Remote Sensing of Sea Surface pCO2 in the Bering Sea in Summer Based on a Mechanistic Semi-Analytical Algorithm (MeSAA)
Abstract
:1. Introduction
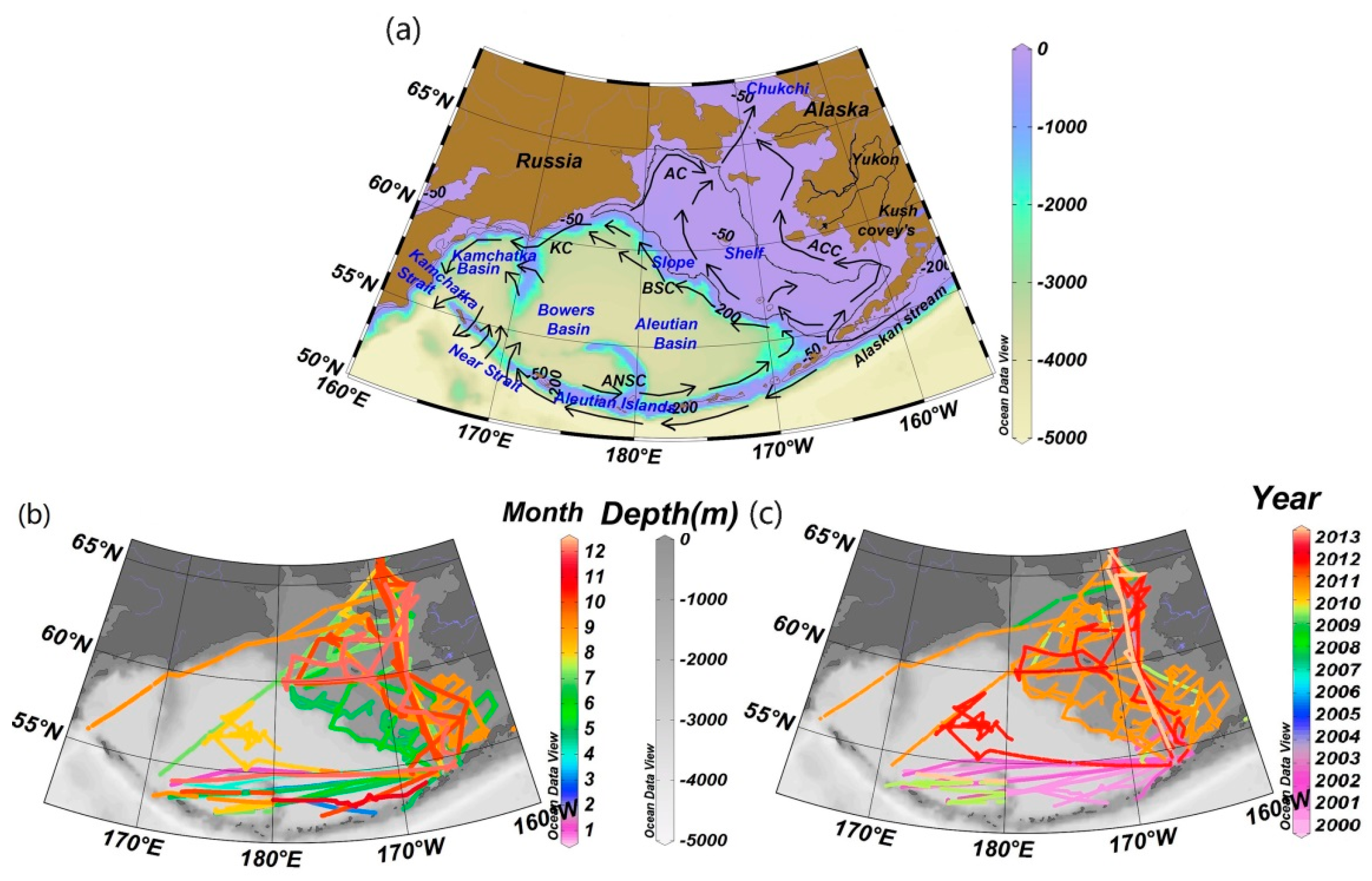
2. Study Area and Data
2.1. Study Area
2.2. Data and Methods
3. Implementation of the MeSAA Algorithm for pCO2 in the Bering Sea
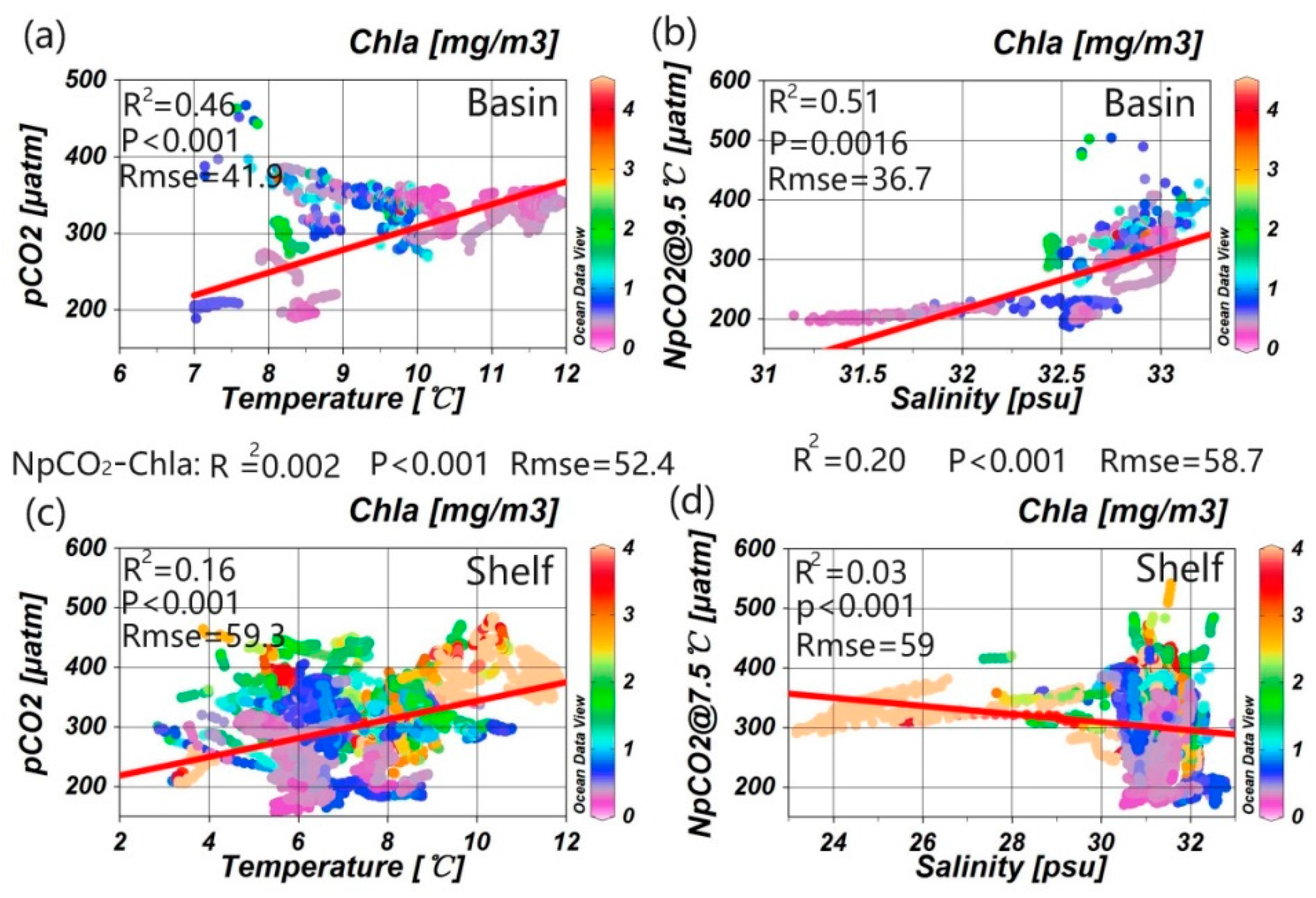
3.1. Correlations between Underway pCO2 and Related Parameters
3.2. The MeSAA Algorithm for pCO2
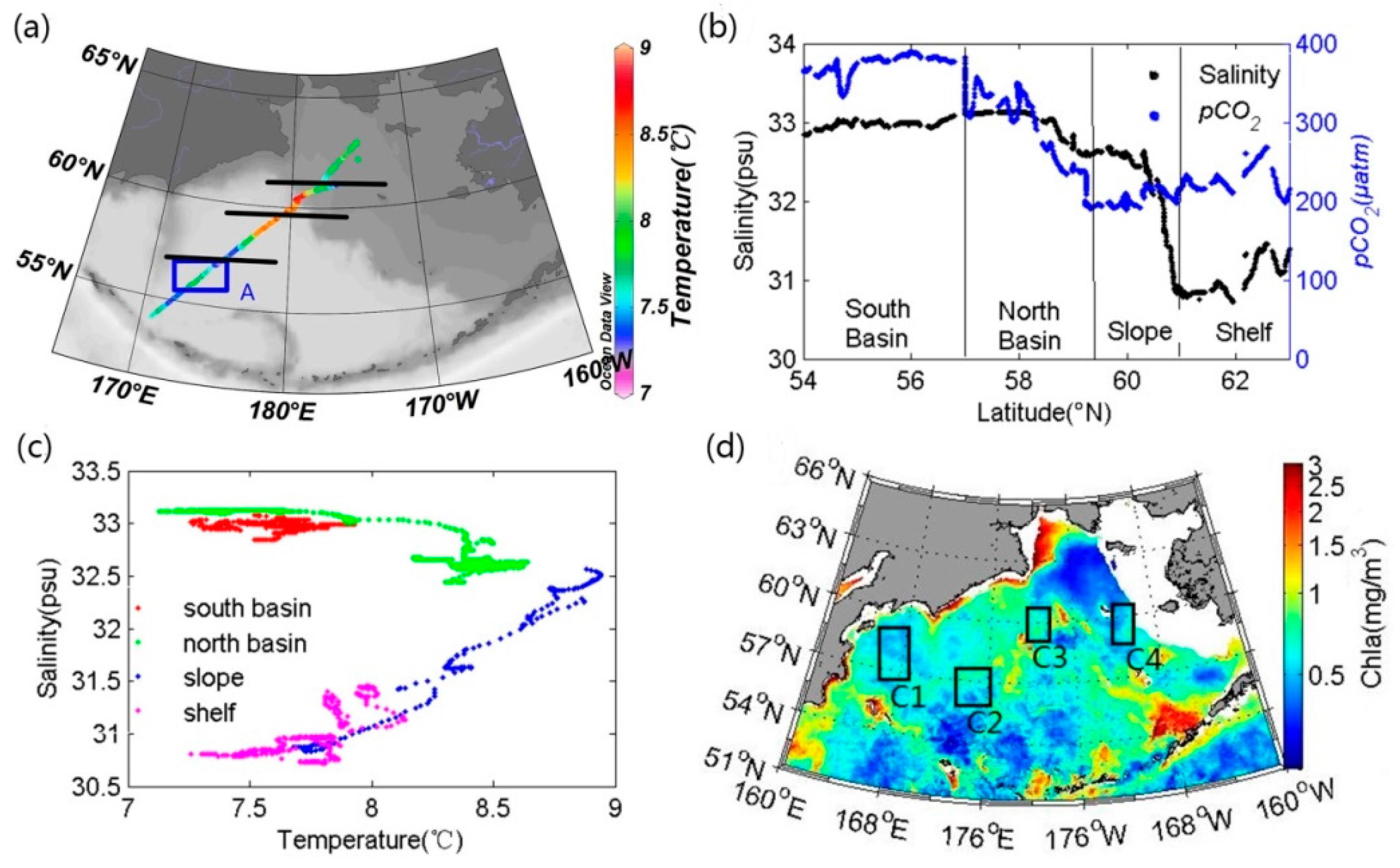
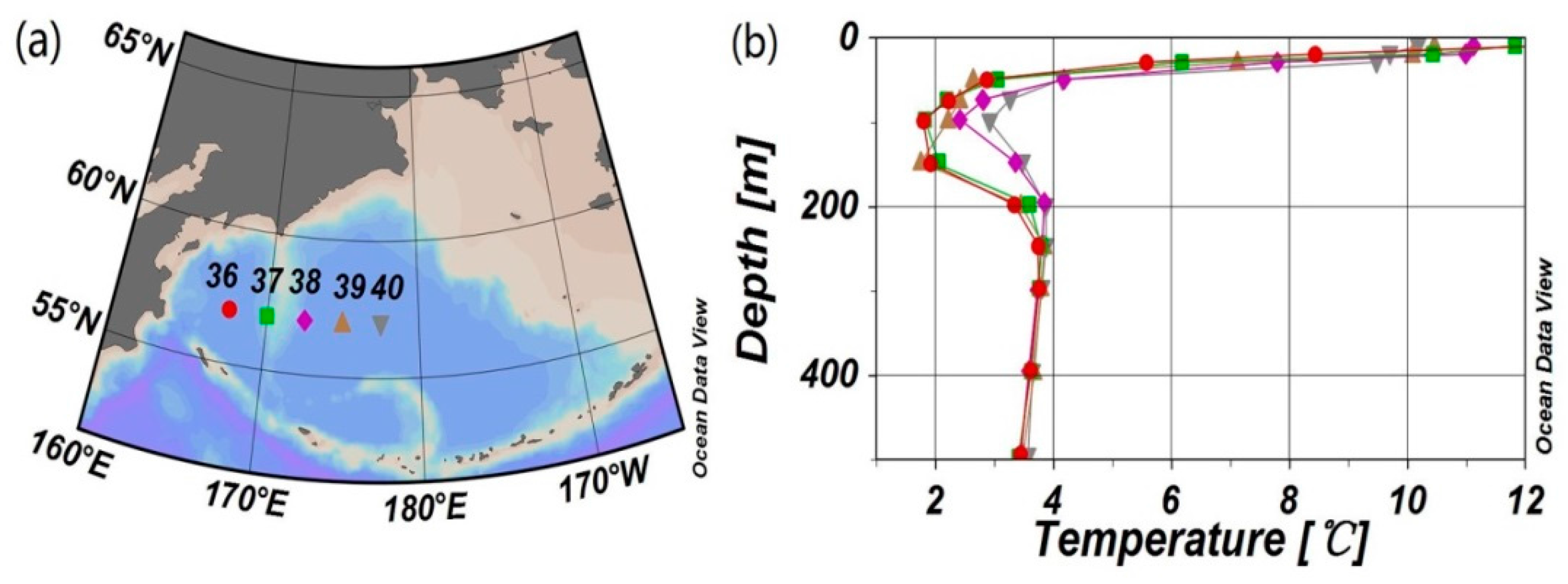
3.2.1. Reference Water Mass and Temperature Effect
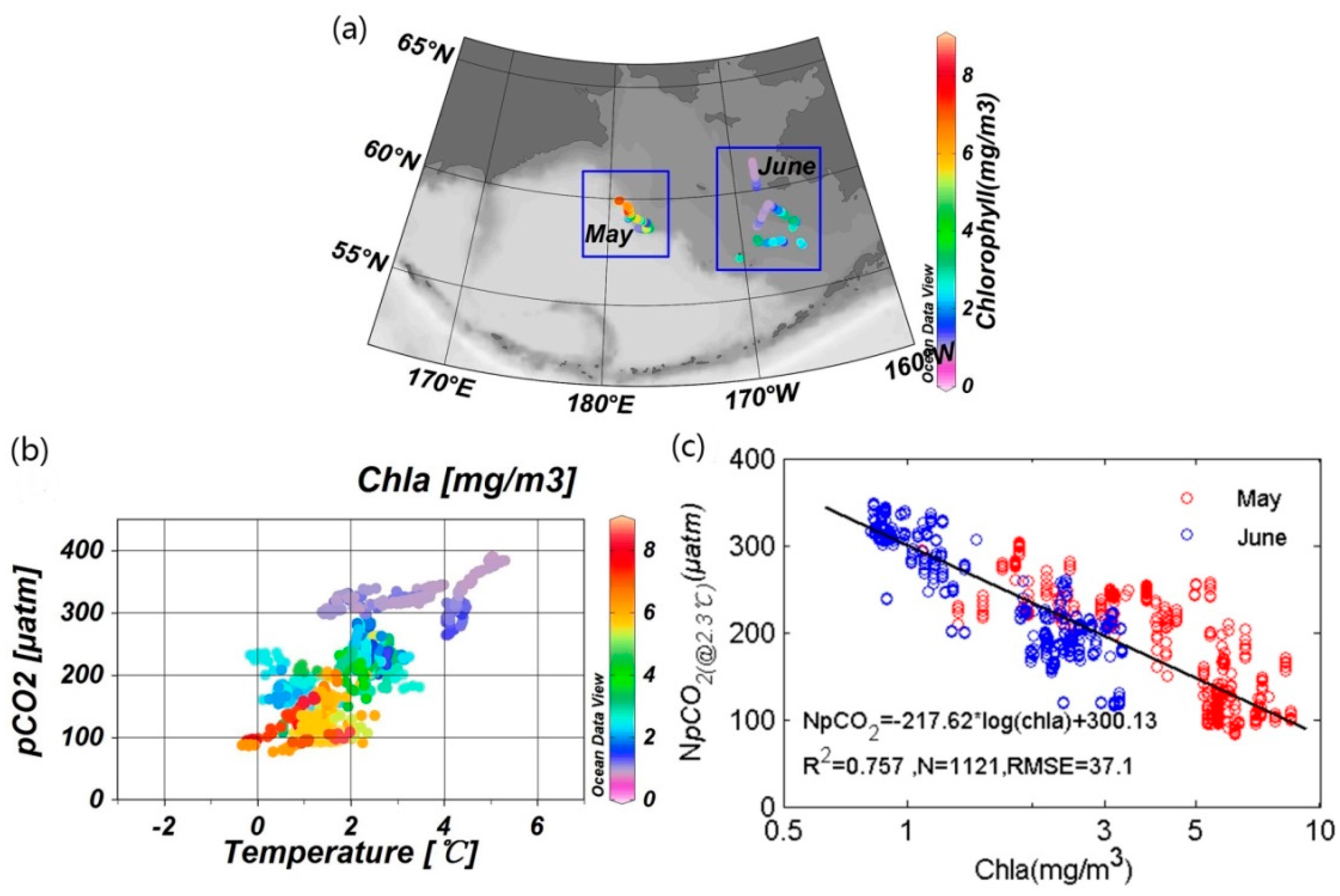
3.2.2. Quantification of Biological Effect
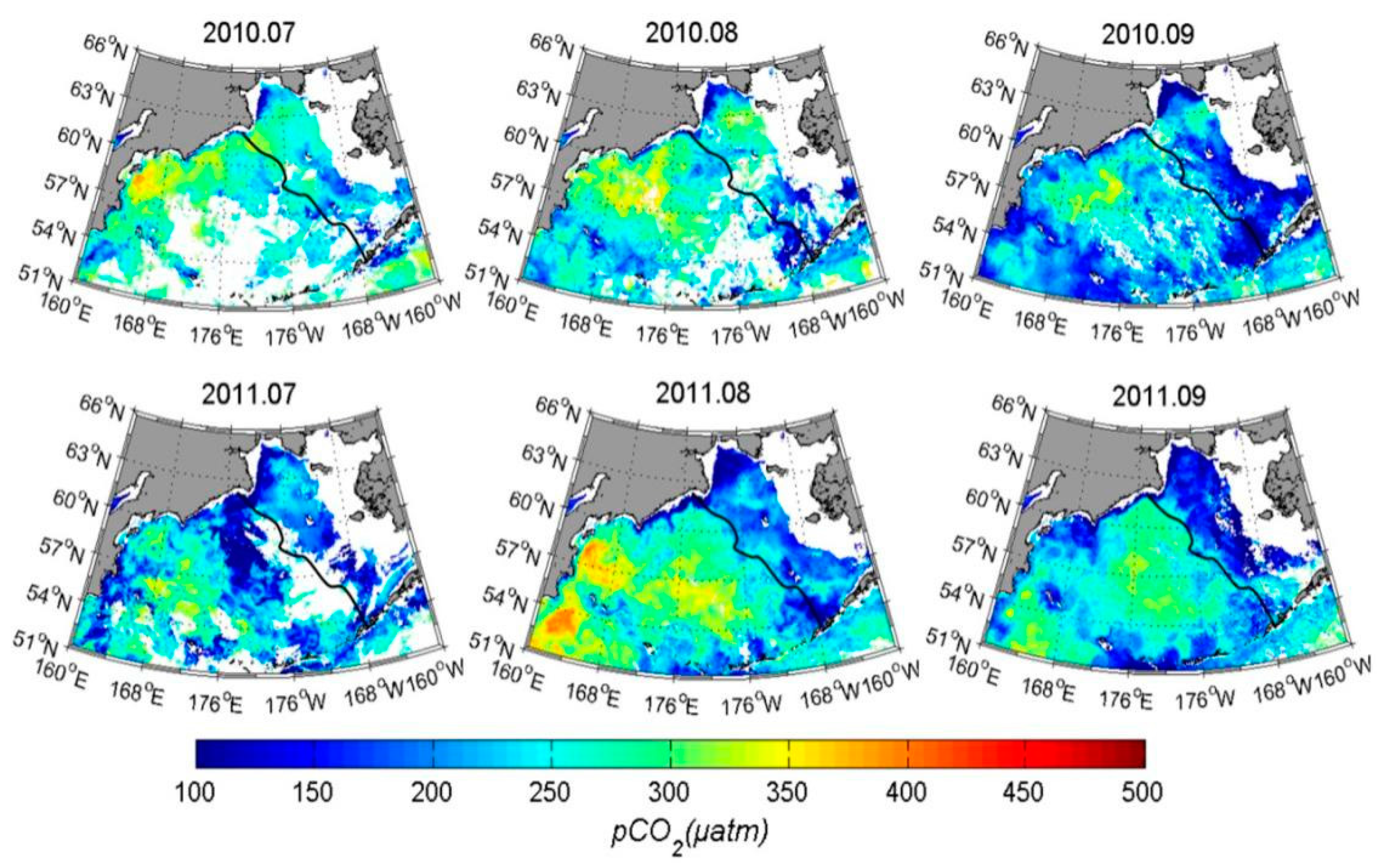
3.3. Results and Validation
3.4. Contribution of Different Controlling Factors to the Variation of pCO2
4. Discussion
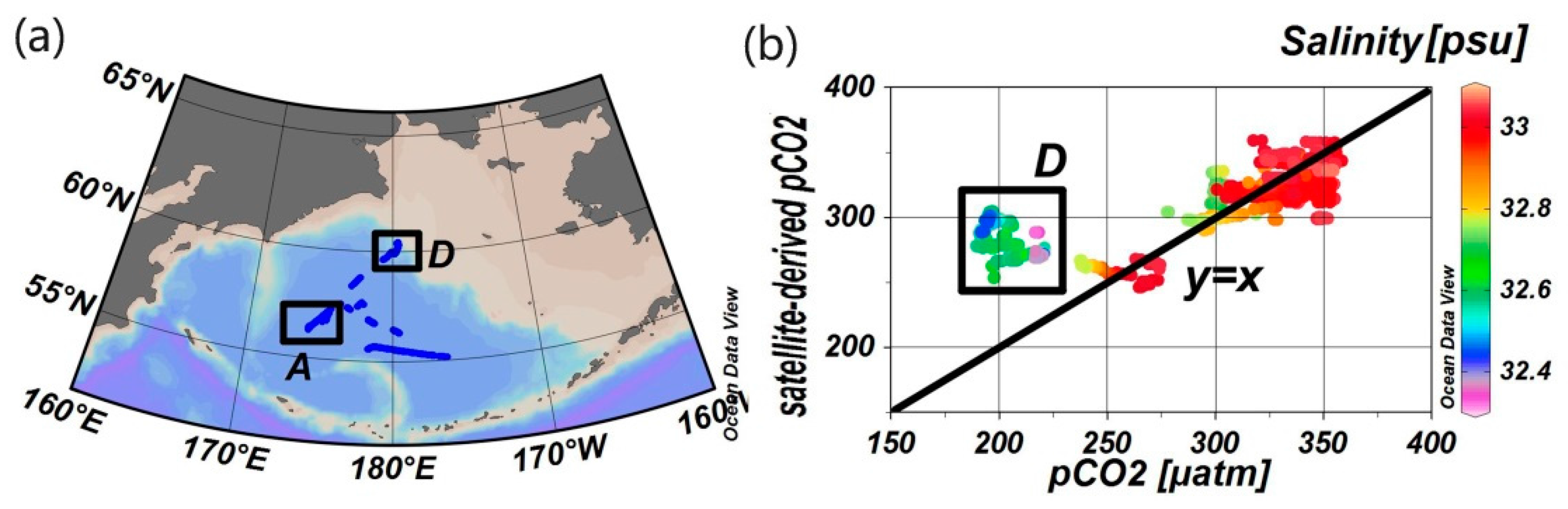
4.1. Stability of Reference Water Mass
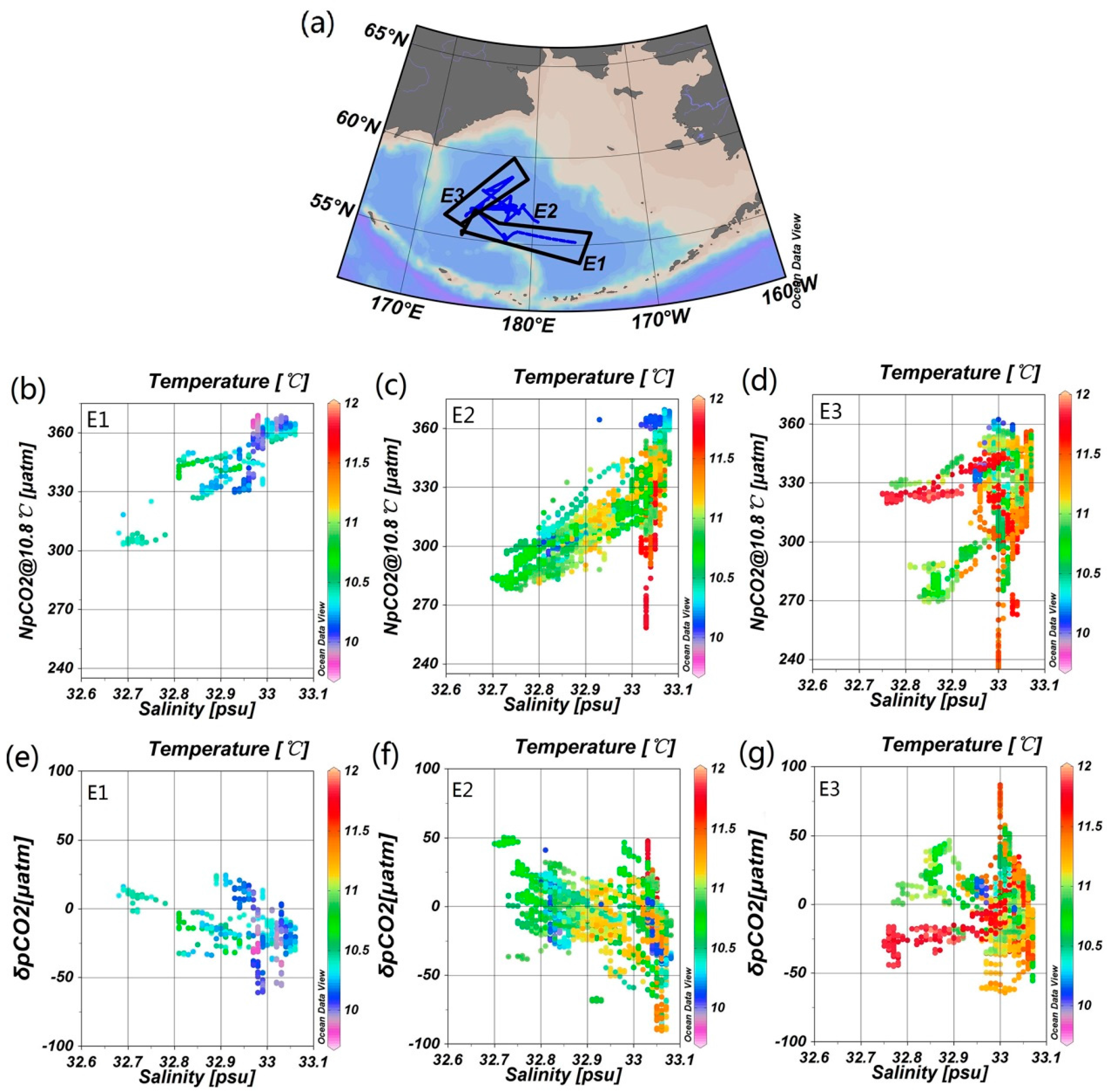
4.2. Influence of the Biological Effect on pCO2
4.3. The Influence of Mixing
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bates, N.R.; Mathis, J.T.; Jeffries, M.A. Air-sea CO2 fluxes on the Bering Sea shelf. Biogeosciences 2011, 8, 1237–1253. [Google Scholar] [CrossRef]
- Roden, G.I. Aleutian Basin of the Bering Sea: Thermohaline, oxygen, nutrient, and current structure in July 1993. J. Geophys. Res. Oceans 1995, 100, 13539–13554. [Google Scholar] [CrossRef]
- Springer, A.M.; Mcroy, C.P.; Flint, M.V. The Bering Sea green belt: Shelf-edge processes and ecosystem production. Fish. Oceanogr. 1996, 5, 205–223. [Google Scholar] [CrossRef]
- Chen, L.; Gao, Z. Spatial variability in the partial pressures of CO2 in the northern Bering and Chukchi seas. Deep Sea Res. Part II Top. Stud. Oceanogr. 2007, 54, 2619–2629. [Google Scholar] [CrossRef]
- Banse, K.; English, D.C. Comparing phytoplankton seasonality in the eastern and western subarctic Pacific and the western Bering Sea. Prog. Oceanogr. 1999, 43, 235–288. [Google Scholar] [CrossRef]
- Wadley, M.R.; Bigg, G.R. Impact of flow through the Canadian Archipelago and Bering Strait on the North Atlantic and Arctic circulation: An ocean modelling study. Q. J. R. Meteorol. Soc. 2002, 128, 2187–2203. [Google Scholar] [CrossRef]
- Chen, C.A.; Andreev, A.; Kim, K.; Yamamoto, M. Roles of continental shelves and marginal seas in the biogeochemical cycles of the North Pacific Ocean. J. Oceanogr. 2004, 60, 17–44. [Google Scholar] [CrossRef]
- Roach, A.T.; Aagaard, K.; Pease, C.H.; Salo, S.A.; Weingartner, T.; Pavlov, V.; Kulakov, M. Direct measurements of transport and water properties through the Bering Strait. J. Geophys. Res. Oceans 1995, 100, 18443–18457. [Google Scholar] [CrossRef]
- Bates, N.R.; Best, M.H.; Hansell, D.A. Spatio-temporal distribution of dissolved inorganic carbon and net community production in the Chukchi and Beaufort Seas. Deep Sea Res. Part II Top. Stud. Oceanogr. 2005, 52, 3303–3323. [Google Scholar] [CrossRef]
- Bates, N.R.; Mathis, J.T. The Arctic Ocean marine carbon cycle: Evaluation of air-sea CO2 exchanges, ocean acidification impacts and potential feedbacks. Biogeosciences 2009, 6, 2433–2459. [Google Scholar] [CrossRef]
- Fransson, A.; Chierici, M.; Nojiri, Y. New insights into the spatial variability of the surface water carbon dioxide in varying sea ice conditions in the Arctic Ocean. Cont. Shelf Res. 2009, 29, 1317–1328. [Google Scholar] [CrossRef]
- Cai, W.; Chen, L.; Chen, B.; Gao, Z.; Sang, H.L.; Chen, J.; Pierrot, D.; Sullivan, K.; Wang, Y.; Hu, X. Decrease in the CO2 uptake capacity in an ice-free Arctic Ocean basin. Science 2010, 329, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.A. Carbonate chemistry of the wintertime Bering Sea marginal ice zone. Cont. Shelf Res. 1993, 13, 67–87. [Google Scholar] [CrossRef]
- Walsh, J.J.; Dieterle, D.A.; Muller-Karger, F.E.; Aagaard, K.; Roach, A.T.; Whitledge, T.E.; Stockwell, D. CO2 cycling in the coastal ocean. II. Seasonal organic loading of the Arctic Ocean from source waters in the Bering Sea. Cont. Shelf Res. 1997, 17, 1–36. [Google Scholar] [CrossRef]
- Murphy, P.P.; Nojiri, Y.; Harrison, D.E.; Larkin, N.K. Scales of spatial variability for surface ocean pCO2 in the Gulf of Alaska and Bering Sea: Toward a sampling strategy. Geophys. Res. Lett. 2001, 28, 1047–1050. [Google Scholar] [CrossRef]
- Mathis, J.T.; Cross, J.N.; Bates, N.R.; Moran, S.B.; Lomas, M.W.; Stabeno, P.J. Seasonal distribution of dissolved inorganic carbon and net community production on the Bering Sea shelf. Biogeosciences 2010, 7, 1769–1787. [Google Scholar] [CrossRef]
- Mathis, J.T.; Cross, J.N.; Bates, N.R. Coupling primary production and terrestrial runoff to ocean acidification and carbonate mineral suppression in the eastern Bering Sea. J. Geophys. Res. Oceans 2011, 116. [Google Scholar] [CrossRef]
- Mathis, J.T.; Cross, J.N.; Bates, N.R. The role of ocean acidification in systemic carbonate mineral suppression in the Bering Sea. Geophys. Res. Lett. 2011, 38. [Google Scholar] [CrossRef]
- Williams, B.; Halfar, J.; Steneck, R.S.; Wortmann, U.G.; Hetzinger, S.; Adey, W.; Lebednik, P.; Joachimski, M. Twentieth century δ13 C variability in surface water dissolved inorganic carbon recorded by coralline algae in the northern North Pacific Ocean and the Bering Sea. Biogeosciences 2011, 8, 165–174. [Google Scholar] [CrossRef] [Green Version]
- Cross, J.N.; Mathis, J.T.; Bates, N.R. Hydrographic controls on net community production and total organic carbon distributions in the eastern Bering Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2012, 65, 98–109. [Google Scholar] [CrossRef]
- Takahashi, T.; Sutherland, S.C.; Wanninkhof, R.; Sweeney, C.; Feely, R.A.; Chipman, D.W.; Hales, B.; Friederich, G.; Chavez, F.; Sabine, C. Climatological mean and decadal change in surface ocean pCO2, and net sea–air CO2 flux over the global oceans. Deep Sea Res. Part II Top. Stud. Oceanogr. 2009, 56, 554–577. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Gao, Z.; Sun, H.; Chen, B.; Cai, W.J. Distributions and air-sea fluxes of CO2 in the summer Bering Sea. Acta Oceanol. Sin. 2014, 33, 1–8. [Google Scholar] [CrossRef]
- Cross, J.N.; Mathis, J.T.; Frey, K.E.; Cosca, C.E.; Danielson, S.L.; Bates, N.R.; Feely, R.A.; Takahashi, T.; Evans, W. Annual sea-air CO2 fluxes in the Bering Sea: Insights from new autumn and winter observations of a seasonally ice-covered continental shelf. J. Geophys. Res. Oceans 2014, 119, 6693–6708. [Google Scholar] [CrossRef]
- Fransson, A.; Chierici, M.; Nojiri, Y. Increased net CO2 outgassing in the upwelling region of the southern Bering Sea in a period of variable marine climate between 1995 and 2001. J. Geophys. Res. Oceans 2006, 111. [Google Scholar] [CrossRef]
- Olsen, A.; Bellerby, R.G.; Johannessen, T.; Omar, A.M.; Skjelvan, I. Interannual variability in the wintertime air-sea flux of carbon dioxide in the northern North Atlantic, 1981–2001. Deep Sea Res. Part I Oceanogr. Res. Pap. 2003, 50, 1323–1338. [Google Scholar] [CrossRef]
- Ono, T.; Saino, T.; Kurita, N.; Sasaki, K. Basin-scale extrapolation of shipboard pCO2 data by using satellite SST and Chla. Int. J. Remote Sens. 2004, 25, 3803–3815. [Google Scholar] [CrossRef]
- Lüger, H.; Wanninkhof, R.; Olsen, A.; Triñanes, J.; Johannessen, T.; Wallace, D.W.R.; Körtzinger, A. The Sea-Air CO2 Flux in the North Atlantic Estimated from Satellite and Argo Profiling Data; Atlantic Oceanographic and Meteorological Laboratory: Miami, FL, USA, 2008. [Google Scholar]
- Hales, B.; Strutton, P.G.; Saraceno, M.; Letelier, R.; Takahashi, T.; Feely, R.; Sabine, C.; Chavez, F. Satellite-based prediction of pCO2 in coastal waters of the eastern North Pacific. Prog. Oceanogr. 2012, 103, 1–15. [Google Scholar] [CrossRef]
- Sarma, V.; Saino, T.; Sasaoka, K.; Nojiri, Y.; Ono, T.; Ishii, M.; Inoue, H.Y.; Matsumoto, K. Basin-scale pCO2 distribution using satellite sea surface temperature, Chla, and climatological salinity in the North Pacific in spring and summer. Glob. Biogeochem. Cycles 2006, 20. [Google Scholar] [CrossRef]
- Else, B.G.; Yackel, J.J.; Papakyriakou, T.N. Application of satellite remote sensing techniques for estimating air-sea CO2 fluxes in Hudson Bay, Canada during the ice-free season. Remote Sens. Environ. 2008, 112, 3550–3562. [Google Scholar] [CrossRef]
- Parard, G.; Charantonis, A.A.; Rutgerson, A. Remote sensing the sea surface CO2 of the Baltic Sea using the SOMLO methodology. Biogeosciences 2015, 12, 3369–3384. [Google Scholar] [CrossRef]
- Parard, G.; Charantonis, A.A.; Rutgersson, A. Using satellite data to estimate partial pressure of CO2 in the Baltic Sea. J. Geophys. Res. Biogeosci. 2016, 121, 1002–1015. [Google Scholar] [CrossRef]
- Bai, Y.; Cai, W.J.; He, X.; Zhai, W.; Pan, D.; Dai, M.; Yu, P. A mechanistic semi-analytical method for remotely sensing sea surface pCO2 in river-dominated coastal oceans: A case study from the East China Sea. J. Geophys. Res. Oceans 2015, 120, 2331–2349. [Google Scholar] [CrossRef]
- Schumacher, J.D.; Reed, R.K. Characteristics of currents over the continental slope of the eastern Bering Sea. J. Geophys. Res. Oceans 1992, 97, 9423–9433. [Google Scholar] [CrossRef]
- Stabeno, P.J.; Reed, R.K. Circulation in the Bering Sea basin observed by satellite-tracked drifters: 1986–1993. J. Phys. Oceanogr. 1994, 24, 848–854. [Google Scholar] [CrossRef]
- Khen, G.V. Oceanographic conditions and Bering Sea biological productivity. Available online: nsgl.gso.uri.edu/aku/akuw88002/akuw88002chap4.pdf (accessed on 29 June 2016).
- Stabeno, P.J.; Schumacher, J.D.; Ohtani, K. The physical oceanography of the Bering Sea. Dyn. Bering Sea 1999, 1999, 1–28. [Google Scholar]
- Denman, K.L.; Gargett, A.E. Multiple thermoclines are barriers to vertical exchange in the subarctic Pacific during SUPER, May 1984. J. Mar. Res. 1988, 46, 77–103. [Google Scholar] [CrossRef]
- Luchin, V.A.; Menovshchikov, V.A.; Lavrentiev, V.M.; Reed, R.K. Thermohaline structure and water masses in the Bering Sea. Dyn. Bering Sea 1999, 61–91. [Google Scholar]
- Stabeno, P.J.; Hunt, G.L. Overview of the inner front and southeast Bering Sea carrying capacity programs. Deep Sea Res. Part II Top. Stud. Oceanogr. 2002, 49, 6157–6168. [Google Scholar] [CrossRef]
- Mysak, L.A.; Manak, D.K. Arctic sea-ice extent and anomalies, 1953–1984. Atmos.-Ocean 1989, 27, 376–405. [Google Scholar] [CrossRef]
- Niebauer, H.J.; Bond, N.A.; Yakunin, L.P.; Plotnikov, V.V. An update on the climatology and sea ice of the Bering Sea. Dyn. Bering Sea 1999, 1999, 29–60. [Google Scholar]
- Wyllie-Echeverria, T.; Ohtani, K. Seasonal sea ice variability and the Bering Sea ecosystem. Dyn. Bering Sea 1999, 1999, 435–451. [Google Scholar]
- Stabeno, P.J.; Bond, N.A.; Salo, S.A. On the recent warming of the southeastern Bering Sea shelf. Deep Sea Res. Part II Top. Stud. Oceanogr. 2007, 54, 2599–2618. [Google Scholar] [CrossRef]
- Hoppema, M.; Goeyens, L. Redfield behavior of carbon, nitrogen, and phosphorus depletions in Antarctic surface water. Limnol. Oceanogr. 1999, 44, 220–224. [Google Scholar] [CrossRef]
- Miura, T.; Suga, T.; Hanawa, K. Winter mixed layer and formation of dichothermal water in the Bering Sea. J. Oceanogr. 2002, 58, 815–823. [Google Scholar] [CrossRef]
- Carbon Dioxide Information Analysis Center (CDIAC). Available online: http://cdiac3.ornl.gov/waves/underway/ (accessed on 29 June 2016).
- Carbon Dioxide Information Analysis Center (CDIAC). Available online: http://cdiac.ornl.gov/oceans/PACIFICA (accessed on 29 June 2016).
- NASA Ocean Color Website. Available online: http://oceancolor.gsfc.nasa.gov/ (accessed on 29 June 2016).
- Wanninkhof, R. Relationship between wind speed and gas exchange over the ocean. J. Geophys. Res. Oceans 1992, 97, 7373–7382. [Google Scholar] [CrossRef]
- Weiss, R.F. Carbon dioxide in water and seawater: The solubility of a non-ideal gas. Mar. Chem. 1974, 2, 203–215. [Google Scholar] [CrossRef]
- Pierrot, D.; Lewis, E.; Wallace, D. MS Excel Program Developed for CO2 System Calculations; ORNL/CDIAC-105a; Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy: Oak Ridge, TN, USA, 2006. [Google Scholar]
- Van Heuven, S.; Pierrot, D.; Rae, J.; Lewis, E.; Wallace, D. MATLAB Program Developed for CO2 System Calculations; ORNL/CDIAC-105b; Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy: Oak Ridge, TN, USA, 2011. [Google Scholar]
- Broecker, W.S.; Peng, T.H. Gas exchange rates between air and sea. Tellus 1974, 26, 21–35. [Google Scholar] [CrossRef]
- Zeebe, R.E.; Wolf-Gladrow, D.A. CO2 in Seawater: Equilibrium, Kinetics, Isotopes; Gulf Professional Publishing: Houston, TX, USA, 2001. [Google Scholar]
- Jones, D.C.; Ito, T.; Takano, Y.; Hsu, W.C. Spatial and seasonal variability of the air-sea equilibration timescale of carbon dioxide. Glob. Biogeochem. Cycles 2014, 28, 1163–1178. [Google Scholar] [CrossRef]
- Chen, M. Chemical Oceanography; Maritime Press: Beijing, China, 2009; pp. 135–136. [Google Scholar]
- Revelle, R.; Suess, H.E. Carbon dioxide exchange between atmosphere and ocean and the question of an increase of atmospheric CO2 during the past decades. Tellus 1957, 9, 18–27. [Google Scholar] [CrossRef]
- Takahashi, T.; Olafsson, J.; Goddard, J.G.; Chipman, D.W. Seasonal variation of CO2 and nutrients in the high-latitude surface oceans: A comparative study. Glob. Biogeochem. Cycles 1993, 7, 843–878. [Google Scholar] [CrossRef]
- NOAA/ESRL Physical Sciences Division. Available online: http://www.esrl.noaa.gov/psd/data/reanalysis/reanalysis.shtml (accessed on 29 June 2016).
- NOAA/ESRL Global Monitoring Division Carbontracker Project. Available online: http://www.esrl.noaa.gov/gmd/ccgg/carbontracker/ (accessed on 29 June 2016).
- Mixed Layer Depth (MLD) Climatology. Available online: http://www.Ifremer.fr/cerweb/deboyer/mld (accessed on 29 June 2016).
- Remote Sensing Systems. Available online: http://www.remss.com/missions/windsat (accessed on 29 June 2016).
- Rangama, Y.; Boutin, J.; Etcheto, J.; Merlivat, L.; Takahashi, T.; Delille, B.; Frankignoulle, M.; Bakker, D.C.E. Variability of the net air-sea CO2 flux inferred from shipboard and satellite measurements in the Southern Ocean south of Tasmania and New Zealand. J. Geophys. Res. Oceans 2005, 110. [Google Scholar] [CrossRef]
- Takahashi, T.; Sutherland, S.C.; Sweeney, C.; Poisson, A.; Metzl, N.; Tilbrook, B.; Bates, N.; Wanninkhof, R.; Feely, R.A.; Sabine, C. Global sea-air CO2 flux based on climatological surface ocean pCO2, and seasonal biological and temperature effects. Deep Sea Res. Part II Top. Stud. Oceanogr. 2002, 49, 1601–1622. [Google Scholar] [CrossRef]
- Antoine, D.; Siegel, D.A.; Kostadinov, T.; Maritorena, S.; Nelson, N.B.; Gentili, B.; Vellucci, V.; Guillocheau, N. Variability in optical particle backscattering in contrasting bio-optical oceanic regimes. Limnol. Oceanogr. 2011, 56, 955–973. [Google Scholar] [CrossRef]
- Darecki, M.; Stramski, D. An evaluation of MODIS and SeaWiFS bio-optical algorithms in the Baltic Sea. Remote Sens. Environ. 2004, 89, 326–350. [Google Scholar] [CrossRef]
- Favorite, F. Flow into the Bering Sea through Aleutian island passes. Occa. Pub. 1974, 2, 59–98. [Google Scholar]
- Onishi, H.; Ohtani, K. On seasonal and year to year variation in flow of the Alaskan Stream in the central North Pacific. J. Oceanogr. 1999, 55, 597–608. [Google Scholar] [CrossRef]
- Reed, R.K.; Schumacher, J.D.; Blaha, J.P. Eulerian measurements in the Alaskan stream near Kodiak Island. J. Phys. Oceanogr. 1981, 11, 1591–1595. [Google Scholar] [CrossRef]
- Chen, C.A. Preliminary observations of oxygen and carbon dioxide of the wintertime Bering Sea marginal ice zone. Cont. Shelf Res. 1985, 4, 465–483. [Google Scholar] [CrossRef]
- Guoping, G.; Maochong, S.; Jinping, Z. Hydrologic features of the Bering Sea in the summer of 1999. Acta Oceanol. Sin. 2002, 24, 8–16. (In Chinese) [Google Scholar]
- Redfield, A.C. The influence of organisms on the composition of sea-water. Sea 1963, 1963, 26–77. [Google Scholar]
- Martiny, A.C.; Pham, C.T.; Primeau, F.W.; Vrugt, J.A.; Moore, J.K.; Levin, S.A.; Lomas, M.W. Strong latitudinal patterns in the elemental ratios of marine plankton and organic matter. Nat. Geosci. 2013, 6, 279–283. [Google Scholar] [CrossRef]
- Anderson, L.A.; Sarmiento, J.L. Redfield ratios of remineralization determined by nutrient data analysis. Glob. Biogeochem. Cycles 1994, 8, 65–80. [Google Scholar] [CrossRef]
- Deutsch, C.; Weber, T. Nutrient ratios as a tracer and driver of ocean biogeochemistry. Annu. Rev. Mar. Sci. 2012, 4, 113–141. [Google Scholar] [CrossRef] [PubMed]
- Geider, R.; La Roche, J. Redfield revisited: Variability of C:N:P in marine microalgae and its biochemical basis. Eur. J. Phycol. 2002, 37, 1–17. [Google Scholar] [CrossRef]
- He, X.Q.; Bai, Y.; Pan, D.L.; Chen, C.T.A. Satellite views of seasonal and inter-annual variability of phytoplankton blooms in the eastern China seas over the past 14 years (1998–2011). Biogeosciences 2013, 10, 4721–4739. [Google Scholar] [CrossRef]
- Bricaud, A.; Babin, M.; Morel, A.; Claustre, H. Variability in the chlorophyll-specific absorption coefficients of natural phytoplankton: Analysis and parameterization. J. Geophys. Res. Oceans 1995, 100, 13–16. [Google Scholar] [CrossRef]
- Okkonen, S.R. Altimeter observations of the Bering Slope Current eddy field. J. Geophys. Res. Oceans 2001, 106, 2465–2476. [Google Scholar] [CrossRef]
- Johnson, G.C.; Stabeno, P.J.; Riser, S.C. The Bering Slope Current System Revisited. J. Phys. Oceanogr. 2004, 34, 384–398. [Google Scholar] [CrossRef]
- Kinney, J.C.; Maslowski, W.; Okkonen, S. On the processes controlling shelf-basin exchange and outer shelf dynamics in the Bering Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2009, 56, 1351–1362. [Google Scholar] [CrossRef]
- Kinder, T.H.; Coachman, L.K.; Galt, J.A. The Bering slope current system. J. Phys. Oceanogr. 1975, 5, 231–244. [Google Scholar] [CrossRef]
- Miller, A.J.; Schneider, N. Interdecadal climate regime dynamics in the North Pacific Ocean: Theories, observations and ecosystem impacts. Prog. Oceanogr. 2000, 47, 355–379. [Google Scholar] [CrossRef]
- Napp, J.M.; Hunt, G.L. Anomalous conditions in the south-eastern Bering Sea 1997: Linkages among climate, weather, ocean, and Biology. Fish. Oceanogr. 2001, 10, 61–68. [Google Scholar] [CrossRef]
- Stabeno, P.J.; Bond, N.A.; Kachel, N.B.; Salo, S.A.; Schumacher, J.D. On the temporal variability of the physical environment over the south-eastern Bering Sea. Fish. Oceanogr. 2001, 10, 81–98. [Google Scholar] [CrossRef]
- Stockwell, D.A.; Whitledge, T.E.; Zeeman, S.I.; Coyle, K.O.; Napp, J.M.; Brodeur, R.D.; Pinchuk, A.I.; Hunt, G.L. Anomalous conditions in the south-eastern Bering Sea, 1997: Nutrients, phytoplankton and zooplankton. Fish. Oceanogr. 2001, 10, 99–116. [Google Scholar] [CrossRef]
- Murata, A.; Takizawa, T. Impact of a coccolithophorid bloom on the CO2 system in surface waters of the eastern Bering Sea shelf. Geophys. Res. Lett. 2002, 29. [Google Scholar] [CrossRef]
- Murata, A. Increased surface seawater pCO2 in the eastern Bering Sea shelf: An effect of blooms of coccolithophorid Emiliania huxleyi? Glob. Biogeochem. Cycles 2006, 20. [Google Scholar] [CrossRef]
- Takahashi, K. Seasonal fluxes of pelagic diatoms in the subarctic Pacific, 1982–1983. Deep Sea Res. Part A Oceanogr. Res. Pap. 1986, 33, 1225–1251. [Google Scholar] [CrossRef]
- Hunt, G.L., Jr.; Stabeno, P.; Walters, G.; Sinclair, E.; Brodeur, R.D.; Napp, J.M.; Bond, N.A. Climate change and control of the southeastern Bering Sea pelagic ecosystem. Deep Sea Res. Part II Top. Stud. Oceanogr. 2002, 49, 5821–5853. [Google Scholar] [CrossRef]
- Morel, A.; Gentili, B. A simple band ratio technique to quantify the colored dissolved and detrital organic material from ocean color remotely sensed data. Remote Sens. Environ. 2009, 113, 998–1011. [Google Scholar] [CrossRef]
- Bai, Y.; Pan, D.; Cai, W.-J.; He, X.; Wang, D.; Tao, B.; Zhu, Q. Remote sensing of salinity from satellite-derived CDOM in the Changjiang River dominated East China Sea. J. Geophys. Res. Oceans 2013, 118, 227–243. [Google Scholar] [CrossRef]
- Bai, Y.; He, X.; Pan, D.; Chen, C.-T.-A.; Kang, Y.; Chen, X.; Cai, W.-J. Summertime Changjiang River plume variation during 1998–2010. J. Geophys. Res. Oceans 2014, 119, 6238–6257. [Google Scholar] [CrossRef]
- Dai, M.H.; Cao, Z.M.; Guo, X.H.; Zhai, W.D.; Liu, Z.Y.; Yin, Z.Q.; Xu, Y.P.; Gan, J.P.; Hu, J.Y.; Du, C.J. Why are some marginal seas sources of atmospheric CO2? Geophys. Res. Lett. 2013, 40, 2154–2158. [Google Scholar] [CrossRef]
- He, X.; Bai, Y.; Pan, D.; Huang, N.; Dong, Xu.; Chen, J.; Chen, C.-T.-A.; Cui, Q. Using geostationary satellite ocean color data to map the diurnal dynamics of suspended particulate matter in coastal waters. Remote Sens. Environ. 2013, 133, 225–239. [Google Scholar] [CrossRef]




| Cruise | Ship/Experiment | Parameters | Period of Record | Observer |
|---|---|---|---|---|
| M/S Alligator Hope Lines 1999–2001 | M/S Alligator Hope | pCO2, Sal., temp. | January 2000 to December 2000 January, February, April, May 2001 | Y. Nojiri NIES Japan |
| VOS Lines 2001–2006 | M/V Pyxis | pCO2, Sal., temp. | July, August, November 2002 January, March 2003 May, July to October 2009 January, February, April 2013 | Y. Nojiri NIES Japan |
| Transit | M/V Turmoil | pCO2, Sal., temp. | July 2010 | / |
| Aleutian Is. | R/V M.G. Langseth 11/3 | pCO2, Sal., temp. | July 2011, August 2011 * September to October 2011 | LDEO Tech |
| XUE_CHINARE2010_Arctic xue_arctic08 | R/V Xue Long | pCO2, Sal., temp. | July 2010 *, August 2010 September 2010 * July to September 2008 | AMOL R. Wanninkhof Group |
| NA | NOAA Miller Freeman | pCO2, Sal., temp. | September, October 2009 September, October 2010 | PMEL Feely Group |
| FOCI2000 FOCI2001a FOCI2001b | R/V Ron Brown 2000 | pCO2, Sal., temp. | September 2000 May, June 2001 | AMOL R. Wanninkhof Group |
| NA | R/V Thomas Thompson | pCO2, Sal., temp. | May 2010 | / |
| NA | R/V N.B Palmer | pCO2, Sal., temp. | July, August 2003 | / |
| Arctic Research | USCGC Healy | pCO2, Sal., temp. | June to December 2011 | USCG Tech |
| NA | USCGC Polar Sea | pCO2, Sal., temp. | October 2013 | / |
| MR04-04 | Mirai | DIC, TA, AOU, sal., temp. | August 2004 | M. Wakita S. Watanabe |
| Title | Location | Temperature (°C) | Salinity (psu) | pCO2 | DIC μmol/kg | TA μmol/kg | AOU μmol/kg |
|---|---|---|---|---|---|---|---|
| Reference water a | 55.7–57°N 171.4–174.2°W | 7.70 (0.11) | 32.98 (0.02) | 381.80 (5.08) | / | / | / |
| Station 38 b | 57°N 172.5°E | 2.42 °C | 33.25 | / | 2154.40 | 2239.70 | 35.98 |
| Calculated winter data c | / | 2.42 °C | 33.25 | 415.10 | 2126.76 | 2239.70 | / |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Bai, Y.; Cai, W.-J.; Chen, C.-T.A.; Pan, D.; He, X.; Zhu, Q. Remote Sensing of Sea Surface pCO2 in the Bering Sea in Summer Based on a Mechanistic Semi-Analytical Algorithm (MeSAA). Remote Sens. 2016, 8, 558. https://doi.org/10.3390/rs8070558
Song X, Bai Y, Cai W-J, Chen C-TA, Pan D, He X, Zhu Q. Remote Sensing of Sea Surface pCO2 in the Bering Sea in Summer Based on a Mechanistic Semi-Analytical Algorithm (MeSAA). Remote Sensing. 2016; 8(7):558. https://doi.org/10.3390/rs8070558
Chicago/Turabian StyleSong, Xuelian, Yan Bai, Wei-Jun Cai, Chen-Tung Arthur Chen, Delu Pan, Xianqiang He, and Qiankun Zhu. 2016. "Remote Sensing of Sea Surface pCO2 in the Bering Sea in Summer Based on a Mechanistic Semi-Analytical Algorithm (MeSAA)" Remote Sensing 8, no. 7: 558. https://doi.org/10.3390/rs8070558






