Role of Dietary Protein and Thiamine Intakes on Cognitive Function in Healthy Older People: A Systematic Review
Abstract
:1. Introduction
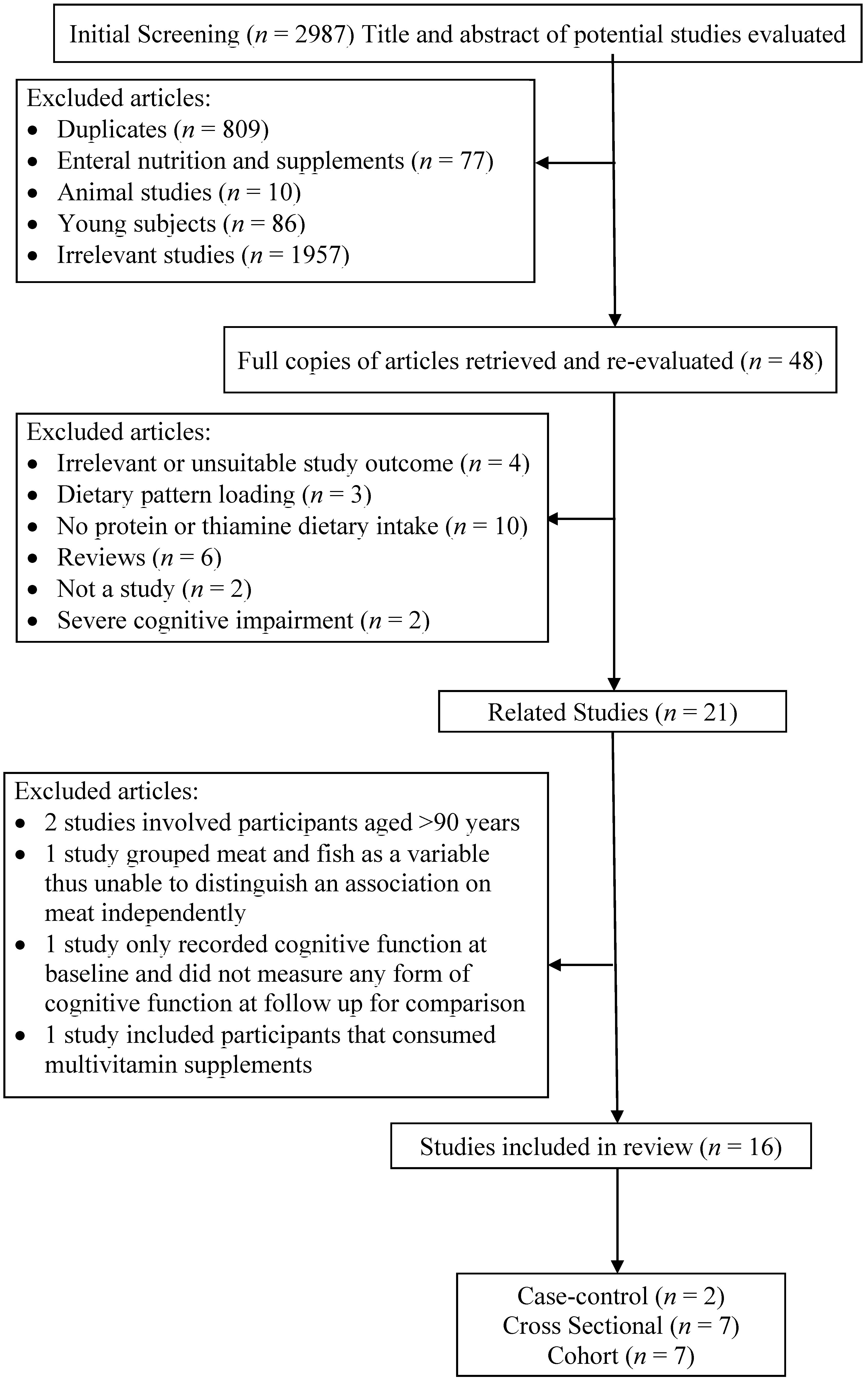
2. Methods
2.1. Identification of Studies
2.2. Data Extraction
3. Results
| Author | Population | Measurement of Cognition (Cutoff Point) and Diet | Protein | Protein Food Source | Thiamine | Adjustments | NHMRC Level of Evidence |
|---|---|---|---|---|---|---|---|
| La Rue et al. 1997 [27], USA | 304 community-dwelling healthy individuals, age between 66 and 90 years (6 years cohort). | The Abstraction Scale from Shipley-Hartford Intelligence Test, The Logical Memory and Visual Reproduction subtests from Wechsler Memory Scale and Rey-Osterrieth Test 3-day Food Dietary records | Protein (g/day) Baseline (median): 75 g/day 6 Years Follow Up (median): 72 g/day Rey Osterrieth Recall Test r = 0.19 p-value < 0.05 Logical Memory Test r = 0.20 p-value < 0.05 Significantly positive association between dietary protein intake and Rey Osterrieth Recall and Logical Memory Test scores. | Thiamine (mg/day) Baseline (median): 1.93 6 years Follow up (median): 2.47 Rey Osterrieth Copy Test Baseline, r = −0.07 6 years Follow up, r = 0.16 p-value < 0.10 Rey Osterrieth Recall Test Baseline, r = 0.08 6 years Follow up, r = 0.15 p-value < 0.10 Shipley Hartford Abstraction Test Baseline, r = −0.08 6 years Follow up, r = 0.29 p-value < 0.01 Significant positive association between dietary thiamine intake and Shipley Hartford Abstraction Test scores. | Body weight | III-2, Neutral | |
| Deschamps et al. 2002 [28], France | 125 community-dwelling non-demented elderly, age >68 years (5 years cohort) 3-day food diary, Diet history and FFQ | MMSE (cognitive decline: reduction of ≥3 MMSE score over 5 years) | Protein Median intake: 1.33 g/kg/day <1.0 g/kg/day (n = 21), OR = 1.00 ≥1.0 g/kg/day (n = 104), OR = 1.92 (0.38–9.62) p-value n.s No significant association between dietary protein intake and cognitive decline. | Age, Gender and Education | III-2, Neutral | ||
| McNeill et al. 2011 [29], UK | 882 subjects living around Edinburg, mean age 70 years. (57 to 60 years cohort) | MHT, MMSE, NART, Verbal Fluency test, Wescler Adult Intelligence Scale–III. Semi-quantitative FFQ | Thiamine (mg/day) Mean Intake from diet: 1.51 ± 0.50 MMSE β = 0.057 p-value n.s Verbal fluency β = 0.004 p-value n.s No significant association between dietary thiamine intake and cognitive function or verbal fluency. | Age, gender, IQ at age 11 years, smoking, social class, education, statin use, presence of APOE allele | III-3, Neutral | ||
| Barberger-Gateau et al. 2007 [24], France | 8085 free-living non-demented elderly, age >65 years (4 years cohort) | DSM- IV by neurologist FFQ | Meat All cause dementia 2–3 times/week: Incidence = 1.13 (0.88–1.97), 4–6 times/week: Incidence = 0.85 (0.68–1.01), Daily: Incidence =1.03 (0.80–1.27) p-value n.s AD 2–3 times/week: Incidence = 0.76 (0.58–0.96) 4–6 times/week: Incidence = 0.53 (0.40–0.66), Daily: Incidence =0.65 (0.46–0.84) p-value n.s No significant association between meat consumption and dementia/AD. | Age | III-2 | ||
| Velho et al. 2008 [30], Portugal | 187 free-living elderly participants with normal cognition, age >65 years (8.5 ± 3.5 months cohort). | MMSE (Improvement: Increase >0 in MMSE score, No Improvement: No increase in MMSE score) 3-day food diary | Protein (g/day) No improvement: 70.9 ± 2.0 Improvement: 73.4 ± 1.8 t-test = 1.04 p-value n.s No significant association between dietary protein intake and improvements in cognitive function. | Age, Total energy | III-2, Neutral | ||
| Vercambre et al. 2009 [31], France | 4809 elderly women from E3N cohort, age between 63 and 68 years (13 years cohort). | DECO scale (<33), Questionnaire for close relative/friend 208 item FFQ, 24 h recalls | Protein (g/day) Mean = 87.70 ± 24.55 Q3-Q1 OR = 0.92 (0.74–1.14) Trend (p-value n.s) No significant association between dietary protein intake and cognitive function. | Beef, pork, lamb (g/day) Mean = 45.45 ± 35.53 Q3-Q1 OR = 0.87 (0.66–1.15) Trend (p-value n.s) Poultry (g/day) Mean = 16.93 ± 17.89 Q3-Q1 OR = 0.73 (0.58–0.91) Trend (p-value = 0.004) No significant association for beef, pork and lamb. Significantly higher poultry intake in participants with better cognition. | Age, Education, BMI, Frequency of average physical activity, Average daily energy intake, Smoking, Supplements, Post-menopausal hormones, Depression, Cancer, CHD, Stroke, T2DM, High cholesterol and Hypertension. | III-2, Neutral | |
| Roberts et al. 2012 [32], USA | 937 cognitively normal participants, age between 70 and 89 (median of 3.7 years cohort) | CDR, Short test of mental status with 9 test assessing 4 domains of memory, executive function , language and visuospatial skills 128 item FFQ | Protein (g/day) All participants (mean): 78 g/day, 18% energy Q1 (20% energy) HR = 0.79 (0.52–1.20) Correlation of trend across quartiles, p-value = 0.03 Significant association between dietary protein intake of 16%–20% of energy intake and reduced risk of MCI or dementia. | Gender, Education, Total daily energy, Non-participation at baseline, Single macronutrient, APOE e4, T2DM, Depression, BMI, Stroke, Marital status, Smoking, Alcohol, Occupation and Frequency of moderate physical activity | III-2, Neutral |
| Author | Population | Measurement of Cognition (Cutoff Point) and Diet | Protein | Protein Food Source | Thiamine | Adjustments | NHMRC Level of Evidence |
|---|---|---|---|---|---|---|---|
| Burns et al. 1989 [33], UK | 78 elderly subjects (28 community-living demented, 21 hospitalized demented, 29 control) | MMSE (control: ≥29, case: ≤24) 3-day weighed food record | Protein (g/day) Community living demented: 61 ± 12.0 Control: 44 ± 15.2 p-value < 0.05 Hospitalized demented: 73 ± 8.3 Control: 44 ± 15.2 p-value < 0.05 Significantly higher dietary protein intake in controls than demented participants. | None | III-3, Positive | ||
| Nes et al. 1998 [34], Norway | 32 community-living elderly (16- case, 16-control), age >75 years. | DSM-III 3-day weighed food record | Protein (g/day) Men (Control =75 ± 12, Dementia = 69 ± 14) p-value n.s Women (Control = 64 ± 14, Dementia = 51 ± 12) p-value ≤ 0.05 Significantly higher dietary protein intake in women controls than women with dementia. No significant difference detected for men. | Thiamine (mg/day) Men (Control =1.0 ± 0.1, Dementia = 1.0 ± 0.1) p-value n.s Women (Control = 1.0 ± 0.3, Dementia = 0.7 ± 0.2) p-value ≤ 0.05 Significantly higher dietary thiamine intake in women controls than women with dementia. No significant difference detected for men. | None | III-3, Positive |
| Author | Population | Measurement of Cognition (Cutoff Point) and Diet | Protein | Protein Food Source | Thiamine | Adjustments | NHMRC Level of Evidence |
|---|---|---|---|---|---|---|---|
| Ortega et al. 1997 [35], Spain | 260 free living elderly (108 men and 152 women) aged between 65 and 90 years. | MMSE (unsatisfactory: 0) 7-day weighed food record, FFQ | Protein (g/day) Men (Unsatisfactory MMSE: 81.0 ± 21.3, Satisfactory MMSE: 81.8 ± 18.7) p-value n.s Women (Unsatisfactory MMSE: 71.8 ± 14.5, Satisfactory MMSE: 73.9 ± 18.8) p-value n.s No significant association between dietary protein intake and cognitive function. | Thiamine (mg/day) Men (Unsatisfactory MMSE: 1.18 ± 0.25, Satisfactory MMSE: 1.19 ± 0.36) Women (Unsatisfactory MMSE: 0.96 ± 0.26, Satisfactory MMSE: 1.06 ± 0.36) r = 0.2225, p-value < 0.01 Significantly higher dietary thiamine intake in participants with satisfactory scores. | Age, Gender | IV, Neutral | |
| Lee et al. 2001 [36],, Korea | 449 free-living participants (210 men & 239 women, age >60 years. | MMSE-Korean Version (Poor: ≤19, Inadequate: 20–23, Normal: ≥24) 24-hour recall | Protein (g/day) Men (Normal: 65.1 ± 25.7, Inadequate: 63.9 ± 26.4, Poor: 60.0 ± 25.0) p-value n.s r = 0.078, p-value n.s Women (Normal: 57.0 ± 24.5, Inadequate: 58.4 ± 29.1, Poor: 42.5 ± 22.3) p-value < 0.05 r = 0.181 (p-value < 0.01) Significant association between higher dietary protein intake and better cognitive function only in women. | Meat (g/day) Men (Normal: 39.2 ± 47.4, Inadequate: 40.9 ± 50.2, Poor: 46.7 ± 47.3) p-value n.s r = −0.004, p-value n.s Women (Normal: 37.7 ± 52.5, Inadequate: 34.6 ± 57.1, Poor: 20.7 ± 31.1) p-value n.s r = 0.096, p-value n.s No significant association between meat intake and cognitive function. | Thiamine (mg/day) Men (Normal: 0.95 ± 0.35, Inadequate: 0.91 ± 0.34, Poor: 0.82 ± 0.27), r = 0.083, p-value n.s Women (Normal: 0.91 ± 0.39, Inadequate: 0.90 ± 0.63, Poor: 0.71 ± 0.35) p-value < 0.05, r = 0.125, p-value n.s Significantly higher intake of dietary thiamine in women normal cognition participants than cognitively impaired participants but not significant in men. | Age | IV, Neutral |
| Requejo et al. 2003 [37], Spain | 168 free-living elderly at day centres with normal cognition, age between 65 and 90 years. | MMSE (unsatisfactory: <28, satisfactory: ≥28) 7-day food diary and 5-day weighted food record of lunch | Meat (g/day) Age ≥ 75 years (MMSE < 28: 126.3 ± 66.6, MMSE ≥ 28: 98.9 ± 32.1) Age < 75 years (MMSE < 28: 127.6 ± 60.9, MMSE ≥ 28: 138.5 ± 77.7) p-value n.s No significant association between higher meat consumption with better cognitive function. | Thiamine Age ≥ 75 years (MMSE < 28: 1.05 ± 0.29, MMSE ≥ 28: 0.96 ± 0.23) Age < 75 years (MMSE < 28: 1.05 ± 0.29, MMSE ≥ 28: 1.12 ± 0.34) p-value < 0.1 almost sig r = 0.2332, p-value < 0.01 Significant association between higher dietary thiamine intake and better cognitive function. | None | IV, Neutral | |
| Rahman et al. 2007 [38], USA | 1056 community dwelling elderly, mean age = 67. | MSQ (cognitive impairment: <9, normal: ≥9) Verbal FFQ (Yes: Once or twice a week, or most days, or everday. No: Less often than once a week, or never.) | Pork, beef, lamb (g/day) Yes (n = 904) No (n = 152) OR = 1.11 (0.67, 1.84) p-value n.s Chicken and turkey Yes (n = 916) No (n = 140) OR= 0.81 (0.48, 1.36) p-value n.s No significant association between pork, beef, lamb, chicken or turkey. | Age, Gender, Education and Other dietary factors | IV | ||
| Mori et al. 2010 [39], Japan | 179 community dwelling elderly, aged ≥65 years | SF-36 MCS (High MCS and Low MCS based on standardised score classified by age, 60–69 years = 52.0 and ≥70 years = 51.7 ) Semi-quantitative FFQ | Protein (g/day) (High: 74.5 ± 1 Low: 73.5 ± 1.6) p-value n.s No significant association between dietary protein intake and cognitive function. | Meat (g/day) (High: 44.0 ± 3.3 Low: 55.3 ± 5.3) p-value n.s No significant association between meat and cognition. | Age, Gender | IV, Neutral | |
| Aparicio Vizuete et al. 2010 [40], Spain | 178 institutionalised elderly, age ≥ 65 years. | SMPSQ (0 = No error, >0 = Error) 7-day Weighed Food Records | Protein (g/day) Age < P50 years (No error: 71.01 ± 14.30, Error: 67.63 ± 12.68) Age ≥ P50 years (No error: 70.02 ± 12.89, Error: 67.98 ± 10.67) p-value n.s r2 = 0.5899 p-value < 0.001 Significantly higher dietary protein and meat intake in participants with better cognitive functioning. | Meat Age < P50 years (No error: 98.14 ± 41.67, Error: 105.55 ± 40.38) Age ≥ P50 years (No error: 93.05 ± 39.81, Error: 88.90 ± 35.73) p-value n.s r2 = 0.1086 p-value < 0.001 Significantly higher meat intake in participants with poorer cognitive functioning. | Thiamine (mg/day) Age < P50 years (No error: 1.11 ± 0.28, Error: 1.10 ± 0.24) Age ≥ P50 years (No error: 1.12 ± 0.25, Error: 1.09 ± 0.25) p-value n.s r2 = 0.3180 p-value < 0.001 Significant association between higher dietary thiamine intake and better cognitive function. | Energy intake and Education level | IV, Neutral |
| Katsiardanis et al. 2013 [41], Greece | 557 free-living elderly (m = 237, w = 320), age > 65 years | MMSE (cognitive impairment: <24, normal: ≥24), GDS. FFQ and Semi-quantitative FFQ | Protein (g/day) Men (Cognitive impairment: 82.5 ± 28.84, Normal: 81.0 ± 23.57) p-value n.s OR = 1.36 (0.92–2.02), p-value n.s Women (Cognitive impairment: 75.5 ± 24.34, Normal: 74.8 ± 28.59) p-value n.s OR = 0.88 (0.56–1.37), p-value n.s No significant association between dietary protein intake and cognitive functioning. | Meat and Meat product Men (Cognitive impairment: 24.0 ± 14.67, Normal: 22.0 ± 10.25) p-value n.s OR = 1.03 (0.84–1.27), p-value n.s Women (Cognitive impairment: 18.8 ± 11.47, Normal: 19.8 ± 12.27) p-value n.s OR = 0.96 (0.81–1.16), p-value n.s No significant association between meat and meat products with cognitive function. | Thiamine Men OR = 1.05 (0.76–1.44) p-value n.s Women OR = 1.16 (0.65–1.38) p-value n.s No significant association between dietary thiamine intake and cognitive function. | Age, Education, Social Activity, Smoking, Metabolic syndrome, Geriatric Depression Scale and MedDiet Score. OR adjusted for core models and energy intake. | IV, Neutral |
3.1. Dietary Protein Intake
3.2. Different Types of Protein Food Sources
3.3. Dietary Thiamine Intake
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Appendix
| Study (1st Author, Year) | Nes et al. 1988 | Burns et al. 1989 | Ortega et al. 1997 | La Rue et al. 1997 | Lee et al. 2001 | Deschamps et al. 2002 | Requejo et al. 2003 | Barberger et al. 2007 | Rahman et al. 2007 | Shatenstein et al. 2007 | Velho et al. 2008 | Vercambre et al. 2009 | Aparicio Vizuete et al. 2010 | Mori et al. 2010 | McNeill et al. 2011 | Roberts et al. 2012 | Katsiardanis et al. 2013 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference Number | [34] | [33] | [35] | [27] | [36] | [28] | [37] | [24] | [38] | [26] | [30] | [31] | [40] | [39] | [29] | [32] | [41] |
| VALIDITY QUESTIONS—PRIMARY STUDIES | |||||||||||||||||
| 1. Was the research question clearly stated? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 2. Was the selection of study subjects/patients free from bias? | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | N | Y | Y | Y |
| 3. Were study groups comparable? | Y | Y | NA | NA | NA | NA | NA | N | NA | Y | NA | NA | NA | NA | NA | NA | NA |
| 4. Were intervention/therapeutic regimens/exposure factor or procedure and any comparison(s) described in detail? Were intervening factors described? | Y | Y | Y | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | Y | Y | Y |
| 5. Were outcomes clearly defined and the measurements valid and reliable? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 6. Was method of handling withdrawals described? | Y | N | N | N | N | Y | N | N | N | Y | Y | Y | N | N | N | Y | Y |
| 7. Was blinding used to prevent introduction of bias? | N | Y | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| 8. Was the statistical analysis appropriate for the study design and type of outcome indicators? | N | N | Y | Y | Y | Y | N | Y | Y | N | Y | Y | Y | Y | Y | Y | Y |
| 9. Were conclusions supported by results with biases and limitations taken into consideration? | Y | N | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 10. Is bias due to study’s funding or sponsorship unlikely? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| OVERALL QUALITY—PRIMARY STUDIES | |||||||||||||||||
| Negative/Neutral/Positive (N/0/P) | P | P | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| If most (six or more) of the answers to the above validity questions are “No,” the report should be designated negative | |||||||||||||||||
| If the answers to validity criteria questions 2, 3, 6, and 7 do not indicate that the study is exceptionally strong, the report should be designated neutral | |||||||||||||||||
| If most of the answers to the above validity questions are “Yes” (including criteria 2, 3, 6, 7 and at least one additional “Yes”), the report should be designated positive | |||||||||||||||||
| Sum | |||||||||||||||||
| Yes (Y) | 7 | 7 | 6 | 7 | 7 | 7 | 5 | 6 | 8 | 8 | 7 | 8 | 7 | 6 | 7 | 8 | 8 |
| No (N) | 3 | 3 | 3 | 2 | 2 | 2 | 4 | 5 | 3 | 2 | 2 | 1 | 2 | 3 | 2 | 1 | 1 |
| Not Applicable (NA) | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
Conflicts of Interest
References
- Spencer, J.P.E. Flavonoids: Modulators of brain function? Br. J. Nutr. 2008, 99, ES60–ES77. [Google Scholar] [PubMed]
- Del Parigi, A.; Panza, F.; Capurso, C.; Solfrizzi, V. Nutritional factors, cognitive decline, and dementia. Brain Res. Bull. 2006, 69, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Luchsinger, J.A.; Tang, M.X.; Shea, S.; Mayeux, R. Antioxidant vitamin intake and risk of Alzheimer disease. Arch. Neurol. 2003, 60, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Otaegui-Arrazola, A.; Amiano, P.; Elbusto, A.; Urdaneta, E.; Martínez-Lage, P. Diet, cognition, and Alzheimer’s disease: Food for thought. Eur. J. Nutr. 2014, 53, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Parsaik, A.K.; Mielke, M.M.; Erwin, P.J.; Knopman, D.S.; Petersen, R.C.; Roberts, R.O. Association of Mediterranean diet with mild cognitive impairment and Alzheimer’s disease: A systematic review and meta-analysis. J. Alzheimer’s Dis. 2014, 39, 271–282. [Google Scholar]
- Morais, J.A.; Chevalier, S.; Gougeon, R. Protein turnover and requirements in the healthy and frail elderly. J. Nutr. Health Aging 2006, 10, 272–283. [Google Scholar] [PubMed]
- Robertson, D.A.; Savva, G.M.; Kenny, R.A. Frailty and cognitive impairment—A review of the evidence and causal mechanisms. Ageing Res. Rev. 2013, 12, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Orsitto, G. Different components of nutritional status in older inpatients with cognitive impairment. J. Nutr. Health Aging 2012, 16, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, L.H.; Kondrup, J.; Zellner, M.; Tetens, I.; Roth, E. Effect of a high protein meat diet on muscle and cognitive functions: A randomised controlled dietary intervention trial in healthy men. Clin. Nutr. 2011, 30, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Walrand, S.; Guillet, C.; Salles, J.; Cano, N.; Boirie, Y. Physiopathological mechanism of sarcopenia. Clin. Geriatr. Med. 2011, 27, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.L.; Green, M.W. Nutritional influences on cognitive function: Mechanisms of susceptibility. Nutr. Res. Rev. 2002, 15, 169–206. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.E.; Hirsch, J.A.; Cirio, R.T.; Jordan, B.D.; Fonzetti, P.; Elder, J. Abnormal thiamine-dependent processes in Alzheimer’s Disease. Lessons from diabetes. Mol. Cell. Neurosci. 2013, 55, 17–25. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, S.T.; Tormey, W.P.; Glasgow, R.; Lavan, J.N. Thiamine deficiency in hospitalized elderly patients. Gerontology 1994, 40, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Pepersack, T.; Garbusinski, J.; Robberecht, J.; Beyer, I.; Willems, D.; Fuss, M. Clinical relevance of thiamine status amongst hospitalized elderly patients. Gerontology 1999, 45, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.E.; Sheu, K.F.R.; Blass, J.P.; Baker, A.; Carlson, K.C.; Harding, B.; Perrino, P. Reduced activities of thiamine-dependent enzymes in the brains and peripheral tissues of patients with Alzheimer’s disease. Arch. Neurol. 1988, 45, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martín, J.L.; Qizilbash, N.; López-Arrieta, J.M. Thiamine for Alzheimer’s disease. Cochrane Database Syst. Rev. 2001, 2, CD001498. [Google Scholar] [PubMed]
- Meador, K.; Loring, D.; Nichols, M.; Zamrini, E.; Rivner, M.; Posas, H.; Thompson, E.; Moore, E. Preliminary findings of high-dose thiamine in dementia of Alzheimer’s type. J. Geriatr. Psychiatry Neurol. 1993, 6, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRSIMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- American Dietetic Association. Evidence Analysis Manual. Steps in the ADA Evidence Analysis Process; Scientific Affairs and Research: Chicago, IL, USA, 2008. [Google Scholar]
- National Health and Medical Research Council. How to Review the Evidence: SystematicIdentification and Review of the Scientific Literature; NHMRC: Canberra, Australia, 2000. [Google Scholar]
- Gao, L.Y.; Dong, B.R.; Hao, Q.K.; Ding, X. Association between cognitive impairment and eating habits in elderly Chinese subjects over 90 years of age. J. Int. Med. Res. 2013, 41, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dong, B.; Zeng, G.; Li, J.; Wang, W.; Wang, B.; Yuan, Q. Is there an association between mild cognitive impairment and dietary pattern in Chinese elderly? Results from a cross-sectional population study. BMC Public Health 2010, 10, 595. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.; Chan, D.; Woo, J. A cross sectional study to examine the association between dietary patterns and cognitive impairment in older Chinese people in Hong Kong. J. Nutr. Health Aging 2013, 17, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Barberger-Gateau, P.; Raffaitin, C.; Letenneur, L.; Berr, C.; Tzourio, C.; Dartigues, JF.; Alpérovitch, A. Dietary patterns and risk of dementia: The Three-City cohort study. Neurology 2007, 69, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Jesus, P.; Desport, J.C.; Massoulard, A.; Villemonteix, C.; Baptiste, A.; Gindre-Poulvelarie, L.; Lorgueuilleux, S.; Javerliat, V.; Fraysse, J.L.; Preux, P.M. Nutritional assessment and follow-up of residents with and without dementia in nursing homes in the Limousin region of France: A health network initiative. J. Nutr. Health Aging 2012, 16, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Shatenstein, B.; Kergoat, M.; Reid, I. Poor nutrient intakes during 1-year follow-up with community-dwelling older adults with early-stage Alzheimer dementia compared to cognitively intact matched controls. J. Am. Diet. Assoc. 2007, 107, 2091–2099. [Google Scholar] [CrossRef] [PubMed]
- LaRue, A.; Koehler, K.M.; Wayne, S.J.; Chiulli, S.J.; Haaland, K.Y.; Garry, P.J. Nutritional status and cognitive functioning in a normally aging sample: A 6-y reassessment. Am. J. Clin. Nutr. 1997, 65, 20–29. [Google Scholar] [PubMed]
- Deschamps, V.; Astier, X.; Ferry, M.; Rainfray, M.; Emeriau, J.P.; Barberger-Gateau, P. Nutritional status of healthy elderly persons living in Dordogne, France, and relation with mortality and cognitive or functional decline. Eur. J. Clin. Nutr. 2002, 56, 305–312. [Google Scholar] [CrossRef] [PubMed]
- McNeill, G.; Jia, X.; Whalley, L.J.; Fox, H.C.; Corley, J.; Gow, A.J.; Brett, C.E.; Starr, J.M.; Deary, I.J. Antioxidant and B vitamin intake in relation to cognitive function in later life in the Lothian Birth Cohort 1936. Eur. J. Clin. Nutr. 2011, 65, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Velho, S.; Marques-Vidal, P.; Baptista, F.; Camilo, M.E. Dietary intake adequacy and cognitive function in free-living active elderly: A cross-sectional and short-term prospective study. Clin. Nutr. 2008, 27, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Vercambre, M.N.; Boutron-Ruault, M.C.; Ritchie, K.; Clavel-Chapelon, F.; Berr, C. Long-term association of food and nutrient intakes with cognitive and functional decline: A 13-year follow-up study of elderly French women. Br. J. Nutr. 2009, 102, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.O.; Roberts, L.A.; Geda, Y.E.; Cha, R.H.; Pankratz, V.S.; O’Connor, H.M.; Knopman, D.S.; Petersen, R.C. Relative intake of macronutrients impacts risk of mild cognitive impairment or dementia. J. Alzheimer’s Dis. 2012, 32, 329–339. [Google Scholar]
- Burns, A.; Marsh, A.; Bender, D.A. Dietary intake and clinical, anthropometric and biochemical indices of malnutrition in elderly demented patients and non-demented subjects. Psychol. Med. 1989, 19, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Nes, M.; Sem, S.W.; Rousseau, B.; Bjorneboe, G.E.A.; Engedal, K.; Trygg, K.; Pedersen, J.I. Dietary intakes and nutritional status of old people with dementia living at home in Oslo. Eur. J. Clin. Nutr. 1988, 42, 581–593. [Google Scholar] [PubMed]
- Ortega, R.M.; Requejo, A.M.; Andres, P.; Lopez-Sobaler, A.M.; Quintas, M.E.; Redondo, M.R.; Navia, B.; Rivas, T. Dietary intake and cognitive function in a group of elderly people. Am. J. Clin. Nutr. 1997, 66, 803–809. [Google Scholar] [PubMed]
- Lee, L.; Kang, S.A.; Lee, H.O.; Lee, B.H.; Park, J.S.; Kim, J.H.; Jung, I.K.; Park, Y.J.; Lee, J.E. Relationships between dietary intake and cognitive function level in Korean elderly people. Public Health (Nat.) 2001, 115, 133–138. [Google Scholar] [CrossRef]
- Requejo, A.M.; Ortega, R.M.; Robles, F.; Navia, B.; Faci, M.; Aparicio, A. Influence of nutrition on cognitive function in a group of elderly, independently living people. Eur. J. Clin. Nutr. 2003, 57, S54–S57. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Baker, P.S.; Allman, R.M.; Zamrini, E. Dietary factors and cognitive impairment in community-dwelling elderly. J. Nutr. Health Aging 2007, 11, 49–54. [Google Scholar] [PubMed]
- Mori, K.; Kawano, Y.; Tada, Y.; Hida, A.; Nagasawa, N.; Inoue, K.; Kamioka, H.; Inoue, K.; Ozeki, T. Relationship of dietary intake and lifestyle factors to health-related quality of life in the community-dwelling elderly. J. Nutr. Sci. Vitaminol. 2010, 56, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Aparicio Vizuete, A.; Robles, F.; Rodríguez-Rodríguez, E.; López-Sobaler, A.M.; Ortega, R.M. Association between food and nutrient intakes and cognitive capacity in a group of institutionalized elderly people. Eur. J. Nutr. 2010, 49, 293–300. [Google Scholar]
- Katsiardanis, K.; Diamantaras, A.A.; Dessypris, N.; Michelakos, T.; Anastasiou, A.; Katsiardani, K.P.; Kanavidis, P.; Papadopoulos, F.C.; Stefanadis, C.; Panagiotakos, D.B.; et al. Cognitive Impairment and Dietary Habits Among Elders: The Velestino Study. J. Med. Food 2013, 16, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Van de Rest, O.; van der Zwaluw, N.L.; de Groot, L.C. Literature review on the role of dietary protein and amino acids in cognitive functioning and cognitive decline. Amino Acids 2013, 45, 1035–1045. [Google Scholar]
- Gibson, G.E.; Blass, J.P.; Huang, H.M.; Freeman, G.B. The cellular basis of delirium and its relevance to age-related disorders including Alzheimer’s disease. Int. Psychogeriatr./IPA 1991, 3, 373–395. [Google Scholar] [CrossRef]
- Gold, M.; Chen, M.F.; Johnson, K. Plasma and red blood cell thiamine deficiency in patients with dementia of the Alzheimer’s type. Arch. Neurol. 1995, 52, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.E.; Blass, J.P. Thiamine-dependent processes and treatment strategies in neurodegeneration. Antioxid. Redox Signal. 2007, 9, 1605–1619. [Google Scholar] [CrossRef] [PubMed]
- Langlais, P.J.; Savage, L.M. Thiamine deficiency in rats produces cognitive and memory deficits on spatial tasks that correlate with tissue loss in diencephalon, cortex and white matter. Behav. Brain Res. 1995, 68, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.E.; Weinstein, S.J.; Lawson, K.A.; Albanes, D.; Subar, A.F.; Dixon, L.B.; Mouw, T.; Schatzkin, A.; Leitzmann, M.F. Supplemental and dietary vitamin E intakes and risk of prostate cancer in a large prospective study. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The selenium and vitamin E cancer prevention trial (SELECT). J. Am. Med. Assoc. 2009, 301, 39–51. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koh, F.; Charlton, K.; Walton, K.; McMahon, A.-T. Role of Dietary Protein and Thiamine Intakes on Cognitive Function in Healthy Older People: A Systematic Review. Nutrients 2015, 7, 2415-2439. https://doi.org/10.3390/nu7042415
Koh F, Charlton K, Walton K, McMahon A-T. Role of Dietary Protein and Thiamine Intakes on Cognitive Function in Healthy Older People: A Systematic Review. Nutrients. 2015; 7(4):2415-2439. https://doi.org/10.3390/nu7042415
Chicago/Turabian StyleKoh, Freda, Karen Charlton, Karen Walton, and Anne-Therese McMahon. 2015. "Role of Dietary Protein and Thiamine Intakes on Cognitive Function in Healthy Older People: A Systematic Review" Nutrients 7, no. 4: 2415-2439. https://doi.org/10.3390/nu7042415






