86, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Carver, J.D.; Benford, V.J.; Han, B.; Cantor, A.B. The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Res. Bull. 2001, 56, 79–85. [Google Scholar] [CrossRef]
- Salem, N.M.; Lin, Y.H.; Moriguchi, T.; Lim, S.Y.; Salem, N., Jr.; Hibbelin, J.R. Distribution of omega-6 and omega-3 polyunsaturated fatty acids in the whole rat body and 25 compartments. Prostaglandins Leukot. Essent. Fat. Acids 2015, 100, 13–20. [Google Scholar] [CrossRef] [PubMed]
- DeMar, J.C., Jr.; DiMartino, C.; Baca, A.W.; Lefkowitz, W.; Salem, N., Jr. Effect of dietary docosahexaenoic acid on biosynthesis of docosahexaenoic acid from alpha-linolenic acid in young rats. J. Lipid Res. 2008, 49, 1963–1980. [Google Scholar] [CrossRef] [PubMed]
- Tyburczy, C.; Kothapalli, K.S.D.; Park, W.J.; Blank, B.S.; Bradford, K.L.; Zimmer, J.P.; Butt, C.M.; Salem, N., Jr.; Brenna, J.T. Heart arachidonic acid is uniquely sensitive to dietary arachidonic acid and docosahexaenoic acid content in domestic piglets. Prostaglandins Leukot. Essent. Fat. Acids 2011, 85, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, J. Receptor-mediated activation of phospholipase A2 and arachidonic acid release in signal transduction. Biochem. Soc. Trans. 1990, 18, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Piomelli, D. Eicosanoids in synaptic transmissions. Crit. Rev. Neurobiol. 1994, 11, 367–373. [Google Scholar]
- Schoenheimer, R.; Rittenberg, D. Deuterium as an indicator in the study of intermediary metabolism V. The desaturation of fatty acids in the organism. J. Biol. Chem. 1936, 113, 505–510. [Google Scholar]
- Nichaman, M.Z.; Olson, R.E.; Sweeley, C.C. Metabolism of linoleic acid-1-14C in normolipidemic and hyperlipidemic humans fed linoleate diets. Am. J. Clin. Nutr. 1967, 20, 1070–1083. [Google Scholar] [PubMed]
- Chambaz, J.; Ravel, D.; Manier, M.-C.; Pepin, D.; Mulliez, N.; Bereziat, G. Essential fatty acids interconversion in the human fetal liver. Neonatology 1985, 47, 136–140. [Google Scholar] [CrossRef]
- El Boustani, S.; Descomps, B.; Monnier, L.; Warnant, J. In vivo conversion of dihommogamma linolenic acid into arachidonic acid in man. Prog. Lipid Res. 1986, 25, 67–71. [Google Scholar] [CrossRef]
- Emken, E.A.; Adlof, R.O.; Rakoff, H.; Rohwedder, W.K. Metabolism of deuterium-labeled linolenic, linoleic, oleic, stearic and palmitic acid in human subjects. In Synthesis and Applications of Isotopically Labelled Compounds, Proceedings of the Third International Symposium, Innsbruck, Austria, 17–21 July 1988; Baillie, T.A., Jones, J.R., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1988; pp. 713–716. [Google Scholar]
- Emken, E.A.; Rohwedder, W.K.; Adlof, R.O.; Gulley, R.M. Metabolism in humans of cis-12, trans-15-octadecadienoic acid relative to palmitic, stearic, oleic and linoleic acids. Lipids 1987, 22, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Demmelmair, H.; von Schenck, U.; Behrendt, E.; Sauerwald, T.; Koletzko, B. Estimation of arachidonic acid synthesis in full-term neonates using natural variation of 13C content. J. Pediatr. Gastroenterol. Nutr. 1995, 21, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Salem, N., Jr.; Wegher, B.; Mena, P.; Uauy, R. Arachidonic and docosahexaenoic acids are biosynthesized from their 18-carbon precursors in human infants. Proc. Natl. Acad. Sci. USA 1996, 93, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Pawlosky, R.J.; Sprecher, H.W.; Salem, N., Jr. High sensitivity negative ion GC/MS method for detection of desaturated and chain-elongated products of deuterated linoleic and linolenic acids. J. Lipid Res. 1992, 33, 1711–1717. [Google Scholar] [PubMed]
- Carnielli, V.P.; Wattimea, D.J.L.; Luijendijk, I.H.T.; Boerlage, A.; Degenhart, H.J.; Sauer, P.J.J. The very low weight premature infant is capable of synthesizing arachidonic and docosahexaenoic acids from linoleic and linolenic acids. Pediatr. Res. 1996, 40, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Pawlosky, R.J.; Lin, Y.H.; Llanos, A.; Mena, P.; Uauy, R.; Salem, N., Jr. Compartmental analysis of plasma 13C- and 2H-labelled n-6 fatty acids arising from oral administrations of 13C-U-18:2n-6 and 2H5-20:3n-6 in newborn infants. Pediatr. Res. 2006, 60, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Sauerwald, T.U.; Hachey, D.L.; Jensen, C.L.; Chen, H.; Andersen, R.E.; Heird, W.C. Effect of dietary α-linolenic intake on incorporation of docosahexaenoic and arachidonic acids into plasma phospholipids of term infants. Lipids 1996, 31, S131–S135. [Google Scholar] [CrossRef] [PubMed]
- Carnielli, V.P.; Simonato, M.; Verlato, G.; Luijendijk, I.; De Curtis, M.; Sauer, P.J.J.; Cogo, P.E. Synthesis of long-chain polyunsaturated fatty acids in preterm newborns fed formula with long-chain polyunsaturated fatty acids. Am. J. Clin. Nutr. 2007, 86, 1323–1330. [Google Scholar] [PubMed]
- EFSA Panel on Dietetic Products. Scientific opinion on nutrient requirements and dietary intakes on infants and young children in the European Union. EFSA J. 2013, 11, 3408. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the essential composition of infant and follow-on formulae. EFSA J. 2014, 12, 3760. [Google Scholar]
- Birch, E.E.; Castañeda, Y.S.; Wheaton, D.H.; Birch, D.G.; Uauy, R.D.; Hoffman, D.R. Visual maturation of term infants fed long-chain polyunsaturated fatty acid-supplemented or control formula for 12 mo. Am. J. Clin. Nutr. 2005, 81, 871–879. [Google Scholar] [PubMed]
- World Health Organization (WHO). Global Strategy on Infant and Young Child Feeding, 2002. Available online: http://www.who.int/nutrition/topics/infantfeeding _recommendation/en/ (accessed on 15 October 2015).
- World Health Organization (WHO). Nutrient Adequacy of Exclusive Breastfeeding for the Term Infant during the First Six Months of Life, 2002. Available online: http://www.who.int/nutrition/publications/infantfeeding/9241562110/en/ (accessed on 15 October 2015).
- American Academy of Pediatrics. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827. [Google Scholar] [CrossRef]
- International Baby Food Action (IBFAN). The State of Breastfeeding in 33 Countries, 2010. Available online: https://www.google.co.uk/?gws_rd=ssl#q=IBFAN+33+countries (accessed on 15 October 2015).
- U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 27. Nutrient Data Laboratory Home Page. 2014. Available online: http://www.ars.usda.gov/nutrientdata (accessed on 15 October 2015). [Google Scholar]
- Centers for Disease Control and Prevention. Clinical Growth Charts, 2015. Available online: http://www.cdc.gov/growthcharts/cdccharts.htm (accessed on 19 November 2015).
- Michaelsen, K.F.; Dewey, K.G.; Perez-Exposito, A.B. Food sources and intake of n-6 and n-3 fatty acids in low-income countries with emphasis on infants, young children (6–24 months), and pregnant and lactating women. Mater. Child Nutr. 2011, 7 (Suppl. S2), 124–140. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C. Docosahexaenoic acid (DHA): From the maternal-foetal dyad to the complementary feeding period. Early Hum. Dev. 2010, 86 (Suppl. S1), 3–6. [Google Scholar] [CrossRef] [PubMed]
- Prentice, A.M.; Paul, A.A. Fat and energy needs of children in developing countries. Am. J. Clin. Nutr. 2000, 72, 1253S–1265S. [Google Scholar] [PubMed]
- Barbarich, B.N.; Willows, N.D.; Wang, L.; Clandinin, M.T. Polyunsaturated fatty acids and anthropometric indices of children in rural China. Eur. J. Clin. Nutr. 2006, 60, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- PAHO/WHO. Guiding Principles for Complementary Feeding of the Breastfed Child; PAHO/WHO: Washington, DC, USA, 2003. [Google Scholar]
- Joshi, N.; Agho, K.E.; Dibley, M.J.; Senarath, U.; Tiwari, K. Determinants of inappropriate complementary feeding practices in young children in Nepal: Secondary data analysis of Demographic and Health Survey 2006. Mater. Child Nutr. 2012, 1, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Dutta, T.; Sywulka, S.M.; Frongillo, E.A.; Lutter, C.K. Characteristics attributed to complementary foods by caregivers in four countries of Latin America and the Caribbean. Food Nutr. Bull. 2006, 27, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Decsi, T.; Fewtrell, M.; Goulet, O.; Kolacek, S.; Koletzko, B.; Michaelsen, K.F.; Moreno, L.; Puntis, J.; Rigo, J.; et al. Complementary feeding: A commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.; Dube, K.; Alexy, U.; Kalhoff, H.; Kersting, M. PUFA and LC-PUFA intake during the first year of life: Can dietary practice achieve a guideline diet? Eur. J. Clin. Nutr. 2010, 64, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.; Dube, K.; Sichert-Hellert, W.; Kannenberg, F.; Kunz, C.; Kalhoff, H.; Kersting, M. Modification of dietary polyunsaturated fatty acids via complementary food enhances n-3 long-chain polyunsaturated fatty acid synthesis in healthy infants: A double blinded randomised controlled trial. Arch. Dis. Child. 2009, 94, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Grote, V.; Verduci, E.; Scalioni, S.; Vecchi, F.; Contarini, G.; Giovannini, M.; Koletzko, B.; Agostoni, C. Breast milk composition and infant nutrient intakes during the first 12 months of life. Eur. J. Clin. Nutr. 2015, 70, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Sioen, I.; Matthys, C.; De Backer, G.; Van Camp, J.; De Henauw, S. Importance of seafood as nutrient source in the diet of Belgian adolescents. J. Hum. Nutr. Diet. 2007, 20, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.J.; Mann, N.J.; Lewis, J.L.; Milligan, G.C.; Sinclair, A.J.; Howe, P.R. Dietary intakes and food sources of omega-6 and omega-3 polyunsaturated fatty acids. Lipids 2003, 38, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M.; Vaghri, Z.; King, D.J. n-6 docosapentaenoic acid is not a predictor of low docosahexaenoic acid status in Canadian preschool children. Am. J. Clin. Nutr. 2004, 80, 768–773. [Google Scholar] [PubMed]
- Lien, V.W.; Clamdinin, M.T. Dietary assessment of arachidonic acid and docosahexaenoic acid intake in 4–7 year-old children. J. Am. Coll. Nutr. 2009, 28, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Keim, S.A.; Branum, A.M. Dietary intake of polyunsaturated fatty acids and fish among US children 12–60 months of age. Mater. Child Health Nutr. 2015, 11, 987–998. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture, Agricultural Research Service. What We Eat in America. 2015. Available online: http://www.ars.usda.gov/Services/docs.htm?docid=13793# (accessed on 15 October 2015). [Google Scholar]
- Birch, E.E.; Khoury, J.C.; Berseth, C.L.; Castaneda, Y.S.; Couch, J.M.; Bean, J.; Tamer, R.; Harris, C.L.; Mitmesser, S.H.; Scalabrin, D.M. The impact of early nutrition on incidence of allergic manifestations and common respiratory illnesses in children. J. Pediatr. 2010, 156, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Polyunsaturated fatty acids and inflammation: From molecular biology to the clinic. Lipids 2003, 38, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [PubMed]
- Jones, P.J.H.; Kubow, S. Lipids, sterols, and their metabolites. In Modern Nutrition in Health and Disease; Shils, M.E., Shike, M., Ross, A.C., Caballero, B., Cousins, B., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; pp. 92–122. [Google Scholar]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, H.; Ahmadi, S.; Depner, U.B.; Layh, B.; Heindl, C.; Hamza, M.; Pahl, A.; Brune, K.; Narumiya, S.; Müller, U.; et al. Spinal inflammatory hyperalgesia is mediated by prostaglandin E receptors of the EP2 subtype. J. Clin. Investig. 2005, 115, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Noda, M.; Kariura, Y.; Pannasch, U.; Nishikawa, K.; Wang, L.; Seike, T.; Ifuku, M.; Kosai, Y.; Wang, B.; Nolte, C.; et al. Neuroprotective role of bradykinin because of the attenuation of pro-inflammatory cytokine release from activated microglia. J. Neurochem. 2007, 101, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Crawford, M.A. Essential fatty acids. In The Eicosanoids; Curtis, P., Ed.; John Wiley & Sons, Ltd.: West Sussex, UK, 2004; pp. 257–276. [Google Scholar]
- McCarthy, T.L.; Casinghino, S.; Mittanck, D.W.; Ji, C.H.; Centrella, M.; Rotwein, P. Promoter-dependent and -independent activation of insulin-like growth factor binding protein-5 gene expression by prostaglandin E2 in primary rat osteoblasts. J. Biol. Chem. 1996, 271, 6666–6671. [Google Scholar] [PubMed]
- Urade, Y.; Hayaishi, O. Prostaglandin D2 and sleep regulation. Biochem. Biophys. Acta 1999, 1436, 606–615. [Google Scholar] [CrossRef]
- Ushikubi, F.; Sergi, E.; Sugimoto, Y.; Murata, T.; Matsuoka, T.; Kobayashi, T.; Hizaki, H.; Tuboi, K.; Katsuyama, M.; Ichikawa, A.; et al. Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3. Nature 1998, 395, 281–284. [Google Scholar] [PubMed]
- Murata, T.; Ushikubi, F.; Matsuoka, T.; Hirata, M.; Yamasaki, A.; Sugimoto, Y.; Ichikawa, A.; Aze, Y.; Tanaka, T.; Yoshida, N.; et al. Altered pain perception and inflammatory response in mice lacking prostacyclin receptor. Nature 1997, 388, 678–682. [Google Scholar] [PubMed]
- Astudillo, A.M.; Balgoma, D.; Balboa, M.A.; Balsinde, J. Dynamics of arachidonic acid mobilization by inflammatory cells. Biochim. Biophys. Acta 2012, 1821, 249–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagga, D.; Wang, L.; Faris-Eisner, R.; Glaspy, J.A.; Reddy, S.T. Differential effects of prostaglandin derived from ω-6 and ω-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc. Natl. Acad. Sci. USA 2003, 100, 1751–1756. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.D.; Clish, C.B.; Schmidt, B.; Gronert, K.; Serhan, C.N. Lipid mediator class switching during acute inflammation signals in resolution. Nat. Immunol. 2001, 2, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, B.; Dahlen, S.E.; Lindgren, J.A.; Rouzer, C.A.; Serhan, C.N. Leukotrienes and lipoxins—Structures, biosynthesis, and biological effects. Science 1987, 237, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Fredman, G.; Serhan, C.N. Specialized proresolving mediator targets for RvE1 and RvD1 in peripheral blood and mechanisms of resolution. Biochem. J. 2011, 437, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.S. Modulation of human and inflammatory responses by dietary fatty acids. Nutrition 2001, 17, 669–673. [Google Scholar] [CrossRef]
- Calder, P.C.; Kew, S. The immune system: A target for functional foods? Br. J. Nutr. 2002, 88 (Suppl. S2), S165–S176. [Google Scholar] [CrossRef] [PubMed]
- Lentz, A.K.; Feezor, R.J. Principles of immunology. Nutr. Clin. Pract. 2003, 18, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Mahadevappa, V.G.; Holub, B.J. The molecular species composition of individual diacyl phospholipids in human platelets. Biochim. Biophys. Acta 1982, 713, 73–79. [Google Scholar] [CrossRef]
- Kaushansky, K. Lineage-specific hematopoietic growth factors. N. Engl. J. Med. 2006, 354, 2034–2045. [Google Scholar] [CrossRef] [PubMed]
- Semple, J.W.; Italiano, J.E., Jr.; Freedman, J. Platelets and the immune system. Nature 2011, 11, 264–274. [Google Scholar]
- Tamagawa-Mineoka, R. Important roles of platelets as immune cells in the skin. J. Dermatol. Sci. 2015, 77, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; DaSilva, D.A.; Cluette-Brown, J.E.; DiMonda, C.; Hamill, A.; Bhutta, A.Q.; Coronel, E.; Wilschanski, M.; Stephens, A.J.; Driscoll, D.F.; et al. Decreased postnatal docosahexaenoic and arachidonic acid blood levels in premature infants are associated with neonatal morbidities. J. Pediatr. 2011, 159, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Lands, W.F.; LeTellier, P.R.; Rome, L.H.; Vanderhoek, J.Y. Inhibition of prostaglandin biosynthesis. Adv. Biosci. 1973, 9, 15–227. [Google Scholar]
- Vane, J.R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature 1971, 231, 232–235. [Google Scholar] [CrossRef]
- Hormones. Available online: http://www.medicinenet.com/script/main/art.asp?articlekey=3783 (accessed on 6 February 2016).
- McMurray, W.C. A Synopsis of Human Biochemistry; Harper and Row Publishers: New York, NY, USA, 1982; pp. 193–199. [Google Scholar]
- Bowen, R.A. Hormone Chemistry, Synthesis and Elimination. Available online: http://www.vivo.colostate.edu/hbooks/pathphys/endocrine/basics/chem.html (accessed on 15 October 2015).
- Thorner, M.O.; Vance, M.L.; Hartman, M.L.; Holl, R.W.; Evans, W.S.; Veldhuis, J.D.; Van Cauter, E.; Copinschi, G.; Bowers, C.Y. Physiological role of somatostatin on growth hormone regulation in humans. Metabolism 1990, 39, 40–42. [Google Scholar] [CrossRef]
- Brochhausen, C.; Neuland, P.; Kirkpatrick, C.J.; Nusing, R.M.; Klaus, G. Cyclooxygenases and prostaglandin E2 receptors in growth plate chondrocytes in vitro and in situ-prostaglandin E2 dependent proliferation of growth plate chondrocytes. Arthritis Res. Ther. 2006, 8, R78. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Sylvia, V.L.; Dean, D.D.; Del Toro, F.; Schwartz, Z. Differential regulation of growth plate chondrocytes by 1alpha,25-(OH)2D3 and 24R,25-(OH)2D3 involves cell-maturation-specific membrane-receptor-activated phospholipid metabolism. Crit. Rev. Oral Biol. Med. 2002, 13, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Specker, B.; Binkley, T. Randomized trial of physical activity and calcium supplementation on bone mineral content in 3- to 5-year-old children. J. Bone Miner. Res. 2003, 418, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Specker, B.L.; Mulligan, L.; Ho, M. Longitudinal study of calcium intake, physical activity, and bone mineral content in infants 6–18 months of age. J. Bone Miner. Res. 1999, 14, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Del Toro, F., Jr.; Sylvia, V.L.; Schubkegel, S.R.; Campos, R.; Dean, D.D.; Boyan, B.D.; Schwartz, Z. Characterization of prostaglandin E(2) receptors and their role in 24,25-(OH)(2)D(3)-mediated effects on resting zone chondrocytes. J. Cell Physiol. 2000, 182, 196–208. [Google Scholar] [CrossRef]
- Boyan, B.D.; Sylvia, V.L.; Dean, D.D.; Pedrozo, H.; Del Toro, F.; Nemere, I.; Posner, G.H.; Schwartz, Z. 1,25-(OH)2D3 modulates growth plate chondrocytes via membrane receptor-mediated protein kinase C by a mechanism that involves changes in phospholipid metabolism and the action of arachidonic acid and PGE2. Steroids 1999, 64, 129–136. [Google Scholar] [PubMed]
- Schwartz, Z.; Sylvia, V.L.; Curry, D.; Luna, M.H.; Dean, D.D.; Boyan, B.D. Arachidonic acid directly mediates the rapid effects of 24,25-dihydroxyvitamin D3 via protein kinase C and indirectly through prostaglandin production in resting zone chondrocytes. Endocrinology 1999, 140, 2991–3002. [Google Scholar] [CrossRef] [PubMed]
- Sylvia, V.L.; Schwartz, Z.; Curry, D.B.; Chang, Z.; Dean, D.D.; Boyan, B.D. 1,25(OH)2D3 regulates protein kinase C activity through two phospholipid-dependent pathways involving phospholipase A2 and phospholipase C in growth zone chondrocytes. J. Bone Miner. Res. 1998, 13, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Kosher, R.A.; Walker, K.H. The effect of prostaglandins on in vitro limb cartilage differentiation. Exp. Cell Res. 1983, 145, 145–153. [Google Scholar] [CrossRef]
- Copray, J.C.; Jansen, H.W. Cyclic nucleotides and growth regulation of the mandibular condylar cartilage of the rat in vitro. Arch. Oral Biol. 1985, 30, 749–752. [Google Scholar] [CrossRef]
- Li, T.F.; Zuscik, M.J.; Ionescu, A.M.; Zhang, X.; Rosier, R.N.; Schwarz, E.M.; Drissi, H.; O’Keefe, R.J. PGE2 inhibits chondrocyte differentiation through PKA and PKC signaling. Exp. Cell Res. 2004, 300, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Raisz, L.G. Prostaglandins and bone: Physiology and pathophysiology. Osteoarthr. Cartil. 1999, 7, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Suzawa, T.; Miyaura, C.; Inada, M.; Maruyama, T.; Sugimoto, Y.; Ushikubi, F.; Ichikawa, A.; Narumiya, S.; Suda, T. The role of prostaglandin E receptor subtypes (EP1, EP2, EP3, and EP4) in bone resorption: An analysis using specific agonists for the respective EPs. Endocrinology 2000, 141, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Baylink, D.J.; Finkelman, R.D.; Mohan, S. Growth factors to stimulate bone formation. J. Bone Min. Res. 1993, 8, S565–S572. [Google Scholar] [CrossRef] [PubMed]
- Paralkar, V.M.; Borovecki, F.; Ke, H.Z.; Cameron, K.O.; Lefker, B.; Grasser, W.A.; Owen, T.A.; Li, M.; DaSilva-Jardine, P.; Zhou, M.; et al. An EP2 receptor-selective prostaglandin E2 agonist induces bone healing. PNAS 2003, 100, 6736–6740. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Sakai, A.; Uchida, S.; Tanaka, S.; Nagashima, M.; Katayama, T.; Yamaguchi, K.; Nakamura, T. Prostaglandin E2 receptor (EP4) selective agonist (ONO-4819.CD) accelerates bone repair of femoral cortex after drill-hole injury associated with local upregulation of bone turnover in mature rats. Bone 2004, 34, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Jee, W.S.; Ueno, K.; Kimmel, D.B.; Woodbury, D.M.; Price, P.; Woodbury, L.A. The role of bone cells in increasing metaphyseal hard tissue in rapidly growing rats treated with prostaglandin E2. Bone 1987, 8, 171–178. [Google Scholar] [CrossRef]
- Agas, D.; Marchetti, L.; Hurley, M.M.; Sabbieti, D. Prostaglandin F2α: A bone remodeling mediator. J. Cell. Physiol. 2013, 228, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Galea, G.L.; Meakin, L.B.; Williams, C.M.; Hulin-Curtis, S.L.; Lanyon, L.E.; Poole, A.W.; Price, J.S. Protein kinase Cα (PKCα) regulates bone architecture and osteoblast activity. J. Biol. Chem. 2014, 289, 25509–25522. [Google Scholar] [CrossRef] [PubMed]
- Zaman, G.; Sunters, A.; Galea, G.L.; Javaheri, B.; Saxon, L.K.; Moustafa, A.; Armstrong, V.J.; Price, J.S.; Lanyon, L.E. Loading-related regulation of transcription factor EGR2/Krox-20 in bone cells is ERK1/2 protein-mediated and prostaglandin, Wnt-signaling pathway-, and insulin-like growth factor-I axis-dependent. J. Biol. Chem. 2012, 287, 3946–3962. [Google Scholar] [CrossRef] [PubMed]
- Kido, S.; Kuriwaka-Kido, R.; Umino-Miyatani, Y.; Endo, I.; Inoue, D.; Taniguchi, H.; Inoue, Y.; Imamura, T.; Matsumoto, T. Mechanical stress activates Smad pathway through PKCδ to enhance interleukin-11 gene transcription in osteoblasts. PLoS ONE 2010, 5, e13090. [Google Scholar] [CrossRef] [PubMed]
- Nakura, A.; Higuchi, C.; Yoshida, K.; Yoshikawa, H. PkCα suppresses osteoblastic differentiation. Bone 2011, 48, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Weiler, H.A.; Fitzpatrick-Wong, S. Dietary long-chain polyunsaturated fatty acids minimize dexamethasone-induced reductions in arachidonic acid status but not bone mineral content in piglets. Pediatr. Res. 2002, 51, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Blanaru, J.L.; Kohut, J.R.; Fitzpatrick-Wong, S.C.; Weiler, H.A. Dose response of bone mass to dietary arachidonic acid in piglets fed cow milk-based formula. Am. J. Clin. Nutr. 2004, 79, 139–147. [Google Scholar] [PubMed]
- Pash, J.M.; Canalis, E. Transcriptional regulation of insulin-like growth factor-binding protein-5 by prostaglandin E2 in osteoblast cells. Endocrinology 1996, 137, 2375–2382. [Google Scholar] [PubMed]
- Almaden, Y.; Canalejo, A.; Ballesteros, E.; Anon, G.; Rodriguez, M. Effect of high extracellular phosphate concentration on arachidonic acid production by parathyroid tissue in vitro. J. Am. Soc. Nephrol. 2000, 11, 1712–1718. [Google Scholar] [PubMed]
- Klein-Nulend, J.; Burger, E.H.; Semeins, C.M.; Raisz, L.G.; Pilbeam, C.C. Pulsating fluid flow stimulates prostaglandin release and inducible prostaglandin G/H synthase mRNA expression in primary mouse bone cells. J. Bone Miner. Res. 1997, 12, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Weiler, H.A. Dietary supplementation of arachidonic acid is associated with higher whole body weight and bone mineral density in growing pigs. Pediatr. Res. 2000, 47, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Weiler, H.; Fitzpatrick-Wong, S.; Schellenberg, J.; McCloy, U.; Veitch, R.; Kovacs, H.; Kohut, J.; Kin Yuen, C. Maternal and cord blood long-chain polyunsaturated fatty acids are predictive of bone mass at birth in healthy term-born infants. Pediatr. Res. 2005, 58, 1254–1258. [Google Scholar] [CrossRef] [PubMed]
- Akatsu, T.; Takahashi, N.; Udagawa, N.; Imamura, K.; Yamaguchi, A.; Sato, K.; Nagata, N.; Suda, T. Role of prostaglandins in interleukin-1-induced bone resorption in mice in vitro. J. Bone Miner. Res. 1991, 6, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Kirschenbaum, A.; Yao, S.; Levine, A.C. Interactive effect of interleukin-6 and prostaglandin E2 on osteoclastogenesis via the OPG/RANKL/RANK system. Ann. N. Y. Acad. Sci. 2006, 1068, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Schwarz, E.M.; Young, D.A.; Puzas, J.E.; Rosier, R.N.; O’Keefe, R.J. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J. Clin. Investig. 2002, 109, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Samoto, H.; Shimizu, E.; Matsuda-Honjyo, Y.; Saito, R.; Nakao, S.; Yamazaki, M.; Furuyama, S.; Sugiya, H.; Sodek, J.; Ogata, Y. Prostaglandin E2 stimulates bone sialoprotein (BSP) expression through cAMP and fibroblast growth factor 2 response elements in the proximal promoter of the rat BSP gene. J. Biol. Chem. 2003, 278, 28659–28667. [Google Scholar] [CrossRef] [PubMed]
- Cherian, P.P.; Cheng, B.; Gu, S.; Sprague, E.; Bonewald, L.F.; Jiang, J.X. Effects of mechanical strain on the function of Gap junctions in osteocytes are mediated through the prostaglandin EP2 receptor. J. Biol. Chem. 2003, 278, 43146–43156. [Google Scholar] [CrossRef] [PubMed]
- Miyaura, C.; Inada, M.; Matsumoto, C.; Ohshiba, T.; Uozumi, N.; Shimizu, T.; Ito, A. An essential role of cytosolic phospholipase A2alpha in prostaglandin E2-mediated bone resorption associated with inflammation. J. Exp. Med. 2003, 197, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Boswell, K.; Koskelo, E.-K.; Carl, L.; Glaze, S.; Hensen, D.J.; Williams, K.D.; Kyle, D.J. Preclinical evaluation of single-cell oils that are highly enriched with arachidonic acid and docosahexaenoic acid. Food Chem. Toxicol. 1996, 34, 585–593. [Google Scholar] [CrossRef]
- Suarez, A.; del Carmen Ramirez, M.; Faus, M.J.; Gil, A. Dietary long-chain polyunsaturated fatty acids influence tissue fatty acid composition in rats at weaning. J. Nutr. 1996, 126, 887–897. [Google Scholar] [PubMed]
- De la Presa-Owens, S.; Innis, S.M.; Rioux, F.M. Addition of triglycerides with arachidonic acid or docosahexaenoic acid to infant formula has tissue- and lipid class-specific effects on fatty acids and hepatic desaturase activities in formula-fed piglets. J. Nutr. 1998, 128, 1376–1384. [Google Scholar] [PubMed]
- Baur, L.A.; O’Connor, J.; Pan, D.A.; Kriketos, A.D.; Storlien, L.H. The fatty acid composition of skeletal muscle membrane phospholipid: Its relationship with the type of feeding and plasma levels in young children. Metabolism 1998, 47, 106–112. [Google Scholar] [CrossRef]
- Blaauw, B.; Del Piccolo, P.; Rodriguez, L.; Hernandez Gonzales, V.H.; Agata, L.; Solagna, F.; Mammano, F.; Pozzan, T.; Schiaffino, S. No evidence for inositol 1,4,5-triphosphate-dependent Ca2+ release in isolated fibers of adult mouse skeletal muscle. J. Gen. Physiol. 2012, 140, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Berthier, C.; Kutchukian, C.; Bouvard, C.; Okamura, Y.; Jacquemond, V. Depression of voltage-activated Ca2+ release in skeletal muscle by activation of a voltage-sensing phosphatase. J. Gen. Physiol. 2015, 145, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Ohizumi, Y.; Hirata, Y.; Suzuki, A.; Kobayashi, M. Two novel types of calcium release from skeletal sarcoplasmic reticulum by phosphatidylinositol 4,5 biphosphate. Can. J. Physiol. Pharmacol. 1999, 77, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Sandow, A. Excitation-contraction coupling in muscular response. Yale J. Biol. Med. 1952, 25, 176–201. [Google Scholar] [PubMed]
- Melzer, W.; Herrmann-Frank, A.; Luttgau, H.C. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim. Biophys. Acta 1995, 1241, 59–116. [Google Scholar] [CrossRef]
- Kobayashi, M.; Muroyama, A.; Ohizumi, Y. Phosphatidylinositol 4,5-bisphosphate enhances calcium release from sarcoplasmic reticulum of skeletal muscle. Biochem. Biophys. Res. Commun. 1989, 29, 1487–1491. [Google Scholar] [CrossRef]
- Ghigo, A.; Perino, A.; Hirsch, E. Phosphoinositides and cardiac function. In Phosphoinositides and Disease, Current Topics in Microbiology and Immunology; Falasca, M., Ed.; Springer Science + Business Media: Dordrecht, The Netherlands, 2012; pp. 43–60. [Google Scholar]
- Rodemann, H.P.; Goldberg, A.L. Arachidonic acid, prostaglandin E2 and F2α influence rates of protein turnover in skeletal and cardiac muscle. J. Biol. Chem. 1982, 25, 1632–1638. [Google Scholar]
- Standley, R.A.; Liu, S.; Jemiolo, B.; Trappe, S.W.; Trappe, T.A. Prostaglandin E2 induces transcription of skeletal mass regulators interleukin-6 and muscle RING finger-1 in humans. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, R.S.; Luxwolda, M.F.; Dijck-Brouwer, J.; Muskiet, F.A.J. Intrauterine, postpartum and adult relationships between arachidonic acid (AA) and docosahexaenoic acid (DHA). Prostaglandins Leukot. Essent. Fat. Acids 2011, 85, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Luxwolda, M.F.; Kuipers, R.S.; Sango, W.S.; Kwesigabo, G.; Dijck-Brouwer, D.A.J.; Muskiet, F.A.J. A maternal erythrocyte DHA content of approximately 6 g% is the DHA status at which intrauterine DHA biomagnifications turns into bioattenuation and postnatal infant DHA equilibrium is reached. Eur. J. Nutr. 2012, 51, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.E.; Werkman, S.H.; Peoples, J.M.; Cooke, R.J.; Tolley, E.A. Arachidonic acid status correlates with first year of growth in preterm infants. Proc. Natl. Acad. Sci. USA 1993, 90, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Clandinin, M.T. Brain development and assessing the supply of polyunsaturated fatty acids. Lipids 1999, 34, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Clandinin, M.T.; Chappell, J.E.; Leong, S.; Heim, T.; Sayer, P.R.; Chance, G.W. Intrauterine fatty acid accretion in infant brain: Implications for fatty acid requirements. Early Hum. Dev. 1980, 4, 121–129. [Google Scholar] [CrossRef]
- Cunnane, S. Problems with essential fatty acids: Time for a new paradigm? Prog. Lipid Res. 2003, 42, 544–568. [Google Scholar] [CrossRef]
- Stoffel, W.; Holz, B.; Jenke, B.; Binczek, E.; Günter, R.H.; Kiss, C.; Karakesisoglou, I.; Thevis, M.; Weber, A.A.; Arnhold, S.; et al. Δ6-desaturase (FADS2) deficiency unveils role of ω3- and ω6-polyunsaturated fatty acids. EMBO J. 2008, 27, 2281–2292. [Google Scholar] [CrossRef] [PubMed]
- Stroud, C.K.; Nara, T.Y.; Roqueta-Rivera, M.; Radlowski, E.C.; Lawrence, P.; Zhang, Y.; Cho, B.H.; Segre, M.; Hess, R.A.; Brenna, J.T.; et al. Disruption of FADS2 gene in mice impairs male reproduction and causes dermal and intestinal ulceration. J. Lipid Res. 2009, 50, 1870–1880. [Google Scholar] [CrossRef] [PubMed]
- Mohrhauer, H.; Holman, R.T. The effect of dose level of essential fatty acids upon fatty acid composition of the rat liver. J. Lipid Res. 1963, 4, 151–159. [Google Scholar] [PubMed]
- Prottey, C. Essential fatty acids and the skin. Br. J. Dermatol. 1976, 94, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.S.; Jensen, B. Essential function of linoleic acid esterified in acylglucosylceramide and acylceramide in maintaining the epiderma; water permeability barrier. Evidence from feeding studies with oleate, linoleate, arachidonate, columbinate and alpha-linolenate. Biochem. Biophys. Acta 1985, 834, 357–363. [Google Scholar] [CrossRef]
- Hartop, P.J.; Prottey, C. Changes in transepidermal water loss and the composition of epidermal lecithin after application of pure fatty acid triglycerides to the skin of essential fatty acid-deficient rats. Br. J. Dermatol. 1976, 95, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Bertram, T.A. Gastrointestinal tract. In Handbook of Toxicologic Pathology; Haschek, W.M., Rousseaux, C.G., Wallig, M., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 121–186. [Google Scholar]
- Miller, C.C.; Ziboh, V.A. Induction of epidermal hyperproliferation by topical n-3 polyunsaturated fatty acids on guinea pig skin linked to decreased levels of 13-hydroxyoctadecadienoic acid (13-Hode). J. Investig. Dermatol. 1990, 94, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.P.; Nakamura, T.; Clarke, S.D. Cloning, expression, and nutritional regulation of the mammalian delta-6 desaturate. J. Biol. Chem. 1999, 274, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Williard, D.E.; Nwankwo, J.O.; Kaduce, T.L.; Harmon, S.D.; Irons, M.; Moser, H.W.; Raymond, G.V.; Spector, A.A. Identification of a fatty acid delta6-desaturase deficiency in human skin fibroblasts. J. Lipid Res. 2001, 42, 501–508. [Google Scholar] [PubMed]
- Fan, Y-Y.; Monk, J.M.; Hou, T.Y.; Callway, E.; Vincent, L.; Weeks, B.; Yang, P.; Chapkin, R.S. Characterization of an arachidonic acid-deficient (Fads1 knockout) mouse model. J. Lipid Res. 2012, 53, 1287–1295. [Google Scholar]
- Hatanaka, E.; Yasuda, H.; Harauma, A.; Watanabe, J.; Konishi, Y.; Nakamura, M.; Salem, N., Jr.; Moriguchi, T. The Effects of Arachidonic Acid and/or Docosahexaenoic Acid on the Brain Development Using Artificial Rearing of Delta-6-Desaturase Knockout Mice. In Proceedings of the Asian Conference of Nutrition, Yokohama, Japan, 14–18 May 2015.
- Tian, C.; Fan, C.; Liu, X.; Xu, F.; Qi, K. Brain histological changes in young mice submitted to diets with different ratios of n-6/n-3 polyunsaturated fatty acids during maternal pregnancy and lactation. Clin. Nutr. 2011, 30, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Wenk, M.R.; Goh, E.L.K.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.H.; Charron, M.J.; Silver, D.L. Major Facilitator superfamily domain-containing protein 2a (MFSD2A) has roles in body growth, motor function, and lipid metabolism. PLoS ONE 2012, 7, e50629. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.D.; Jeffrey, N.M.; Sanderson, P.; Newholme, E.A.; Calder, P.C. Eicosapentaenoic and docosahexaenoic acids alter rat spleen leukocyte fatty acid composition and prostaglandin E2 production but have different effects on lymphocyte functions and cell-mediated immunity. Lipids 1998, 33, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Jolly, C.A.; Jiang, Y-H.; Chapkin, R.S.; McMurray, D.N. Dietary (n-3) polyunsaturated fatty acids suppress murine lymphoproliferation, interleukin-2 secretion, and the formation of diacylglycerol and cereamide. J. Nutr. 1997, 127, 37–43. [Google Scholar] [PubMed]
- Kelley, D.S.; Taylor, P.C.; Nelson, G.J.; Schmidt, P.C.; Mackey, B.E.; Kyle, D. Effects of dietary arachidonic acid on human immune response. Lipids 1997, 32, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Blikslager, A.T.; Moeser, A.J.; Gookin, J.L.; Jones, S.L.; Odle, J. Restoration of barrier function in injured intestinal mucosa. Physiol. Rev. 2007, 87, 545–564. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, R.; Moreno, J.J. Role of eicosanoids on intestinal epithelial homeostatis. Biochem. Pharmacol. 2010, 80, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, S.K.; Moeser, A.J.; Corl, B.A.; Harrell, R.J.; Bilksager, A.T. Dietary long-chain PUFA enhances acute repair of ischemia-injured intestine of suckling pigs. J. Nutr. 2012, 142, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Le, H.D.; Meisel, J.A.; de Meijer, V.E.; Fallon, E.M.; Gura, K.M.; Nose, V.; Bistrian, B.R.; Puder, M. Docosahexaenoic acid and arachidonic acid prevent essential fatty acid deficiency and hepatic steatosis. J. Parenter. Enter. Nutr. 2012, 36, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Bassaganya-Riera, J.; Guri, A.J.; Noble, A.M.; Reynolds, K.A.; King, J.; Wood, C.M.; Ashby, M.; Rai, D.; Hontecillas, R. Arachidonic acid- and docosahexaenoic acid-enriched formulas modulate antigen-specific T cell responses to influenza virus in neonatal piglets. Am. J. Clin. Nutr. 2007, 85, 824–836. [Google Scholar] [PubMed]
- Weisinger, H.S.; Vingrys, A.J.; Sinclair, A.J. The effect of docosahexaenoic acid on the electroretinogram of the guinea pig. Lipids 1996, 31, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Champoux, M.; Hibbeln, J.R.; Shannon, C.; Majchrzak, S.; Suomi, S.J.; Salem, N., Jr.; Higley, J.D. Fatty acid formula supplementation and neuromotor development in rhesus monkey neonates. Pediatr. Res. 2002, 51, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, A.; Ohishi, M.; Sato, Y.; Hata, N.; Misawa, Y.; Fujii, Y.; Okuyama, H. Reversibility of n-3 fatty acid deficiency-induced alterations of learning behavior in the rat: Level of n-6 fatty acids as another factor. J. Lipid Res. 2001, 42, 1655–1663. [Google Scholar] [PubMed]
- Wainwright, P.E.; Xing, H.-C.; Mutsaers, L.; McCutcheon, D.; Kyle, D. Arachidonic acid offsets the effects on mouse brain and behavior of a diet with a low (n-6):(n-3) ratio and very high levels of docosahexaenoic acid. J. Nutr. 1997, 127, 184–193. [Google Scholar] [PubMed]
- Wainwright, P.E.; Xing, H.-C.; Ward, G.R.; Huang, Y.-S.; Bobik, E.; Auestad, N.; Montalto, M. Water maze performance is unaffected in artificially reared rats fed diets supplemented with arachidonic acid and docosahexaenoic acid. J. Nutr. 1999, 129, 1079–1089. [Google Scholar] [PubMed]
- Wainwright, P.E.; Huang, Y.S.; Bulman-Fleming, B.; Dalby, B.; Mills, D.E.; Redden, P.; McCutcheon, D. The effect of dietary n-3/n-6 ration on brain development in the mouse: A dose response study with long-chain n-3 fatty acids. Lipids 1992, 27, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Lowry, C.; Ghebremeskel, K.; Thomas, B.; Offley-Shore, B.; Crawford, M. Unfavorable effect of type 1 and type 2 diabetes on maternal and fetal essential fatty acid status: A potential marker of fetal insulin resistance. Am. J. Clin. Nutr. 2005, 82, 1162–1168. [Google Scholar] [PubMed]
- Siddappa, A.M.; Georgieff, M.K.; Wewerka, S.; Worwa, C.; Nelson, C.A.; Deregnier, R.A. Iron deficiency alters auditory recognition memory in newborn infants of diabetic mothers. Pediatr. Res. 2004, 55, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- DeBoer, T.; Wewerka, S.; Bauer, P.J.; Geogieff, M.K.; Nelson, C.A. Explicit memory performance in infants of diabetic mothers at 1 year of age. Dev. Med. Child Neurol. 2005, 47, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Holman, R.T.; Johnson, S.B.; Gerrand, J.M.; Mauer, S.M.; Kupcho-Sandberg, S.; Brown, D.M. Arachidonic acid deficiency in streptozotocin-induced diabetes. Proc. Natl. Acad. Sci. USA 1983, 80, 1375–2379. [Google Scholar] [CrossRef]
- Hadders-Algra, M. Prenatal long-chain polyunsaturated fatty acid status: The importance of a balanced intake of docosahexaenoic acid and arachidonic acid. J. Perinat. Med. 2008, 36, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Clandinin, M.T.; Chappell, J.E.; Leong, S.; Heim, T.; Sayer, P.R.; Chance, G.W. Extrauterine fatty acid accretion in infant brain: Implications for fatty acid requirements. Early Hum. Dev. 1980, 4, 131–138. [Google Scholar] [CrossRef]
- Zhao, J.; Bigio, M.R.; Weiler, H.A. Maternal arachidonic acid supplementation improves neurodevelopment in young adult offspring from rat dams with and without diabetes. Prostaglandins Leukot Essent. Fat. Acids 2011, 84, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Amusquivar, E.; Ruperez, F.J.; Barbas, C.; Herrera, E. Low arachidonic acid rather than α-tocopherol is responsible for the delayed postnatal development of offspring of rats fed fish oil instead of olive oil during pregnancy and lactation. J. Nutr. 2000, 13, 2855–2865. [Google Scholar]
- Haubner, L.; Sullivan, J.; Ashmeade, T.; Saste, M.; Wiener, D.; Carver, J. The effects of maternal dietary docosahexaenoic acid intake on rat pup myelin and the auditory startle response. Dev. Neurosci. 2007, 29, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Elsherbiny, M.E.; Goruk, S.; Monckton, E.A.; Richard, C.; Brun, M.; Emara, M.; Field, C.J.; Godbout, R. Long-term effect of docosahexaenoic acid feeding on lipid composition and brain fatty acid-binding protein expression in rats. Nutrients 2015, 7, 8802–8817. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, M.; Takashima, N.; Matsumata, M.; Ikegami, S.; Kontani, M.; Hara, Y.; Kawashima, H.; Owada, Y.; Kiso, Y.; Yoshikawa, T.; et al. Arachidonic acid drives postnatal neurogenesis and elicits a beneficial effect on prepulse inhibition, a biological trait of psychiatric illnesses. PLoS ONE 2009, 4, e5085. [Google Scholar] [CrossRef] [PubMed]
- Fleith, M.; Clandinin, T. Dietary PUFA for preterm and term infants: Review of clinical studies. Crit. Rev. Food Sci. Nutr. 2005, 3, 205–229. [Google Scholar] [CrossRef]
- Jensen, C.L.; Prager, T.C.; Fraley, J.K.; Chen, H.; Anderson, R.E.; Heird, W.C. Effect of dietary linoleic/alpha-linolenic acid ratio on growth and visual function of term infants. J. Pediatr. 1997, 131, 200–209. [Google Scholar] [CrossRef]
- Makrides, M.; Neumann, M.A.; Jeffrey, B.; Lien, E.L.; Gibson, R.A. A randomized trial of different ratios of linoleic to alpha-linolenic acid in the diet of term infants: Effect on visual function and growth. Am. J. Clin. Nutr. 2000, 71, 120–129. [Google Scholar] [PubMed]
- Makrides, M.; Neumann, M.A.; Simmer, K.; Gibson, R.A. Erythrocyte fatty acids of term infants fed either breast milk, standard formula, or formula supplemented with long-chain polyunsaturates. Lipids 1995, 30, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Makrides, M.; Neumann, M.A.; Simmer, K.; Pater, J.; Gibson, R. Are long-chain polyunsaturated fatty acids essential nutrients in infancy? Lancet 1995, 345, 1463–1468. [Google Scholar] [CrossRef]
- Makrides, M.; Neumann, M.A.; Simmer, K.; Gibson, R.A. Dietary long-chain polyunsaturated fatty acids do not influence growth in term infants: A randomized clinical trial. Pediatrics 1999, 104, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.T.; Janowsky, J.S.; Carroll, R.E.; Taylor, J.A.; Auestad, N.; Montalto, M.B. Formula supplementation with long-chain polyunsaturated fatty acids: Are there developmental benefits? Pediatrics 1998, 102, e59. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.A.; Neumann, M.; Makrides, M. The effects of diets rich in docosahexaenoic acid and/or gamma-linolenic acid on plasma fatty acid profiles in term infants. In Lipids in Infant Nutrition; Huang, Y.S., Sinclair, A., Eds.; AOCS Press: Champaign, IL, USA, 1998; pp. 19–28. [Google Scholar]
- Simmer, K. Longchain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database Syst. Rev. 2001, 4. [Google Scholar] [CrossRef]
- Uauy, R.D.; Hoffman, D.R.; Birch, E.E.; Birch, D.G.; Jameson, D.M.; Tyson, J.E. Safety and efficacy of omega-3 fatty acids in the nutrition of very low birth weight infants: Soy oil and marine oil supplementation of formula. J. Pediatr. 1994, 124, 612–620. [Google Scholar] [CrossRef]
- Clandinin, M.T.; Van Aerde, J.E.; Parrott, A.; Field, C.J.; Euler, A.R.; Lien, E.L. Assessment of the efficacious dose of arachidonic and docosahexaenoic acids in preterm infant formula: Fatty acid composition of erythrocyte membrane lipids. Pediatr. Res. 1997, 42, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Vanderhoof, J.; Gross, S.; Hegyi, T.; Clandinin, T.; Porcelli, P.; DeCristofaro, J.; Rhodes, T.; Tsang, R.; Shattuck, K.; Cowett, R.; et al. Evaluation of a long-chain polyunsaturated fatty acid supplemented formula on growth, tolerance, and plasma lipids in preterm infants up to 48 weeks postconceptional age. J. Pediatr. Gastr. Nutr. 1999, 29, 318–326. [Google Scholar] [CrossRef]
- Vanderhoof, J.; Gross, S.; Hegyi, T. A multicenter long-term safety and efficacy trial of preterm formula with long-chain polyunsaturated fatty acids. J. Pediatr. Gastr. Nutr. 2000, 31, 121–127. [Google Scholar] [CrossRef]
- Food and Drug Administration. Agency Response Letter. GRAS Notice No. GRN 000041; U.S. Food and Drug Administration, Department of Health and Human Services: Washington, DC, USA, 2001.
- Health Canada. Novel Food Decision, DHASCO and ARASCO Oils as Sources of Docosahexaenoic (DHA) and Arachidonic Acid (ARA) in Human Milk Substitutes, 2002. Available online: http://www.novelfoods.gc.ca (accessed on 19 October 2015).
- Ryan, A.S.; Zeller, S.; Nelson, E.B. Safety evaluation of single cell oils and the regulatory requirements for use as food ingredients. In Single Cell Oils. Microbial and Algal Oil, 2nd ed.; Cohen, Z., Ratledge, C., Eds.; AOCS Press: Urbana, IL, USA, 2010; pp. 317–350. [Google Scholar]
- Dobbing, J. Vulnerable periods in developing brain. In Brain, Behavior, and Iron in the Infant Diet; Dobbing, J., Ed.; Springer-Verlag: London, UK, 1990; pp. 1–25. [Google Scholar]
- Uauy, R.D.; Birch, D.G.; Birch, E.E.; Hoffman, D.R.; Tyson, J.E. Effect of dietary essential ω-3 fatty acids on retinal and brain development in premature infants. In Essential Fatty Acids and Eicosanoids; Sinclair, A., Gibson, E., Eds.; American Oil Chemists’ Society: Champaign, IL, USA, 1992; pp. 197–202. [Google Scholar]
- Uauy, R.D.; Treen, M.; Hoffman, D.R. Essential fatty acid metabolism and requirements during development. Semin. Perinatol. 1989, 13, 118–130. [Google Scholar] [PubMed]
- Carlson, S.E.; Crooke, R.J.; Werkman, S.H.; Tolley, E.A. First year growth of preterm infants fed standard compared to marine oil n-3 supplemented formula. Lipids 1992, 27, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.E.; Werkman, S.H. A randomized trial of visual attention of preterm infants fed docosahexaenoic acid until two months. Lipids 1996, 31, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Diersen-Schade, D.A.; Hansen, J.W.; Harris, C.L.; Merkel, K.L.; Wisont, K.D.; Boettcher, J.A. Docosahexaenoic acid plus arachidonic acid enhance preterm infant growth. In Essential Fatty Acids and Eicosanoids: Invited Papers from the Fourth International Congress; Riemersma, R.A., Armstrona, R., Kelly, W., Wilson, R., Eds.; AOCS Press: Champaign, IL, USA, 1998; pp. 123–127. [Google Scholar]
- Ryan, A.S.; Montalto, M.B.; Groh-Wargo, S.; Mimouni, F.; Sentipal-Walerius, J.; Doyle, J.; Siegman, J.; Thomas, A.J. Effect of DHA-containing formula on growth of preterm infants to 59 weeks postmenstrual age. Am. J. Hum. Biol. 1999, 11, 457–467. [Google Scholar] [CrossRef]
- Carlson, S.E.; Werkman, S.H.; Peeples, J.M.; Wilson, W.M. Long-chain fatty acids and early visual and cognitive development of preterm infants. Eur. J. Clin. Nutr. 1994, 48, S27–S30. [Google Scholar] [PubMed]
- Werkman, S.H.; Carlson, S.E. A randomized trial of visual attention of preterm infants fed docosahexaenoic acid until nine months. Lipids 1996, 31, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.E.; Werkman, S.H.; Tolley, E.A. Effect of long-chain n-3 fatt acid supplementation on visual acuity and growth of preterm infants with and without bronchopulmonary dysplasia. Am. J. Clin. Nutr. 1996, 63, 687–697. [Google Scholar] [PubMed]
- Root, A.W. Mechanisms of hormone action: General Principals. In Clinical Pediatric Endocrinology; Hung, W., Ed.; Mosby-Year Book: St. Louis, MO, USA, 1992; pp. 1–12. [Google Scholar]
- Watkins, B.A.; Shen, C.-L.; Allen, K.G.D.; Siefert, M.F. Dietary (n-3) and (n-6) polyunsaturates and acetylsalicylic acid alter ex vivo PGE2 biosynthesis, tissue IGF-I levels, and bone morphometry in chicks. J. Bone Miner. Res. 1996, 11, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.; Carlson, S.E.; Cheatham, C.L.; Fitzgerald-Gust Afson, K.M.; Kepler, A.; Doty, T. Long-chain polyunsaturated fatty acid supplementation in infancy reduces heart rate and positively affects distribution of attention. Pediatr. Res. 2011, 70, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.; Carlson, S.E.; Cheatham, C.L.; Shaddy, D.J.; Kerling, E.H.; Thodosoff, J.M.; Gustafson, K.M.; Brez, C. Long-term effects of LCPUFA supplementation on childhood cognitive outcomes. Am. J. Clin. Nutr. 2013, 98, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Alshweki, A.; Munuzuri, A.P.; Bana, A.M.; de Castro, J.; Andrade, F.; Aldamiz-Echevarria, L.; de Pipaon, M.S.; Fraga, J.M.; Couce, M.L. Effects of different arachidonic acid supplementation on psychomotor development in very preterm infants; a randomized trial. Nutr. J. 2015, 14, 101. [Google Scholar] [CrossRef] [PubMed]
- Beyerlein, A.; Hadders-Algra, M.; Kennedy, K.; Fewtrell, M.; Singhal, A.; Rosenfeld, E.; Lucas, A.; Bouwstra, H.; Koletzko, B.; von Kries, R. Infant formula supplementation with long-chain polyunsaturated fatty acids has no effect on Bayley developmental scores at 18 months of age-IPD meta-analysis of 4 large clinical trials. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M.; Auestad, N.; Siegman, J.S. Blood lipid docosahexaenoic and arachidonic acid in term gestation infants fed formula with high docosahexaenoic, low eicosapentaenoic acid fish oil. Lipids 1996, 31, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Desci, T.; Keleman, B.; Minda, H.; Burus, I.; Kohn, G. Effect of type of early infant feeding on fatty acid composition of plasma lipid classes in full-term infants during the second 6 months of life. J. Pediatr. Gastroenterol. Nutr. 2000, 30, 547–551. [Google Scholar]
- Hoffman, D.R.; Boettcher, J.A.; Diersen-Schade, D.A. Toward optimizing vision and cognition in term infants by dietary docosahexaenoic and arachidonic acid supplementation: A review of randomized clinical trials. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.S.; Entin, E.K.; Hoffman, J.P.; Kuratko, C.N.; Nelson, E.B. Role of fatty acids in the neurological development of infants. In Nutrition in Infancy, Volume 2, Nutrition and Health; Watson, R.R., Ed.; Springer Science + Business Media: New York, NY, USA, 2013; pp. 331–346. [Google Scholar]
- Drover, J.R.; Hoffman, D.R.; Casteneda, Y.S.; Morale, S.E.; Garfield, S.; Wheaton, D.H.; Birch, E.E. Cognitive function in 18-month-old term infants of the DIAMOND study: A randomized, controlled trial with multiple dietary levels of docosahexaenoic acid. Early Hum. Dev. 2011, 87, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Makrides, M.; Gibson, R.A.; McPhee, A.J.; Collins, C.T.; Davis, P.G.; Doyle, L.W.; Simmer, K.; Colditz, P.B.; Morris, S.; Smithers, L.G.; et al. Neurodevelopment outcomes of preterm infants fed high-dose docosahexaenoic acid: A randomized controlled trial. JAMA 2009, 301, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Makrides, M.; Gibson, R.A.; McPhee, A.J.; Yelland, L.; Quinlivan, J.; Ryan, P. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: A randomized controlled trial. JAMA 2010, 304, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.; Stafford, M.; Morley, R.; Abbott, R.; Stephenson, T.; MacFadyen, U.; Elias-Jones, A.; Clements, H. Efficacy and safety of long-chain polyunsaturated fatty acid supplementation of infant formula milk: A randomized trial. Lancet 1999, 354, 1948–1954. [Google Scholar] [CrossRef]
- Fewtrell, M.S.; Morley, R.; Abbott, R.A.; Singhal, A.; Isaacs, E.B.; Stephenson, T.; MacFadyen, U.; Lucas, A. Double-blind, randomized trial of long-chain polyunsaturated fatty acid supplementation in formula fed to preterm infants. Pediatrics 2002, 110, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, C.; Haugholt, K.; Lindgren, M.; Aurvåg, A.K.; Rønnestad, A.; Grønn, M.; Solberg, R.; Moen, A.; Nakstad, B.; Berge, R.K.; et al. Improved cognitive development among preterm infants attributable to early supplementation of human milk with docosahexaenoic acid and arachidonic acid. Pediatrics 2008, 121, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Westerberg, A.C.; Schei, R.; Henriksen, C.; Smith, L.; Veierød, M.B.; Drevon, C.A.; Iversen, P.O. Attention among very low birth weight infants following early supplementation with docosahexaenoic acid and arachidonic acid. Acta Pediatr. 2011, 100, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Clandinin, M.T.; Van Aerde, J.E.; Merkel, K.L.; Harris, C.L.; Springer, M.A.; Hansen, J.W.; Diersen-Schade, D.A. Growth and development of preterm infants fed infant formulas containing docosahexaenoic acid and arachidonic acid. J. Pediatr. 2005, 146, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Birch, E.E.; Garfield, S.; Hoffman, D.R.; Uauy, R.; Birch, D.G. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev. Med. Child Neurol. 2000, 42, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.E.; Montalto, M.B.; Ponder, D.L.; Werkman, S.H.; Korones, S.B. Lower incidence of necrotizing enterocolitis in infants fed a preterm formula with egg phospholipids. Pediatr. Res. 1998, 44, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Seki, H.; Sasaki, T.; Ueda, T.; Arita, M. Resolvins as regulators of the immune system. Sci. World J. 2010, 10, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Perinatal supplementation of long-chain polyunsaturated fatty acids, immune response and adult diseases. Med. Sci. Monit. 2004, 10, HY19–HY25. [Google Scholar] [PubMed]
- D’Vas, N.; Meldrum, S.J.; Dunstan, J.A.; Lee-Pullen, T.F.; Metcalfe, J.; Holt, B.J.; Serralha, M.; Tulic, M.K.; Mori, T.A.; Prescott, S.L. Fish oil supplementation in early infancy modulates developing infant immune responses. Clin. Exp. Allergy 2012, 42, 1206–1216. [Google Scholar]
- Khader, S.A.; Gaffen, S.L.; Kolls, J.K. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2009, 2, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Muc, M.; Kreiner-Moller, E.; Larsen, J.M.; Birch, S.; Brix, S.; Bisgaard, H.; Lauritzen, L. Maternal fatty acid desaturase genotype correlates with infant immune responses at 6 months. Br. J. Nutr. 2015, 114, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Barakat, R.; Abou El-Ela, N.E.; Sharaf, S.; El Sagheer, O.; Selim, S.; Tallima, H.; Bruins, M.J.; Hadley, K.B.; El Ridi, R. Efficacy and safety of arachidonic acid for treatment of school-aged children in Schistosoma mansoni high-endemicity regions. Am. J. Trop. Med. Hyg. 2015, 92, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Standl, M.; Lattka, E.; Stach, B.; Koletzko, S.; Bauer, C.P.; von Berg, A.; Berdel, D.; Kramer, U.; Schaaf, B.; Roder, S.; et al. FADS1 FADS2 gene cluster, PUFA intake and blood lipids in children: Results from the GINIplus and LISAplus Study Group. PLoS ONE 2012, 7, e37780. [Google Scholar]
- Makajima, H.; Hirose, K. Role of IL-23 and Th17 cells in airway inflammation in asthma. Immune Netw. 2010, 10, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Dong, C. Regulation and pro-inflammatory function of interleukin-17 family cytokines. Immunol. Rev. 2008, 226, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Sapone, A.; Lammers, K.M.; Casolaro, V.; CAmmarota, M.; Giulano, M.T.; De Rosa, M.; Stefanile, R.; Mazzarella, G.; Tolone, C.; Russo, M.I.; et al. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: Celiac disease and gluten sensitivity. BMC Med. 2011, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Pastor, N.; Soler, B.; Mitmesser, S.H.; Ferguson, P.; Lifschitz, C. Infants fed docosahexaenoic acid- and arachidonic acid-supplemented formula have decreased incidence of bronchiolitis/bronchitis the first year of life. Clin. Pediatr. 2006, 45, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Lapillone, A.; Pastor, N.; Zhuang, W.; Scalabrin, D.M.F. Infants fed formula with added long chain polyunsaturated fatty acids have reduced incidence of respiratory illnesses and diarrhea during the first year of life. BMC Pediatr. 2014, 14, 168. [Google Scholar] [CrossRef] [PubMed]
- Birch, E.E.; Carlson, S.E.; Hoffman, D.R.; Fitzgerald-Gustafson, K.M.; Fu, V.L.; Drover, J.R.; Castañeda, Y.S.; Minns, L.; Wheaton, D.K.; Mundy, D.; et al. The DIAMOND (DHA Intake and Measurement of Neural Development) Study: A double-masked, randomized controlled clinical trial of the maturation of infant visual acuity as a function of the dietary level of docosahexaenoic acid. Am. J. Clin. Nutr. 2010, 91, 848–859. [Google Scholar] [CrossRef] [PubMed]
- Foiles, A.M.; Kerling, E.H.; Wick, J.A.; Scalabrin, D.M.; Colombo, J.; Carlson, S.E. Formula with long chain polyunsaturated fatty acids reduces incidence of allergy in early childhood. Pediatr. Allergy Immunol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Hindenes, J.O.; Nerdal, W.; Guo, W.; Di, L.; Small, D.M.; Holmsen, H. Physical properties of the transmembrane signal molecule, sn-1-stearoyl-2-arachidonylglycerol. Acyl chain segregation and its biochemical implications. J. Biol. Chem. 2000, 275, 6857–6867. [Google Scholar] [CrossRef] [PubMed]
- Moodley, T.; Vella, C.; Djahanbakhch, O.; Branford-White, C.J.; Crawford, M.A. Arachidonic and docosahexaenoic acid deficits in preterm neonatal mononuclear cell membranes. Implications for the immune response at birth. Nutr. Health 2009, 20, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Van Goor, S.A.; Schaafsma, A.; Erwich, J.J.; Dijck-Brouwer, D.A.; Muskiet, F.A. Mildly abnormal general movement quality in infants is associated with high Mead acid and lower arachidonic acid shows a U-shaped relation with the DHA/AA ratio. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Groen, S.E.; de Blecourt, A.C.; Postema, K.; Hadders-Algra, M. General movements in early infancy predict neuromotor development at 9 to 12 years of age. Dev. Med. Child Neurol. 2005, 47, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Field, C.J.; Thompson, C.A.; Van Aerde, J.E.; Parrott, A.; Euler, A.; Lien, E.; Clandinin, M.T. Lower proportion of CD45R0+ cells and deficient interleukin-10 production by formula-fed infants, compared with human-fed, is correlated with supplementation of long-chain polyunsaturated fatty acids. J. Pediatr. Gastroenterol. Nutr. 2000, 31, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.J.; Calvin, C.M.; Clisby, C.; Schoenheimer, D.R.; Montgomery, P.; Hall, J.A. Fatty acid deficiency signs predict the severity of reading and related difficulties in dyslexic children. Prostaglandins Leukot. Essent. Fat. Acids 2000, 63, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.R.; Stevens, L.; Zhang, W.; Peck, L. Long-chain polyunsaturated fatty acids in children with attention-deficit hyperactivity disorder. Am. J. Clin. Nutr. 2000, 71, 327S–330S. [Google Scholar] [PubMed]
- Chen, J.R.; Hsu, S.F.; Hsu, C.D.; Hwang, L.H.; Yang, S.C. Dietary patterns and blood fatty acid composition in children with attention-deficit hyperactivity disorder in Taiwan. J. Nutr. Biochem. 2004, 15, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Young, G.S.; Maharaj, N.J.; Conquer, J.A. Blood phospholipid fatty acid analysis of adults with and without attention deficit/hyperactivity disorder. Lipids 2004, 39, 117–123. [Google Scholar] [PubMed]
- Morse, N.L. A meta-analysis of blood fatty acids in people with learning disorders with particular interest in arachidonic acid. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Yamada, J.; Banks, A. Evidence for and characteristics of dyslexia among Japanese children. In Annals of Dyslexia; Schatschneider, C., Compton, D., Eds.; Springer: New York, NY, USA, 1994; pp. 103–119. [Google Scholar]
- FAO. Dietary Fats and Oils in Human Nutrition; FAO/WHO: Rome, Italy, 1978. [Google Scholar]
- FAO/WHO. Fats and Oils in Human Nutrition; Report of a Joint FAO/WHO Expert Consultation, 19 to 26 October 1993; FAO/WHO: Rome, Italy, 1994. [Google Scholar]
- FAO. Fats and Fatty Acids in Human Nutrition; FAO/WHO: Rome, Italy, 2010. [Google Scholar]
- Codex Alimentarius Commission. Standards for Infant Formula and Formulas for Special Medical Purposes Intended for Infants; CODEX STAN 72-1981, Last Revised 2007; Codex Alimentarius Commission: Rome, Italy, 2007. [Google Scholar]
- Codex Alimentarius Commission. Amendments, 2015. Available online: http://www.fao.org/fao-who-codexalimentarius/en (accessed on 26 January 2016).
- Infant Formula Act. H.R.6940—An Act to Amend the Federal Food, Drug, and Cosmetic Act to Strengthen the Authority under that Act to Assure the Safety and Nutrition of Infant Formulas, and for Other Purposes. Fed. Regist. 1980, 50, 45106–45108. [Google Scholar]
- European Commission. Commission Directive 2006/141/EC of 22 December 2006 on Infant Formulae and Follow-on Formulae and Amending Directive 1999/21/EC; L.401/1; Official Journal of the European Union: Brussels, Belgium, 2008. [Google Scholar]
- Koletzko, B.; Boey, C.C.; Campoy, C.; Carlson, S.E.; Chang, N.; Guillermo-Tuazon, M.A.; Joshi, S.; Prell, C.; Quak, S.H.; Sjarif, D.R.; et al. Current information and Asian perspectives on long-chain polyunsaturated fatty acids in pregnancy, lactation, and infancy: Systematic review and practice recommendations from an early nutrition academy workshop. Ann. Nutr. Metab. 2014, 65, 49–80. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.A.; Wang, Y.; Forsyth, S.; Brenna, J.T. The European Food Safety Authority recommendation for polyunsaturated fatty acid composition of infant formula overrules breast milk, puts infants at risk, and should be revised. Prostaglandins Leukot. Essent. Fat. Acids 2015, 102–103, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Leaf, A.A.; Leighfield, M.J.; Costeloe, K.L.; Crawford, M.A. Long chain polyunsaturated fatty acids and fetal growth. Early Hum. Dev. 1992, 30, 183–191. [Google Scholar] [CrossRef]
- Forsyth, J.S.; Willatts, P.; Agostoni, C.; Bissenden, J.; Casaer, P.; Boehm, G. Long chain polyunsaturated fatty acid supplementation in infant formula and blood pressure in later childhood: Follow up of a randomized controlled trial. Br. Med. J. 2003, 326, 953. [Google Scholar] [CrossRef] [PubMed]
- Makrides, M.; Gibson, R.A.; Udell, T.; Ried, K. Supplementation of infant formula with long-chain polyunsaturated fatty acids does not influence the growth of term infants. Am. J. Clin. Nutr. 2005, 81, 1094–1101. [Google Scholar] [PubMed]


| Country | Age | Method | Mean ARA Intake (mg/Day) (mg/kg/Day) 1 |
|---|---|---|---|
| Australia [91] | 2–3 years | 1-day weighed food record | 16 (1.3) |
| 1-day weighed food record | 22 (1.8) | ||
| Belgium [90] | 2–5 years | 3 days food record | 17 (1.4) |
| 4–6.5 years | 3 days food record | 18 (1.0) | |
| Canada [92,93] | 1.5–2 years | 1 day food frequency | 133 (11.0) |
| 2.1–3 years | I day food frequency | 260 (22.0) | |
| 3.1–5 years | 1 day food frequency | 226 (15.0) | |
| 4–7 years | 3-days food records | 57 (2.9) | |
| China [82] | 1–3 years | 3 days 24 h recall | 55 (4.6) |
| 4–5 years | 3 days 24 h recall | 50 (2.5) | |
| Gambia [82] | 0–6 months | 1 day weighed food monthly | 90 (15.0) |
| 7–12 months | 1 day weighed food monthly | 70 (7.8) | |
| 13–17 months | 1 day weighed food monthly | 60 (6.7) | |
| 24 months | 1 day weighed food monthly | 10 (0.8) | |
| Germany [87,88] | 6 months | 3 days weighed food record | 72 (12.0) |
| 9 months | 3 days weighed food record | 24 (2.7) | |
| Italy [89] | 1 month | Human milk composition | 95.6 (29.0) |
| 2 months | Human milk composition | 109.6 (33.0) | |
| 3 months | Human milk composition | 101.1 (16.9) | |
| 6 months | Human milk composition | 58.7 (9.8) | |
| U.S. 2003–2008 [94] | 1–4 years | 1 day weighed food record | 60 (5.0) |
| U.S. 2015 [95] | 2–5 years | 1 day weighed food record | 80 (6.7) |
| Eicosanoid | Effects |
|---|---|
| PGE2 | Proinflammatory |
| Induces fever | |
| Increases vascular permeability | |
| Increases vasodilatation | |
| Causes pain | |
| Enhances pain caused by other agents | |
| Anti-inflammatory | |
| Inhibits production of TNF and IL-1 | |
| Inhibits 5-LOX (decreases 4-series LT production | |
| Induces 15-LOX (increases lipoxin production) | |
| LTB4 | Proinflammatory |
| Increases vascular permeability | |
| Enhances local blood flow | |
| Chemotactic agent for leukocytes | |
| Induces release of lysomal enzymes | |
| Induces release of oxygen species by granulocytes | |
| Increases production of TNF, IL-8, and IL-6 |
| Metabolic Effector | Physiological Roles of ARA |
|---|---|
| ARA [149] | Maintain normal balance between bone mineral accrual and bone resorption during infant development |
| ARA, growth hormones [150,151] | Increase insulin-like growth factor gene expression and induction of osteoblast-dependent bone formation |
| Vitamin D3 [128,133] | Mediate vitamin D3 coordination of chondrocyte proliferation in the epiphyseal growth plates of long bones. Parathyroid hormone secretion |
| Calcium and phosphorous [152] | Regulation of parathyroid hormone secretion in response to blood mineral concentrations |
| Parathyroid hormone [149,150] | ARA mediated/activated pathway involved in the activation and secretion of vitamin D3 by kidneys |
| Physical activity [153] | ARA mediates bone adaptation to changes in physical stress through mechanisms which mediate resorption and remodeling |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadley, K.B.; Ryan, A.S.; Forsyth, S.; Gautier, S.; Salem, N. The Essentiality of Arachidonic Acid in Infant Development. Nutrients 2016, 8, 216. https://doi.org/10.3390/nu8040216
Hadley KB, Ryan AS, Forsyth S, Gautier S, Salem N. The Essentiality of Arachidonic Acid in Infant Development. Nutrients. 2016; 8(4):216. https://doi.org/10.3390/nu8040216
Chicago/Turabian StyleHadley, Kevin B., Alan S. Ryan, Stewart Forsyth, Sheila Gautier, and Norman Salem. 2016. "The Essentiality of Arachidonic Acid in Infant Development" Nutrients 8, no. 4: 216. https://doi.org/10.3390/nu8040216





