A Hyperspectral Imaging Approach to White Matter Hyperintensities Detection in Brain Magnetic Resonance Images
Abstract
:1. Introduction
2. Methods
2.1. Nonlinear Band Dimensionality Expansion
- (i)
- = set of auto-correlated band images
- (ii)
- = set of cross-correlated band images
- (i)
- = set of auto-correlated band images
- (ii)
- = set of two cross-correlated band images
- (iii)
- = set of three cross-correlated band images
- (i)
- = set of band images stretched out by the square-root.
- (ii)
- = set of band images stretched out by the logarithmic function.
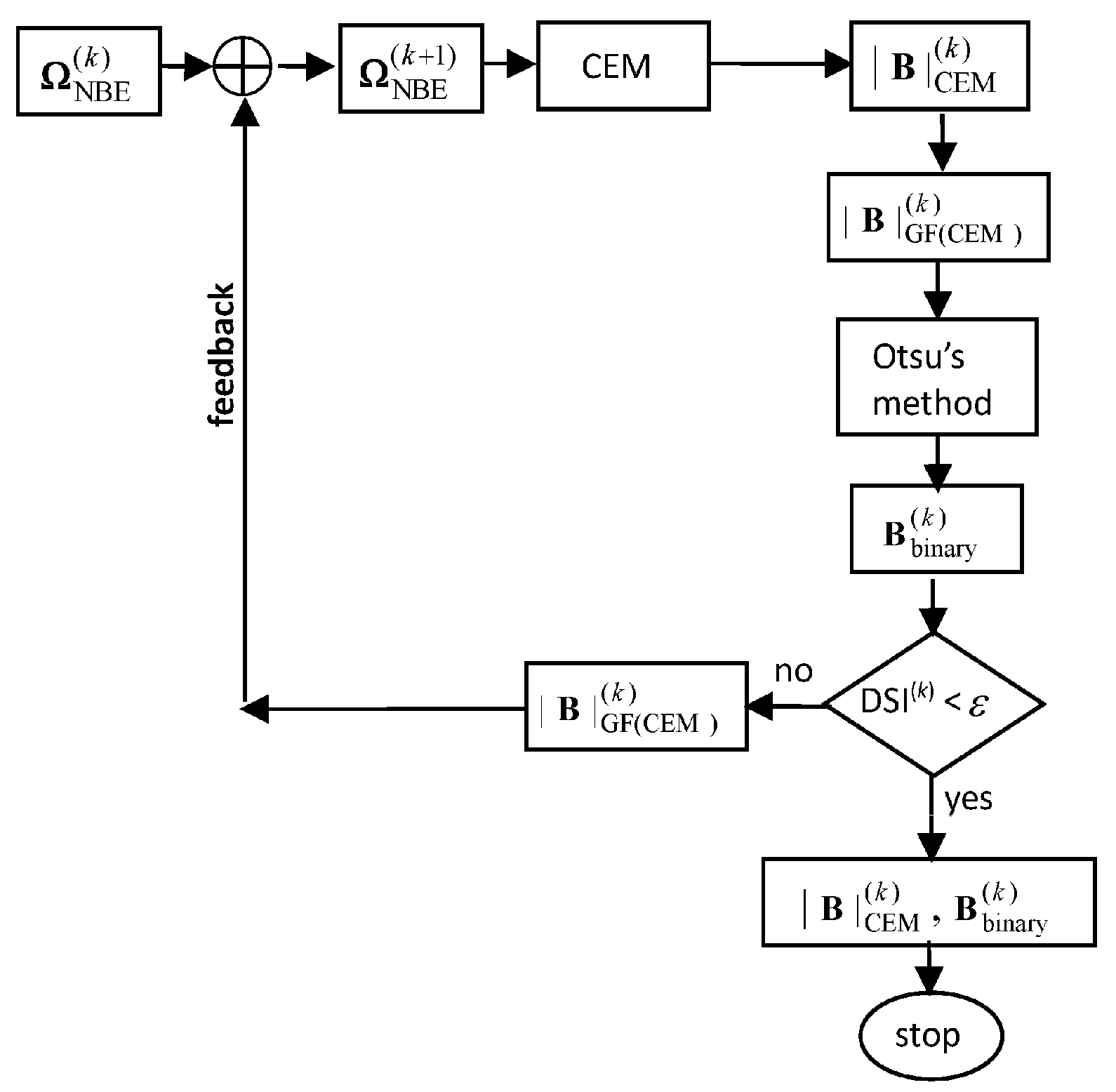
2.2. Iterative CEM
- Initial condition: Let be the original set of band images.
- Use an NBE process to create a new set of nonlinear band images, where nNB is the number of new band images by the NBE process.
- Form a new set of band images, . Let be the desired target pixels in . Let be CEM using d(0) and R(0) which are obtained from . Let .
- At the kth iteration, update and from .
- Use new generated d(k) and R(k) for to be implemented on . Let be the detection abundance fractional map produced by .
- Use a Gaussian filter to blur where is the absolute value of . The resulting image is denoted by Gaussian-filter .
- Check if satisfies a given stopping rule. If no, continue. Otherwise, go to Step 9.
- Form . Let and go to Step 4.
- is the desired detection abundance fractional map and ICEM is terminated.
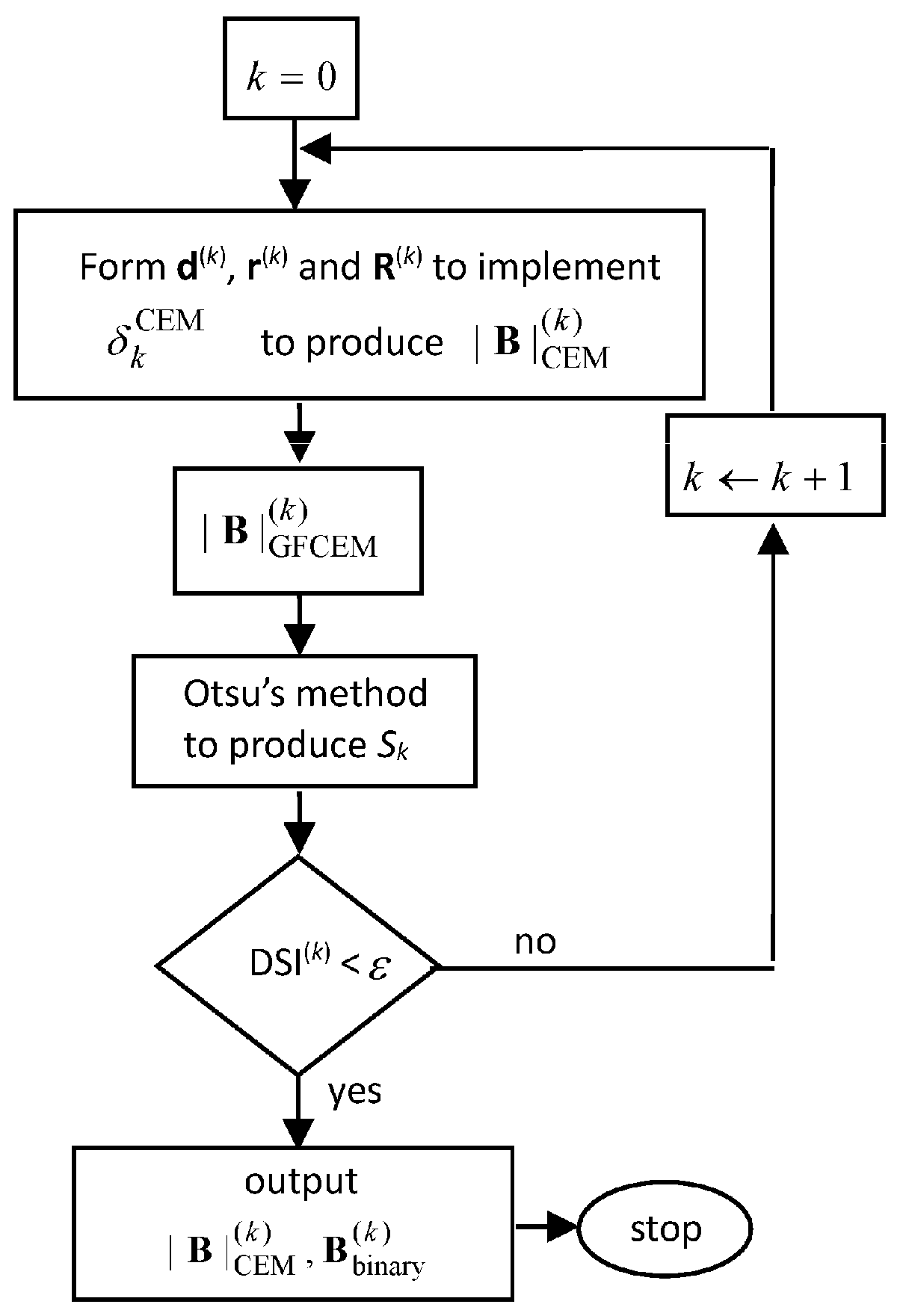
2.3. Stopping Rule for ICEM
2.4. Algorithm for NBE-ICEM
- Initial conditions:
- For each class, find its sample mean to calculate the desired signature d for the particular class.
- Select the values of the parameter σ used for Gaussian filters in ICEM,
- Prescribe an error threshold for DSI in Equation (1)
- Use the NBE process described in Section 2.1 to generate a set of nonlinear band images, .
- Apply ICEM described in Figure 1 to .
- Use DSI described in Figure 2 as a stopping rule to terminate ICEM.
- Output , which is real-valued, and , which is binary-valued, to produce a confusion matrix for classification.
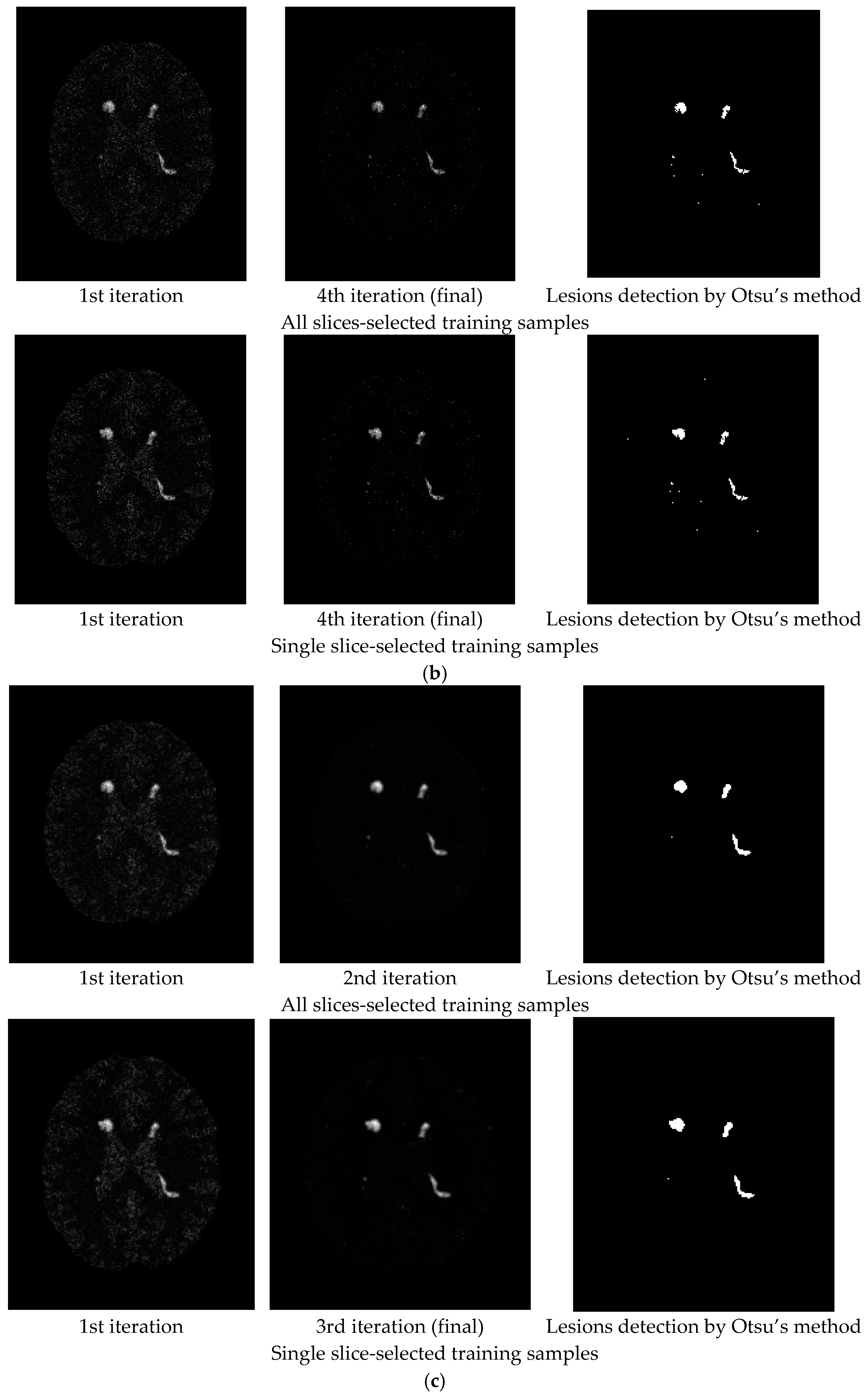
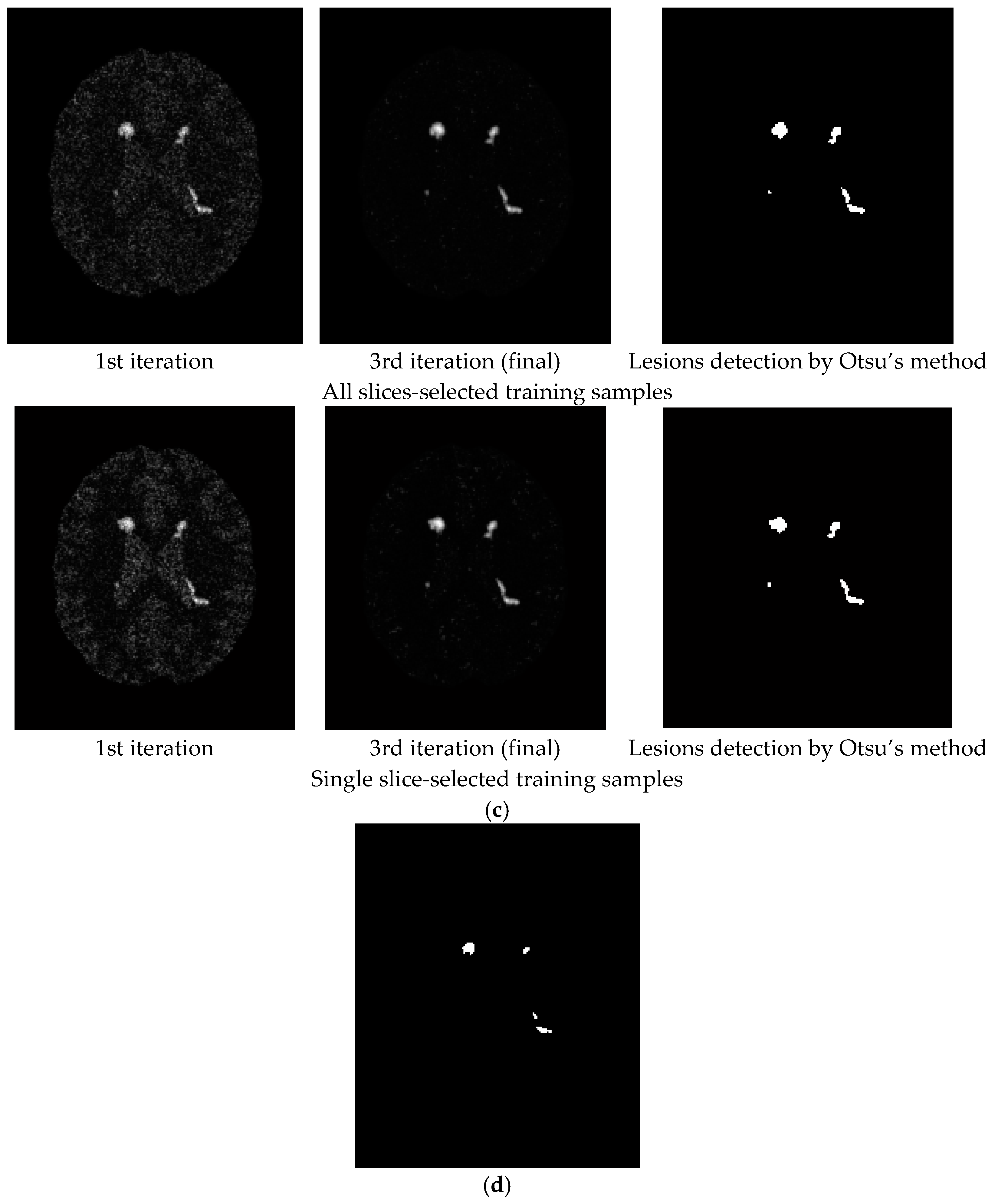
3. Results
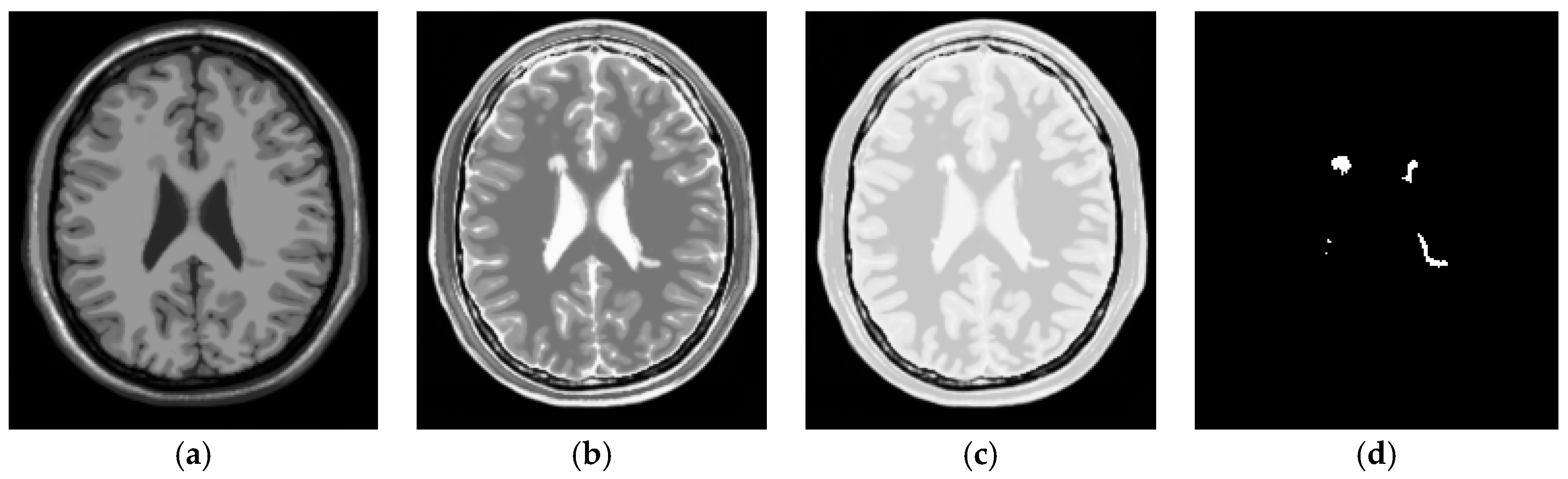
3.1. Synthetic Image Experiments
- Despite the fact that the training samples used for our proposed NBE-ICEM were selected based on 2D images such as by all slices and a single slice, these training samples were either stacked as voxels from all slices or extrapolated from a single slice as voxels by VSA. Accordingly, NBE-ICEM is actually run on 3D images as image cubes.
- There is an issue in implementing LST. Since it is packaged as a software algorithm, there is no flexibility for users to choose parameters at their discretion. Besides, it cannot implement T1W, T2W or FLAIR images alone. Instead, it must require T1W images as reference images to segment WMHs [26]. Most importantly, it produces real valued gray level images, which require users selecting a threshold value from a range from 0.05 to 0.95 with a step size of 0.05 to detect WMHs. In [26], this threshold value was suggested between 0.25 and 0.4. However, in practical applications, the best value is generally selected manually. Thus, technically speaking, LST is not fully automatic. Specifically, when synthetic images from the BainWeb were used for experiments, it was found that using both T1W and T2W could not segment WMHs. It must use T1W and PD to detect WMHs and the threshold value must be set to around 0.2 to segment WMHs.
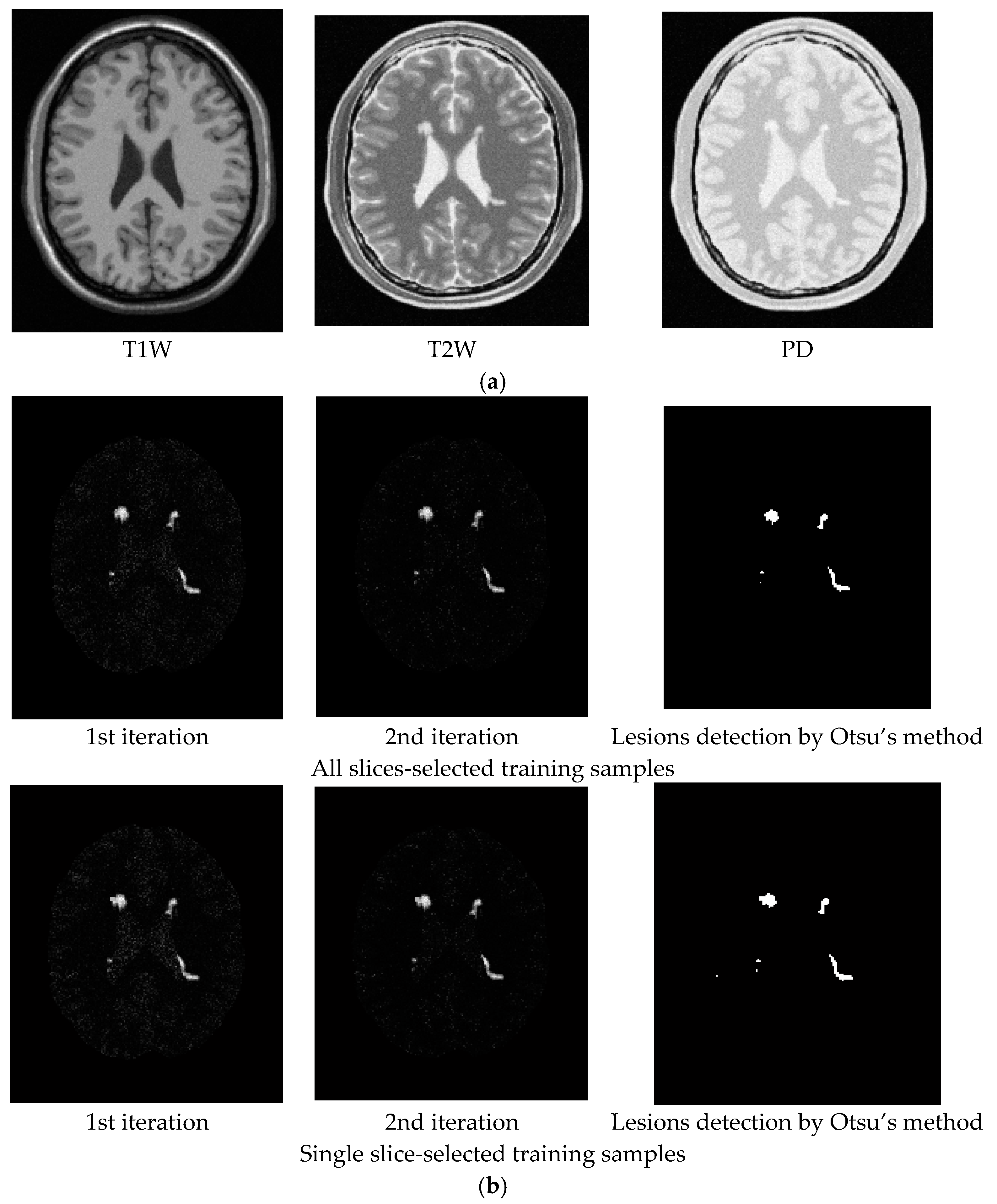
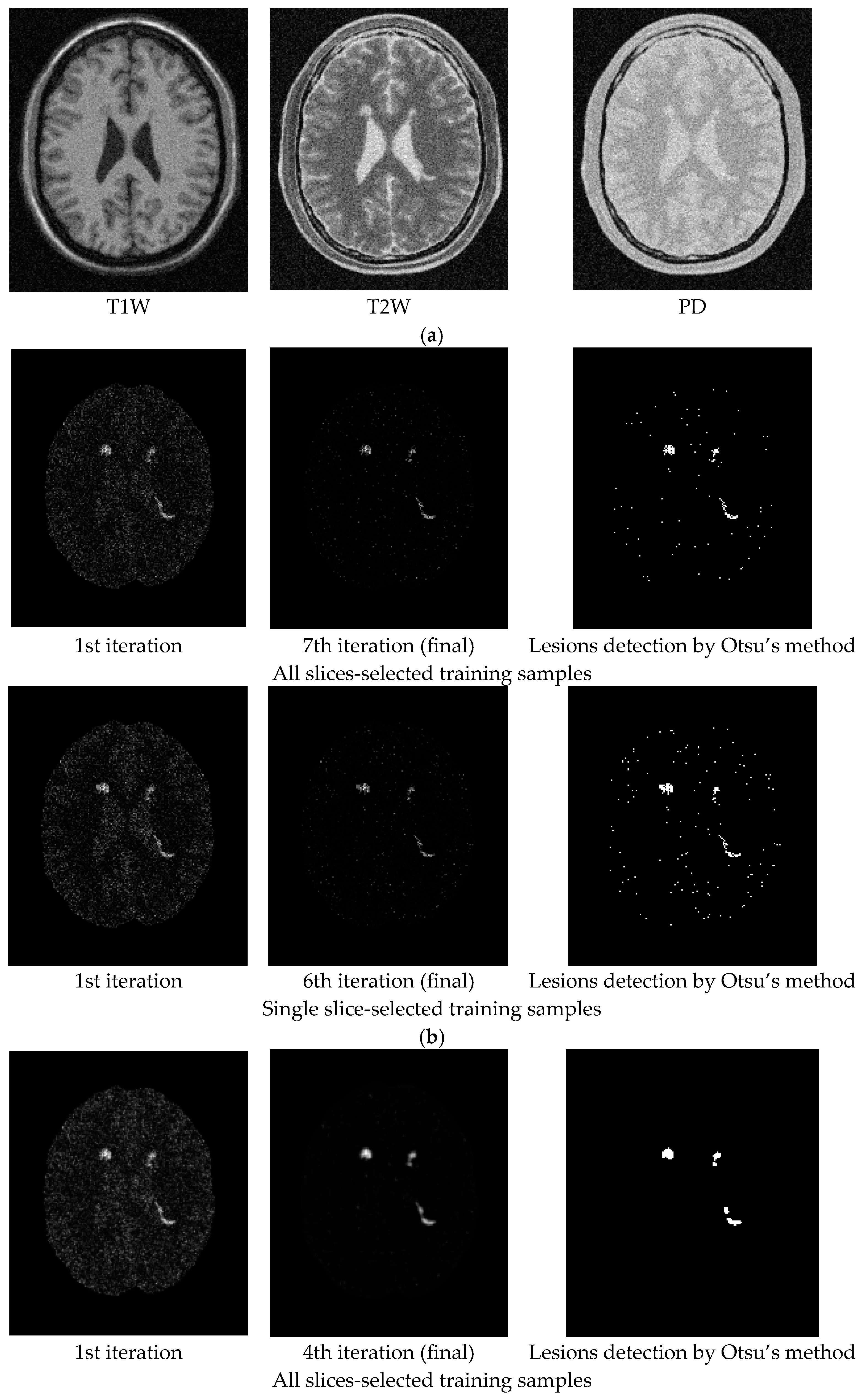
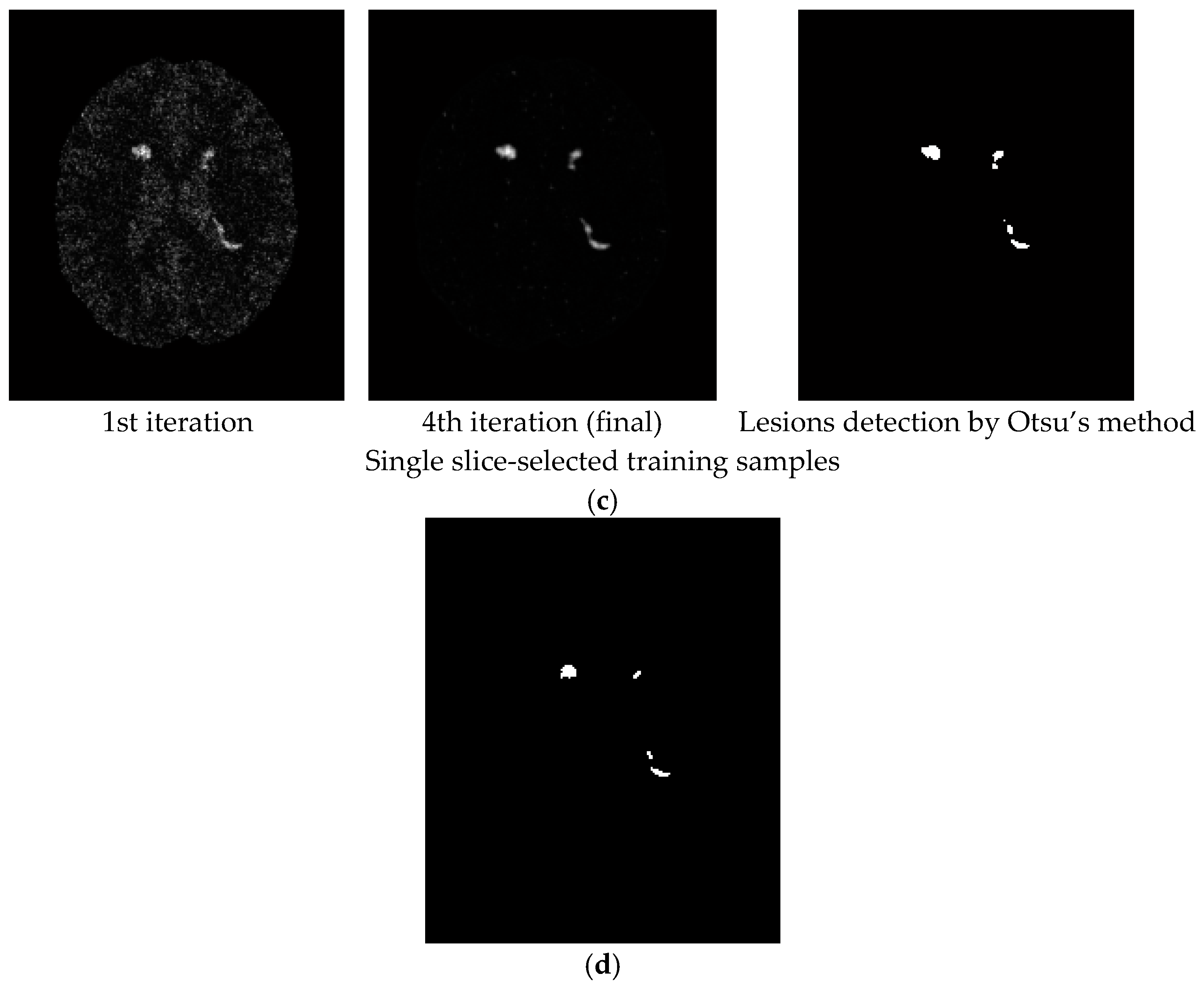
3.2. Real Image Experiments
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Callisaya, M.L.; Beare, R.; Phan, T.; Blizzard, L.; Thrift, A.G.; Chen, J.; Srikanth, V.K. Progression of White Matter Hyperintensities of Presumed Vascular Origin Increases the Risk of Falls in Older People. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Hachinski, V.C.; Potter, P.; Merskey, H. Leuko-araiosis: an ancient term for a new problem. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 1986, 13, 533–534. [Google Scholar] [CrossRef]
- Boutet, C.; Rouffiange-Leclair, L.; Schneider, F.; Camdessanché, J.-P.; Antoine, J.-C.; Barral, F.-G. Visual Assessment of Age-Related White Matter Hyperintensities Using FLAIR Images at 3 T: Inter- and Intra-Rater Agreement. Neurodegener. Dis. 2015. [Google Scholar] [CrossRef] [PubMed]
- Valverde, S.; Oliver, A.; Roura, E.; González-Villà, S.; Pareto, D.; Vilanova, J.C.; Ramió-Torrentà, L.; Rovira, À.; Lladó, X. Automated tissue segmentation of MR brain images in the presence of white matter lesions. Med. Image Anal. 2017, 35, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Roura, E.; Oliver, A.; Cabezas, M.; Valverde, S.; Pareto, D.; Vilanova, J.C.; Ramió-Torrentà, L.; Rovira, À.; Lladó, X. A toolbox for multiple sclerosis lesion segmentation. Neuroradiology 2015, 57, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Samaille, T.; Fillon, L.; Cuingnet, R.; Jouvent, E.; Chabriat, H.; Dormont, D.; Colliot, O.; Chupin, M. Contrast-Based Fully Automatic Segmentation of White Matter Hyperintensities: Method and Validation. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.; Gao, F.; Black, S.E.; Lobaugh, N.J. Automatic segmentation of white matter hyperintensities in the elderly using FLAIR images at 3T. J. Magn. Reson. Imaging 2010, 31, 1311–1322. [Google Scholar] [CrossRef] [PubMed]
- Vannier, M.W.; Butterfield, R.L.; Jordan, D.; Murphy, W.A.; Levitt, R.G.; Gado, M. Multispectral analysis of magnetic resonance images. Radiology 1985, 154, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-I. Hyperspectral Imaging: Techniques for Spectral Detection and Classification; Springer: New York, NY, USA, 2003; ISBN 978-0-306-47483-5. [Google Scholar]
- Nakai, T.; Muraki, S.; Bagarinao, E.; Miki, Y.; Takehara, Y.; Matsuo, K.; Kato, C.; Sakahara, H.; Isoda, H. Application of independent component analysis to magnetic resonance imaging for enhancing the contrast of gray and white matter. NeuroImage 2004, 21, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.-C.; Chen, H.-M.; Chai, J.-W.; Chen, C.-C.; Chen, C.C.-C.; Poon, S.-K.; Yang, C.-W.; Lee, S.-K. Independent Component Analysis for Magnetic Resonance Image Analysis. EURASIP J. Adv. Signal Process. 2008, 2008. [Google Scholar] [CrossRef]
- Ouyang, Y.-C.; Chen, H.-M.; Chai, J.-W.; Chen, C.C.-C.; Poon, S.-K.; Yang, C.-W.; Lee, S.-K.; Chang, C.-I. Band Expansion-Based Over-Complete Independent Component Analysis for Multispectral Processing of Magnetic Resonance Images. IEEE Trans. Biomed. Eng. 2008, 55, 1666–1677. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.-W.; Chen, C.C.-C.; Chiang, C.-M.; Ho, Y.-J.; Chen, H.-M.; Ouyang, Y.-C.; Yang, C.-W.; Lee, S.-K.; Chang, C.-I. Quantitative analysis in clinical applications of brain MRI using independent component analysis coupled with support vector machine. J. Magn. Reson. Imaging 2010, 32, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.-W.; Chen, C.C.; Wu, Y.-Y.; Chen, H.-C.; Tsai, Y.-H.; Chen, H.-M.; Lan, T.-H.; Ouyang, Y.-C.; Lee, S.-K. Robust Volume Assessment of Brain Tissues for 3-Dimensional Fourier Transformation MRI via a Novel Multispectral Technique. PLOS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Chiou, Y.-J.; Chen, C.C.-C.; Chen, S.-Y.; Chen, H.-M.; Chai, J.-W.; Ouyang, Y.-C.; Su, W.-C.; Yang, C.-W.; Lee, S.-K.; Chang, C.-I. Magnetic resonance brain tissue classification and volume calculation. J. Chin. Inst. Eng. 2015, 38, 1055–1066. [Google Scholar] [CrossRef]
- Ren, H.; Chang, C.-I. A generalized orthogonal subspace projection approach to unsupervised multispectral image classification. IEEE Trans. Geosci. Remote Sens. 2000, 38, 2515–2528. [Google Scholar]
- Harsanyi, J.C. Detection and Classification of Subpixel Spectral Signatures in Hyperspectral Image Sequences; Department of Electrical Engineering, University of Maryland, Baltimore County: Baltimore, MD, USA, 1993. [Google Scholar]
- Farrand, W.H.; Harsanyi, J.C. Mapping the distribution of mine tailings in the Coeur d’Alene River Valley, Idaho, through the use of a constrained energy minimization technique. Remote Sens. Environ. 1997, 59, 64–76. [Google Scholar] [CrossRef]
- Chang, C.-I. Target signature-constrained mixed pixel classification for hyperspectral imagery. IEEE Trans. Geosci. Remote Sens. 2002, 40, 1065–1081. [Google Scholar] [CrossRef]
- Xue, B.; Wang, L.; Li, H.-C.; Chen, H.M.; Chang, C.-I. Lesion Detection in Magnetic Resonance Brain Images by Hyperspectral Imaging Algorithms. In Proceedings Volume 9874, Remotely Sensed Data Compression, Communications, and Processing XII; International Society for Optics and Photonics: Baltimore, MD, USA, 2016; Vol. 9874, p. 98740M. [Google Scholar]
- Otsu, N. A threshold selection method from gray-level histgram. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Dice, L.R. Measures of the amount of ecologic association between species. Ecology 1945, 26, 297–302. [Google Scholar] [CrossRef]
- Kang, X.; Li, S.; Benediktsson, J.A. Spectral-Spatial Hyperspectral Image Classification with Edge-Preserving Filtering. IEEE Trans. Geosci. Remote Sens. 2014, 52, 2666–2677. [Google Scholar] [CrossRef]
- BrainWeb: Simulated Brain Database. Available online: http://www.bic.mni.mcgill.ca/brainweb/ (accessed on 15 November 2017).
- LST: A Lesion Segmentation Tool for SPM. Available online: http://www.statistical-modelling.de/lst.html (accessed on 15 November 2017).
- Schmidt, P.; Gaser, C.; Arsic, M.; Buck, D.; Förschler, A.; Berthele, A.; Hoshi, M.; Ilg, R.; Schmid, V.J.; Zimmer, C.; et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. NeuroImage 2012, 59, 3774–3783. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerman, R.A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef] [PubMed]









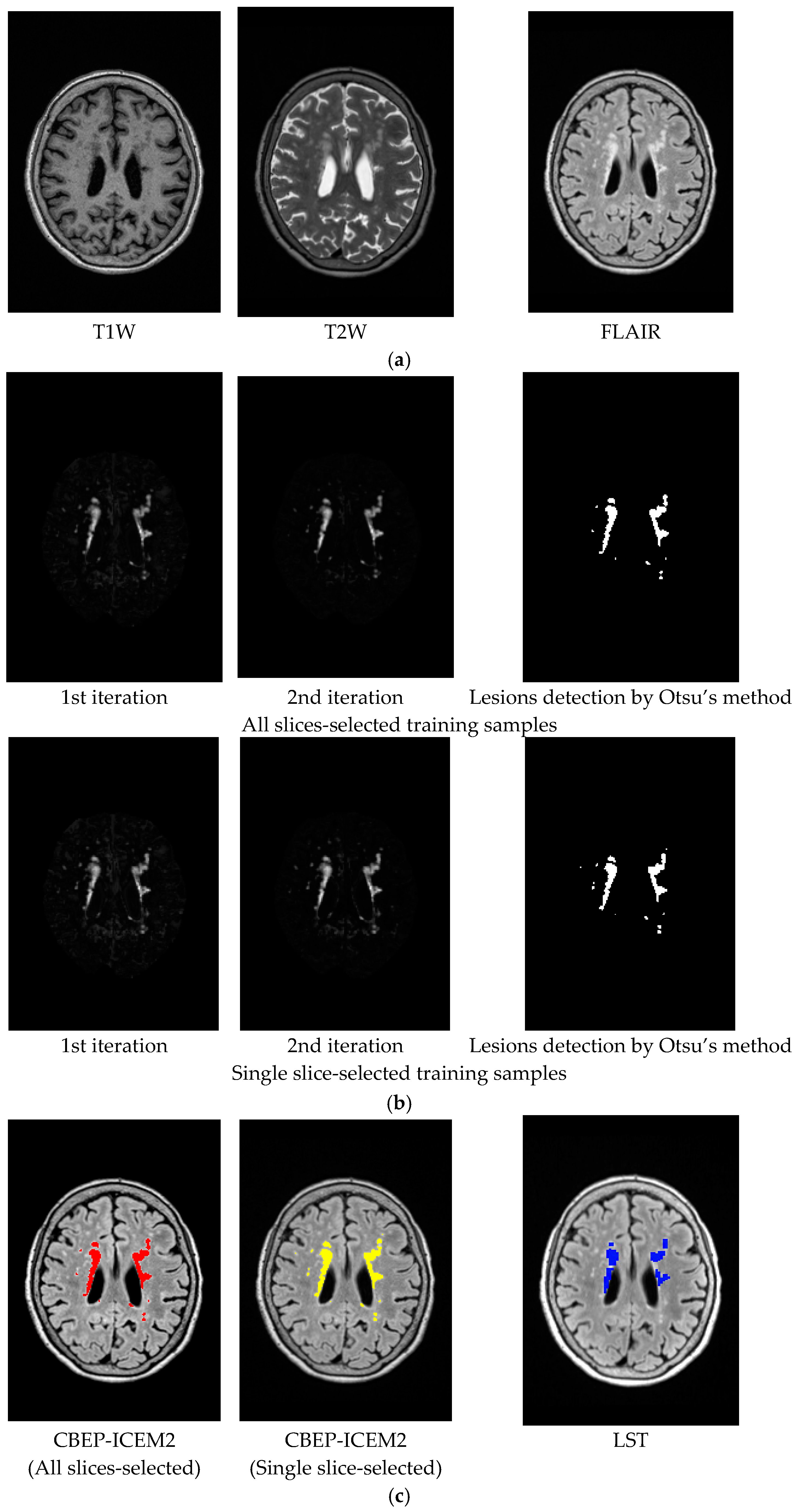

| Band images | T1W, T2W, PD (3 bands) |
| Correlation Band Expansion Process (CBEP) | 3rd order correlated band images |
| d | found by all slices-selected or single slice-selected training samples |
| Gaussian window size | () |
| σ used in Gaussian filter | 0.1 with window size (0.5 with window size ) |
| Thresholding method | Otsu’s method |
| error threshold (DSI) | 0.80 |
| Methods | CBEP-ICEM1 | CBEP-ICEM2 | LST | |
|---|---|---|---|---|
| Noise/INU Level | ||||
| n0/rf0 | 0.865 | 0.808 | 0.739 | |
| n1/rf0 | 0.886 | 0.864 | 0.749 | |
| n3/rf0 | 0.893 | 0.863 | 0.750 | |
| n5/rf0 | 0.806 | 0.839 | 0.731 | |
| n7/rf0 | 0.652 | 0.822 | 0.693 | |
| n9/rf0 | 0.579 | 0.801 | 0.714 | |
| n0/rf20 | 0.861 | 0.829 | 0.733 | |
| n1/rf20 | 0.867 | 0.834 | 0.753 | |
| n3/rf20 | 0.881 | 0.827 | 0.746 | |
| n5/rf20 | 0.814 | 0.831 | 0.732 | |
| n7/rf20 | 0.714 | 0.825 | 0.694 | |
| n9/rf20 | 0.540 | 0.806 | 0.655 | |
| Methods | CBEP-ICEM1 | CBEP-ICEM2 | LST | |
|---|---|---|---|---|
| Noise/INU Level | ||||
| n0/rf0 | 0.798 | 0.784 | 0.739 | |
| n1/rf0 | 0.848 | 0.847 | 0.749 | |
| n3/rf0 | 0.871 | 0.858 | 0.750 | |
| n5/rf0 | 0.776 | 0.836 | 0.731 | |
| n7/rf0 | 0.625 | 0.816 | 0.693 | |
| n9/rf0 | 0.389 | 0.778 | 0.714 | |
| n0/rf20 | 0.844 | 0.834 | 0.733 | |
| n1/rf20 | 0.859 | 0.837 | 0.753 | |
| n3/rf20 | 0.854 | 0.814 | 0.746 | |
| n5/rf20 | 0.811 | 0.819 | 0.732 | |
| n7/rf20 | 0.710 | 0.804 | 0.694 | |
| n9/rf20 | 0.549 | 0.799 | 0.655 | |
| Band | T1W, T2W, FLAIR (3 bands) | ||
| CBEP | 3rd order correlated band images | ||
| d | found by all slices-selected or single slice-selected training samples | ||
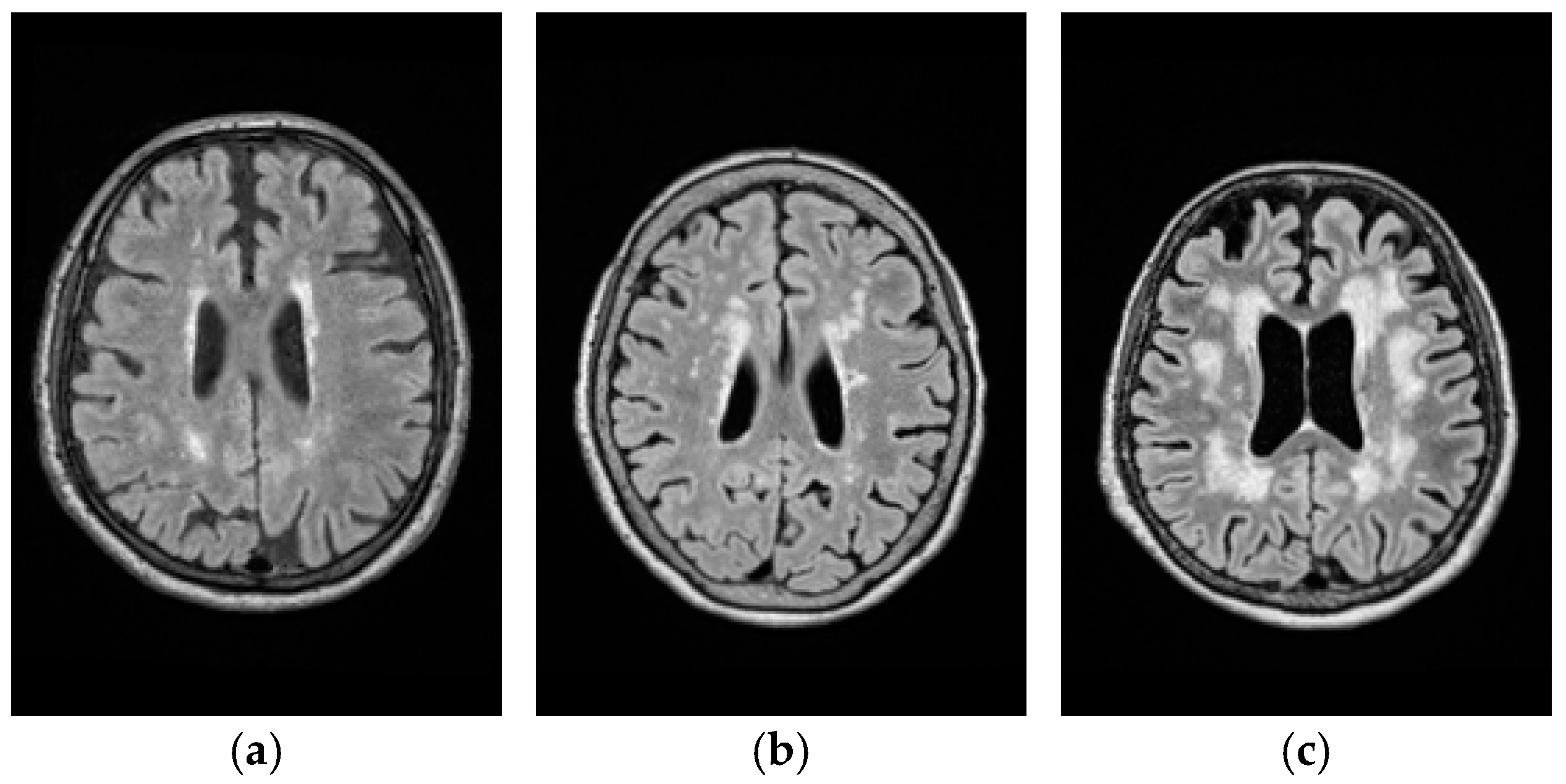
| Fazekas grade | 1 | 2 | 3 |
| Gaussian window size | 5 × 5 | ||
| σ used in Gaussian filter | 0.5 with window size 5 × 5 | ||
| Thresholding method | Otsu’s method | ||
| stopping threshold (DSI) | 0.80 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-M.; Wang, H.C.; Chai, J.-W.; Chen, C.-C.C.; Xue, B.; Wang, L.; Yu, C.; Wang, Y.; Song, M.; Chang, C.-I. A Hyperspectral Imaging Approach to White Matter Hyperintensities Detection in Brain Magnetic Resonance Images. Remote Sens. 2017, 9, 1174. https://doi.org/10.3390/rs9111174
Chen H-M, Wang HC, Chai J-W, Chen C-CC, Xue B, Wang L, Yu C, Wang Y, Song M, Chang C-I. A Hyperspectral Imaging Approach to White Matter Hyperintensities Detection in Brain Magnetic Resonance Images. Remote Sensing. 2017; 9(11):1174. https://doi.org/10.3390/rs9111174
Chicago/Turabian StyleChen, Hsian-Min, Hsin Che Wang, Jyh-Wen Chai, Chi-Chang Clayton Chen, Bai Xue, Lin Wang, Chunyan Yu, Yulei Wang, Meiping Song, and Chein-I Chang. 2017. "A Hyperspectral Imaging Approach to White Matter Hyperintensities Detection in Brain Magnetic Resonance Images" Remote Sensing 9, no. 11: 1174. https://doi.org/10.3390/rs9111174






