1. Introduction
Red leaf blotch of almond (
Prunus amigdalus) is a fungal foliar disease widely extended throughout almond production regions of Europe and Asia and it is considered of high economic importance [
1,
2,
3]. Nowadays, the spread of almond red leaf blotch is increasing in importance in Spain [
4] due to the intensification of production in recently-planted almond orchards,
i.e., incorporation of new varieties, irrigation, higher tree density, light pruning, and occupation of sites with temperate climate that could be advantageous for the disease development [
5]. There are noticeable cultivar differences in genetic resistance to the disease, this being a crucial factor determining its potential impact on almond production. Some varieties such as Guara and Tarraco are considered very susceptible, while Vayro and Constantí have shown high resistance [
5].
Red leaf blotch disease is caused by the fungus
Polystigma amygdalinum PF Cannon (Phyllachoraceae), formerly known as
Polystigma ochraceum [
2], which is mainly present in the Mediterranean Basin [
2]. As the other species of the same genus, in their teleomorph phase, it forms brightly colored stromata on living leaves and overwinters as a saprophyte on fallen ones. It is on these fallen leaves where the fungus forms reproductive structures that will release ascospores the following spring, the maximum discharge occurring at petal fall [
3,
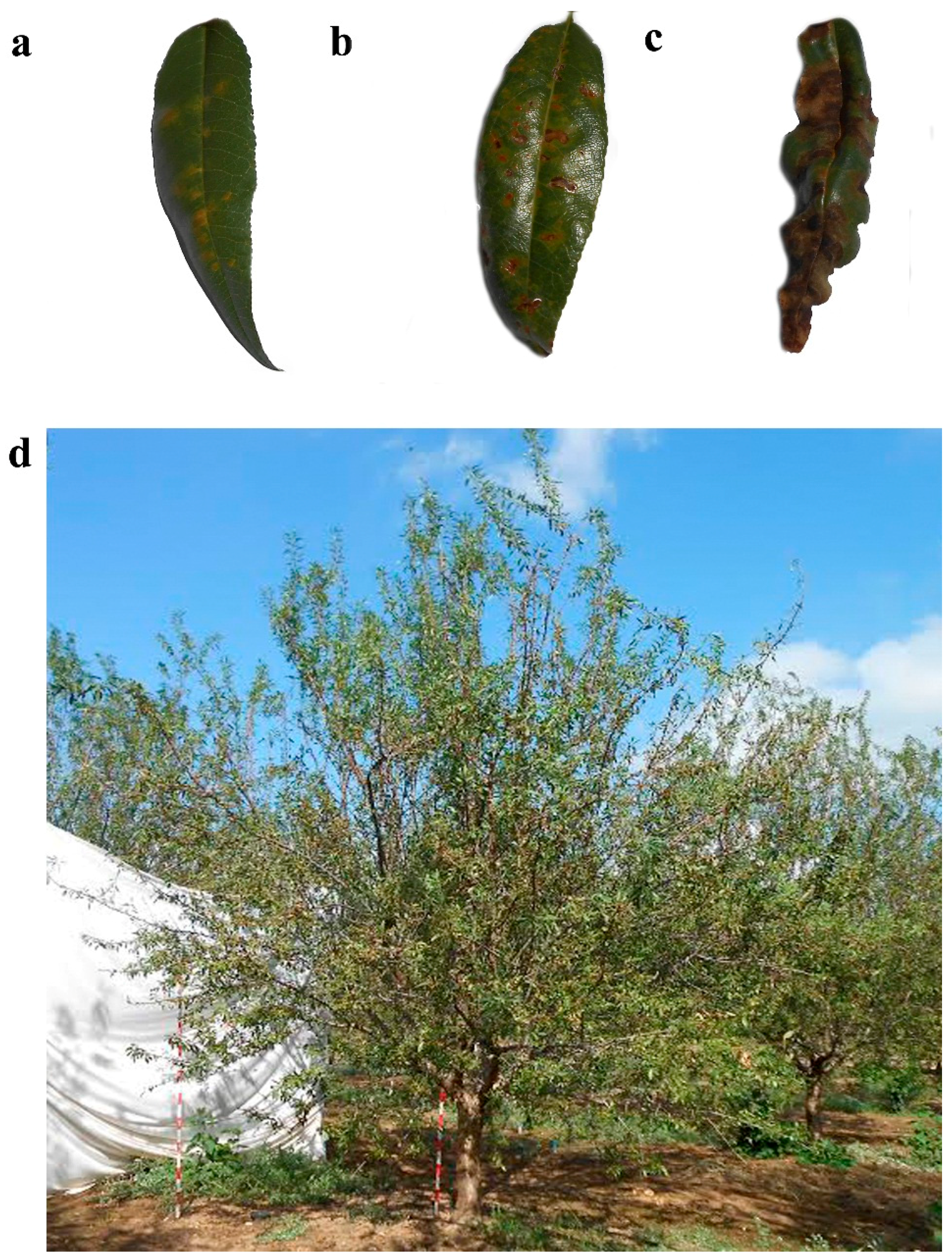
6]. Such released spores begin the infection of young leaves; thus, the removal of fallen leaves is recommended as a cultural means of disease control. Symptoms start as pale green spots on both sides of the leaves, and turn into yellowish-orange afterwards (
Figure 1a). Spots grow in size throughout the spring and end up covering almost the whole leaf surface in late summer (
Figure 1b). Conidia formed in summer are not known to have a relevant impact on the epidemic [
7]. At advanced stages of disease development, leaves curl and become necrotic (
Figure 1c). Red leaf blotch can lead to a premature defoliation (
Figure 1d) [
2,
3], hence causing a decrease in photosynthetic capacity and, thus, possibly affecting yield [
3,
4]. Although triforine, a systemic active ingredient, has been proven to be effective against
P. amygdalinum [
6], until now disease control is mainly based on the application of preventive fungicides at petal fall, vegetative bud break and leaf sprout stages [
4]. Remote sensing could be used to map fields and identify areas with little or no red leaf blotch incidence and, therefore, limit the fungicide applications under precision agriculture strategies. Furthermore, research on new, effective systemic fungicides for disease control could take advantage of early detection via remote sensing by restricting the use of pesticides to areas of the fields where it would be required.
Remote sensing methods have been used to detect the effects of foliar diseases on plant responses, such as changes in leaf color due to pigment degradation, reduction in green canopy biomass, and an increase of canopy temperature because of lower transpiration rates [
8]. Indeed, remote sensing has already been used to detect, monitor, and quantify a variety of diseases in different crops, and several reviews are available [
8,
9,
10,
11]. The majority of these studies are focused on foliar pathogens in annual crops, such as powdery mildew, downy mildew, and leaf rust. These diseases show characteristic leaf color changes caused by a reduction of pigments that absorb light, thus increasing reflectance in the visible region. Moreover, plants infected by foliar pathogens reveal a decrease in green biomass detectable through a reduction in the near-infrared region. These disease effects associated with pigment degradation and structural changes can be detected by multispectral and hyperspectral imagery. Multispectral imagery enabled the detection of downy mildew in opium poppy [
12], and powdery mildew and leaf rust in wheat [
13], while hyperspectral technology was used at the leaf level for early detection [
14] and quantification of Cercospora leaf spot, leaf rust, and powdery mildew in sugar beet [
15], as well as to discriminate among yellow, leaf and stem rust in wheat [
16]. Although much less information exists on the use of multispectral imagery for detecting diseases in tree crops, a recent study realized early detection of laurel wilt in avocado trees using vegetation indices related to pigment content and crown structure [
17]. Hyperspectral airborne sensors have been successfully used to detect a root disease caused by
Verticillium dahliae in olive trees [
18,
19] and the citrus greening disease (Huanglongbing) [
20,
21,
22]. Other approaches have included the use of thermal imagery, which is particularly suitable to detect lower transpiration rates caused by root diseases [
18,
19].
To our knowledge, no previous studies using remote sensing for detection of red leaf blotch in almond have been published. The hypotheses of the current study were, on the one hand, that red leaf blotch symptoms were related to physiological changes at the canopy level that can be detected using high-resolution hyperspectral and thermal imagery, and, on the other hand, that the fungus affects only the symptom-bearing leaf areas while the green parts of infected leaves may continue to function normally. Therefore, the objectives of this study were (i) to early detect and quantify the incidence and severity of almond red leaf blotch using high-resolution hyperspectral and thermal imagery; and (ii) to assess the effects of P. amygdalinum infection on some physiological variables at the leaf level.
2. Materials and Methods
2.1. Study Site Description
The experimental area was located in Córdoba, in Southern Spain, a region of Mediterranean climate characterized by warm and dry summers and cool and wet winters, with an average annual rainfall exceeding 550 mm. The study was conducted in a 5.5-ha cv. Guara almond orchard at Alameda del Obispo Research Station (37°52’N, 4°49’W). The orchard was drip irrigated with two laterals per tree row and conventionally managed: no soil ploughing, weed control through herbicide applications and weeding machine passes and pest and disease control following a fungicide-and-insecticide application calendar. The orchard became naturally infected by P. amygdalinum. The fungicide applications based on thiram and captan were not enough for complete control of red leaf blotch in 2014. Consequently, the field showed a range of levels of disease incidence and severity.
Spring of 2014 was characterized by a precipitation of 93 mm, which was temporally-distributed along the spring period, and an average temperature of 17 °C, with temperatures from 10 °C in March to 25 °C in May [
23].
2.2. Leaf-Level Measurements
One infected tree was chosen for detailed leaf measurements in order to avoid tree-to-tree variations. Chlorophyll meter readings (SPAD units), chlorophyll fluorescence (Fs), photochemical reflectance index (PRI570), and stomatal conductance (gs) were measured on 24 asymptomatic, 29 ochre, and nine necrotic areas in affected leaves, and 20 locations within healthy leaves, located in the same stems as the affected leaves.
Chlorophyll content was measured with a chlorophyll meter (SPAD 502 Plus Chlorophyll Meter, Spectrum Technologies, Inc., Aurora, IL, USA), which was used preferentially because of the strong relationship between its digital readings and real leaf chlorophyll content, as demonstrated by several authors (e.g., [
24,
25]). Steady-state chlorophyll fluorescence and PRI
570 data were taken with instruments designed to measure chlorophyll fluorescence (FluorPen FP 100, Photon Systems Instruments, Drasov, Czech Republic), and R531 and R570 spectral bands with a bandwidth of 10 nm (PlantPen PRI 200, Photon Systems Instruments, Drasov, Czech Republic), respectively. For stomatal conductance measurements, a steady-state diffusion porometer (model SC-1, Decagon Devices, Washington, DC, USA) was employed. These devices have been successfully used for the detection of Verticillium wilt in olive plants at leaf level under controlled conditions [
26]. Measurements were taken on fully-expanded sunny leaves according to operational requirements of the devices, between 11:30 and 12:30 GMT (so that there were no noticeable variations in sun radiation intensity), on 18 July. Measured leaves were 1–1.5 meters high in the canopy.
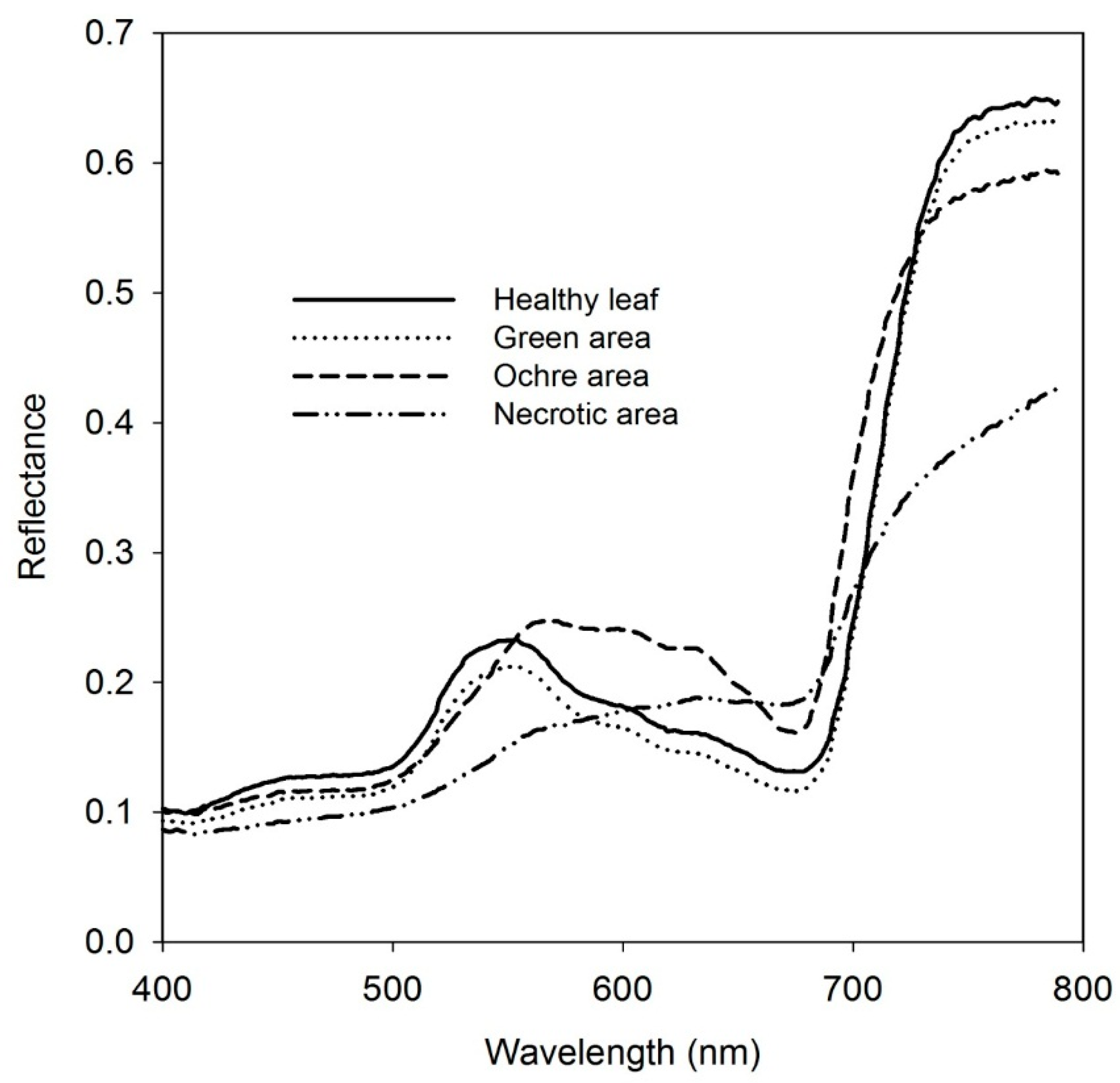
Spectral reflectance within the visible and near-infrared regions was measured on 11 asymptomatic, 11 ochre, and 10 necrotic areas in affected leaves, and on six locations within healthy leaves nearby with a device designed for measuring spectral reflectance on flat leaves (PolyPen RP400, Photon Systems Instruments, Drasov, Czech Republic), operating in the spectral mode of 256 bands in the 400–790 nm region with 8 nm FWHM. Average values of spectral reflectance for asymptomatic leaves and green, ochre, and necrotic areas of symptomatic leaves resulted in the spectrum shown in
Figure 2. Moreover, data coming from PolyPen measurements were used to calculate pigment (chlorophyll, carotenes, and xanthophyll) indices as well as visible R/G/B ratios and disease-related indices (
Table 1).
2.3. Disease Assessment at Tree Crown Level
Red leaf blotch incidence and severity were assessed by visually inspecting 52 almond trees for disease symptoms described above on 11 and 30 July 2014, the same days when the two flights took place. Disease severity was evaluated by visual observation of foliar symptoms in each individual tree and rated on a 0–3 rating scale according to the percentage of foliage with disease symptoms, where: 0 = 0% (asymptomatic tree), 1 = 1% to 10% (initially symptomatic), 2 = 11% to 50% (moderately symptomatic), and 3 = 51% to 100% (severely symptomatic). Four operators performed the evaluation over the 52 almond trees. The observations from the operators that recorded the highest and lowest overall evaluations were discarded, and as a result, the final score was obtained by averaging the observations from the two remaining operators.
The disease developed unevenly throughout the field, some trees showing very advanced stages while others were unaffected or with only initial symptoms at the end of summer. Generally, the first pale-green spots appeared in mid-March and turned ochre by the end of the month. Throughout the spring, ochre spots continued to appear, growing in size and merging with other spots. The symptoms were usually more visible in the inner and lower parts of the canopy. The first necrotic lesions appeared in early May. At the end of June, the youngest stems and leaves were the only asymptomatic parts of the most severely affected trees. Among the 52 trees evaluated on 11 July, there were 16 asymptomatic trees (30.8% of the total evaluated trees), 16 presenting red leaf blotch disease at its initial stage (30.8%), 14 with moderate symptoms (26.9%), and six were severely affected (11.5%). On 30 July, the tree ratings frequencies were of 14 (26.9%), 10 (19.2%), 17 (32.7%), and 11 (21.2%), respectively.
2.4. Airborne Imagery
Imagery was acquired from the study site on 11 and 30 July 2014 using a hyperspectral sensor and a thermal camera on board a Cessna aircraft operated by the Laboratory for Research Methods in Quantitative Remote Sensing of the Consejo Superior de Investigaciones Científicas (Quantalab, IAS-CSIC, Spain). Both cameras were flown at 550 m above ground level (AGL) at 10:00 GMT on both dates to minimize differences due to sun angle effects between airborne flights. Hyperspectral and thermal imagery were acquired at 30 cm and 40 cm pixel resolution, respectively.
The hyperspectral sensor used in this experiment was the visible and near-infrared micro-hyperspectral imager (Micro-Hyperspec VNIR model, Headwall Photonics, Fitchburg, MA, USA) operating in the spectral mode of 260 bands at 1.85 nm/pixel and 12-bit radiometric resolution, yielding 6.4 nm FWHM with a 25-micron slit in the 400–885 nm region. Frame storage rate on board the aircraft was set to 50 fps (frames per second) with 18 ms integration time. The 8-mm focal length lens yielded an IFOV of 0.93 mrad and an angular FOV of 49.82°. The hyperspectral sensor was radiometrically calibrated in the laboratory with an integrating sphere (CSTMUSS2000C Uniform Source System, LabSphere, North Sutton, NH, USA) using coefficients derived from a calibrated uniform light source at four illumination and six integration times. Atmospheric correction enabled the conversion of radiance values to reflectance using total incoming irradiance simulated with the SMARTS model [
27,
28] and the aerosol optical depth was measured at 550 nm with a Micro-Tops II sunphotometer (Solar LIGHT Co., Philadelphia, PA, USA) at the study site concurrently with the flight. Ortho-rectification of the hyperspectral imagery was performed with PARGE (ReSe Applications Schläpfer, Wil, Switzerland), using inputs from an inertial measuring unit (IMU) (MTiG model, Xsens, Enschede, The Netherlands) installed onboard and synchronized with the micro-hyperspectral imager.
The narrow-band indices described in
Table 1 were calculated from the mean radiance and reflectance spectra obtained from the 260 spectral bands acquired by the hyperspectral imager [
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57]. Many of these indices have been shown to be closely related to specific features of leaf physiology [
18,
19], thus, according to the effects of red leaf blotch on almond trees, namely, a decrease in green leaf biomass, pigment degradation, leaf color changes, and an expected increase of fluorescence emission by PS-I photosystem, we selected five types of indices related to: (i) structural effects; (ii) pigment concentration (chlorophyll, carotenoid and xanthophyll); (iii) blue/green/red ratios; (iv) chlorophyll fluorescence, and (v) spectral disease indices.
The fluorescence emission was assessed from the radiance spectra by the Fraunhofer Line Depth (FLD) principle calculated from a total of two bands for the in (L762 nm) and out band of the O
2-A feature (L750 nm) (FLD2), using the equation described by Zarco-Tejada
et al. [
54].
The thermal camera used in the two flights (FLIR SC655, FLIR Systems, Wilsonville, OR, USA) has a 640 × 480 pixel resolution and was equipped with a 24.5 mm f1.0 lens, connected to a computer via USB 2.0 protocol. This camera has a spectral response in the range of 7.5–13 µm and operates with a thermoelectric cooling stabilization, giving us a high sensitivity below 50 mK. Radiometric calibration was conducted in the laboratory using a black body (model P80P, Land Instruments, Dronfield, UK) at varying target and ambient temperatures and through vicarious calibrations using surface temperature measurements [
19]. Canopy temperature (Tc), extracted from thermal imagery, minus air temperature (Ta) was calculated as a water stress indicator of almond trees (Tc-Ta).
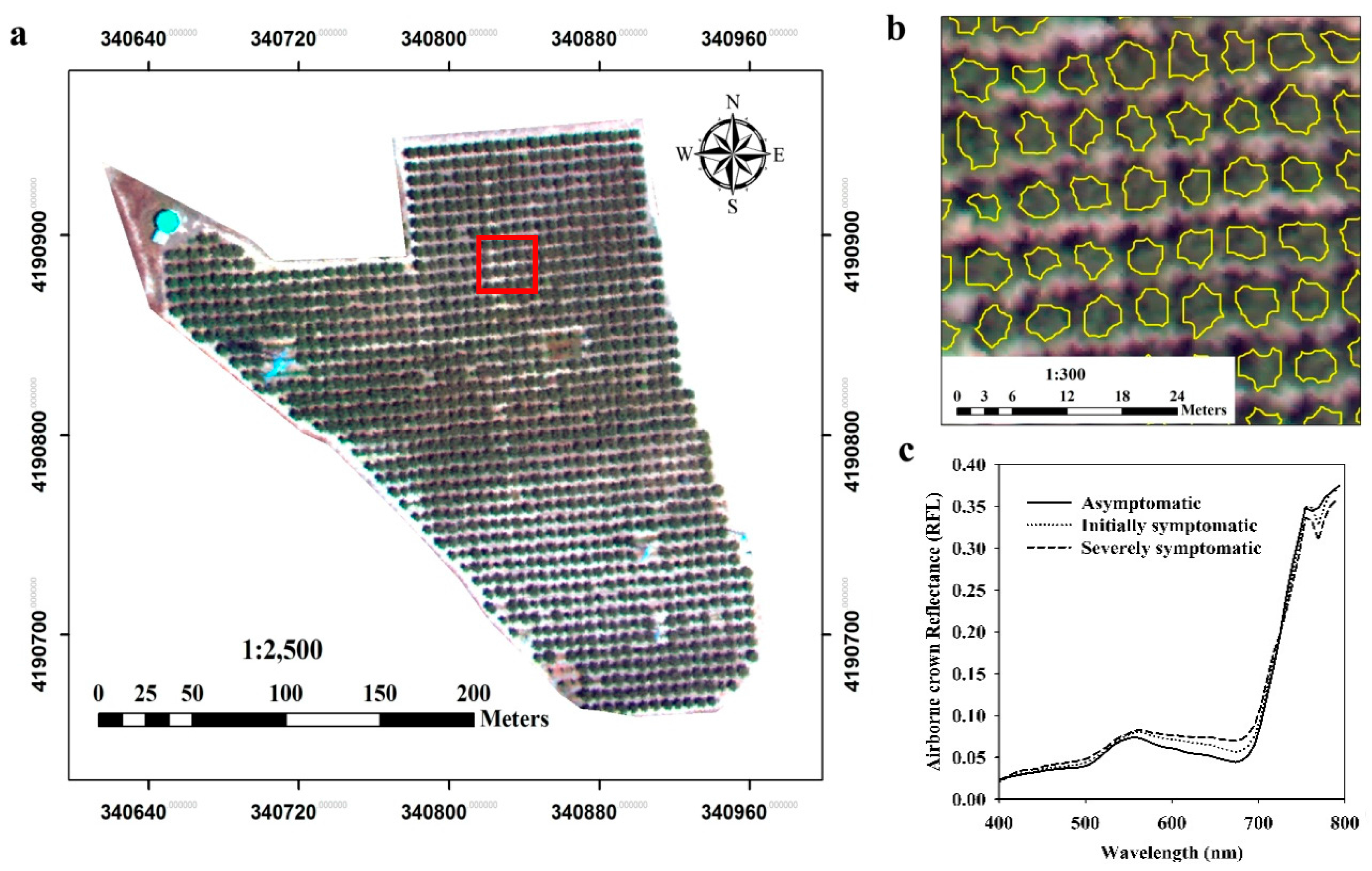
The high-resolution hyperspectral and thermal imagery (
Figure 3a and
Figure 4a) acquired over the orchard allowed single tree identification using automatic object-based crown detection algorithms (
Figure 3b and
Figure 4b), which calculated mean temperature and hyperspectral reflectance at the pure crown-level (
Figure 3c). An image segmentation procedure was used as described Calderón
et al. [
19].
2.5. Data Analyses
Leaf-level data were subjected to a completely randomized standard analysis of the variance (ANOVA) followed by a HSD Tukey test to examine significant differences at p < 0.05 for each measured parameter among the four classes of leaf areas (asymptomatic, ochre, and necrotic areas of symptomatic leaves, and asymptomatic leaves nearby). Leaf-level measured parameters with either a non-normal data distribution or with not homogenous variances underwent a Kruskal–Wallis analysis of variance (Kruskal–Wallis AOV) instead of standard ANOVA. The software Statistix 9.0 (Analytical Software, Tallahassee, FL, USA) was used to conduct these analyses.
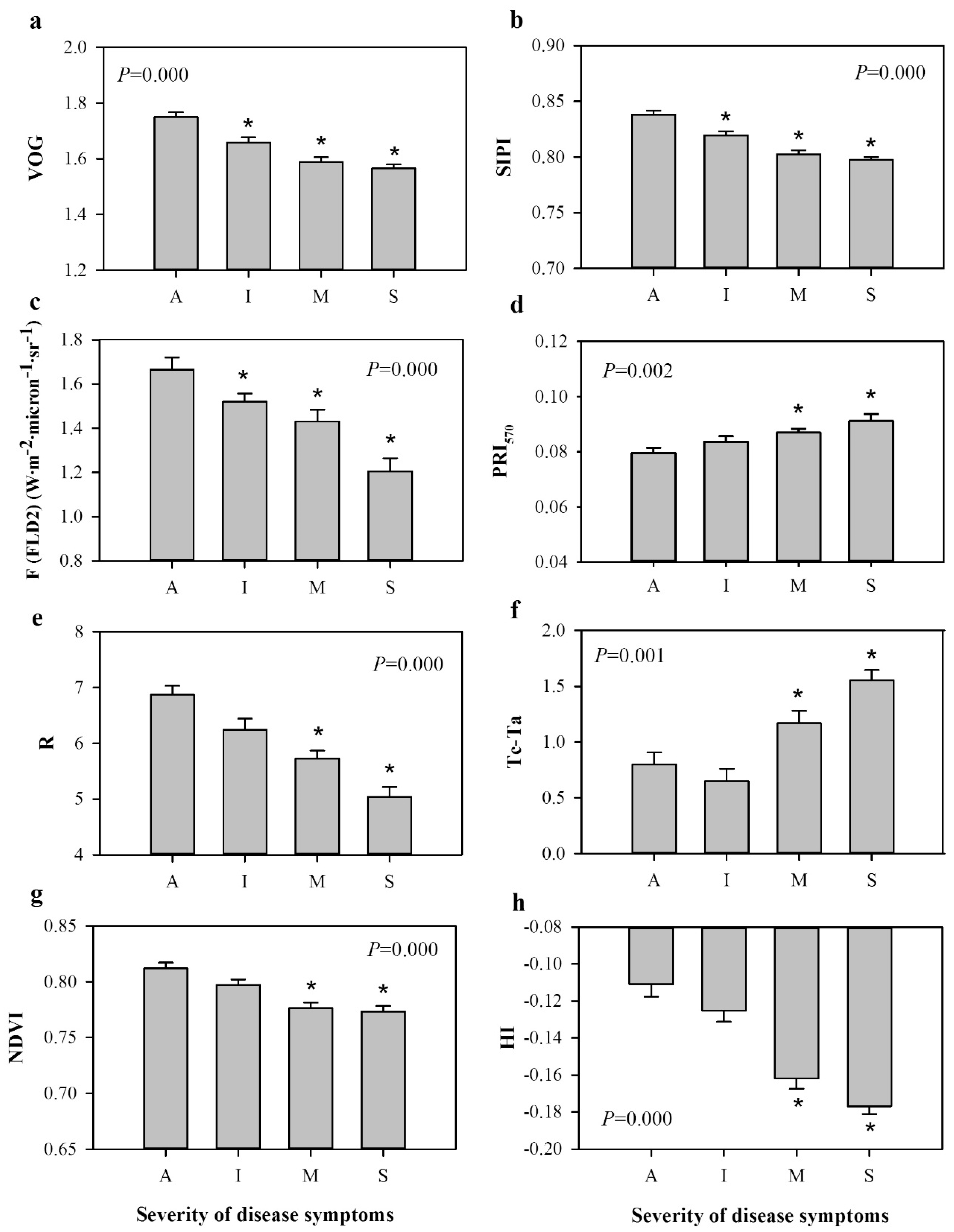
At canopy scale, the level of disease severity was related to Tc-Ta values and to the indices calculated from hyperspectral imagery obtained from each of the two flights. In order to check the sensitivity of Tc-Ta and each of the vegetation indices to the levels of disease severity evaluated in the field, standard ANOVA was conducted on the indices that were normally distributed and showed uniform variance, and again Kruskal–Wallis AOV was used for those which did not meet these requirements. The Dunnett’s two-tailed test (for indices analyzed with ANOVA) and Mann–Whitney U test (for the ones analyzed with Kruskal–Wallis AOV) with Bonferroni’s correction for α value were used to determine significant differences between asymptomatic trees and each of the disease severity levels at p < 0.05.
Afterwards, the combined information from the indices underwent a multivariate analysis and was used to develop models able to quantify disease incidence and severity, so as to separate each severity class from the rest and detect early symptomatic trees. The data calculated from thermal and hyperspectral imagery acquired on 11 July were the training dataset and the data from the 30 July flight were the test dataset. The classification models were trained to classify disease severity groups and the test data was used to evaluate the model classification accuracy. First, the STEPDISC procedure of SAS software (version 9.4; SAS Institute, Cary, NC, USA) was applied to the training set to eliminate variables within the model that did not provide additional information or were redundant according to the Wilk’s lambda method, as well as to add variables outside the model that contribute most to it. The data obtained from the stepwise analysis were further subjected to two different classification methods: linear discriminant analysis (LDA) and support vector machine (SVM). LDA was conducted with the DISCRIM procedure of SAS to generate a discriminant function capable of determining the classification accuracy of the dataset, based on the pooled covariance matrix and the prior probabilities of the classification groups [
58]. Due to the non-normal distribution of the data, a non-parametric discriminant analysis was conducted specifying a
k-value of 4 for the k-nearest-neighbor rule [
19]. Then, a canonical discriminant analysis using the CANDISC procedure of SAS was conducted to separate red leaf blotch severity classes based on linear combinations of the spectral indices selected by the stepwise analysis. The linear combinations of variables (canonical variates) were then correlated with the original disease severity classes. Individual values for each canonical variate were plotted in a bi-plot for the first and second canonical variables.
Linear and non-linear SVM classification methods were conducted using R software (R Foundation for Statistical Computing), following the procedures described in Calderón
et al. [
19] for non-linear SVM classification methods. The linear SVM classification was carried out using a linear kernel, while the non-linear SVM classification used the radial basis function kernel. Likewise, a grid search method using cross validation approach was applied, as suggested by Hsu
et al. [
59].
The classification accuracies of the models were evaluated by calculating the overall accuracy value and the kappa (κ) coefficient, which gives an overall accuracy assessment for the classification based on commission and omission error for all classes.
4. Discussion
Remote sensing has been proposed as a useful tool for disease management, primarily at early stages of disease development in order to design appropriate disease control measurements [
8]. However, red leaf blotch of almond trees is presently managed by preventive applications of fungicide. Thus, remote sensing could be used to build up field maps where areas with little or no risk of infection can be identified, and so implement precision agriculture strategies which restrict the application of fungicides to areas where disease incidence is expected. Early detection of this disease could also help reduce the use of fungicide once effective active ingredients against this disease are available.
For technical reasons, it was impossible to fly in spring, at the start of the epidemics, which would be appropriate for early detection. Nonetheless, the disease presented a wide range of disease severity values throughout the field, healthy and initially-symptomatic trees also being present even in July in some areas of the field, which allowed us to check the model ability to distinguish red leaf blotch early symptoms. Moreover, since quantification and segregation among higher disease severity levels were objectives of the present work as well, it was not optimum to fly until mid-summer, when more severe symptoms can be observed. Even if leaf area density is much lower in early-April than from May onwards, the model was developed from pure-crown pixels, so there is no interference of soil pixels. In addition, this procedure prevents any effect of inter-tree and inter-row spacing, training, and canopy architecture when modeling other fields.
Furthermore, the aircraft was flown at 550 m AGL so as to minimize the effect of sun angle variation between flying dates, cameras underwent radiometric calibration before every flight, and atmospheric correction was applied when calculating reflectance values from radiance values measured. Therefore, the scrupulous methodology followed in the acquisition of data from the hyperspectral and thermal cameras and its processing ensures the adequate correlation between the model and the field visual evaluation as well as its transferability to other fields and dates, two flights being enough—one for training and other one for validating the model. The main conclusion of the present work regarding early detection, that is, “early symptoms can be detected from chlorophyll indices and fluorescence”, is supported by our measurements at leaf level and by the individual analysis of variance conducted on each index.
Up to now, few studies using remote sensing have been conducted for detection of diseases at the canopy scale in tree crops, with the exception of wilt diseases in olive [
18,
19] and avocado trees [
17] and greening disease of citrus [
20,
21,
22]. This work has demonstrated the potential of high-resolution hyperspectral imagery for the early detection and quantification of red leaf blotch in almond orchards. Chlorophyll a+b and carotenoid indices and chlorophyll fluorescence, estimated with the FLD principle, were identified as the best indicators to detect red leaf blotch at leaf level and at early stages of disease development, while canopy temperature (Tc-Ta) and structural, xanthophyll, R/G/B, and disease indices proven to be good indicators to detect the presence of moderate and severe symptoms.
Calderón
et al. [
18,
19] studied the infection and severity caused by
Verticillium dahliae in olive orchards, identifying Tc-Ta and chlorophyll fluorescence (FLD3) as early remote sensing indicators of the disease. Here, Tc-Ta only detected moderate and severe red leaf blotch symptoms due to the appearance of ochre and necrotic spots which cause an increase in leaf temperature. In another fungal foliar disease (downy mildew in opium poppy), Tc-Ta was significantly affected when symptoms reached a moderate stage [
12]. While Tc-Ta is useful to detect root impairment caused by soil-borne pathogens due to the colonization of xylem vessels and the subsequent reduction in the transpiration rate, it is not such a good indicator of fungal foliar diseases at early stages of disease development. This may be because the disease does not affect the green area of symptomatic leaves so the reduction in stomatal conductance is only restricted to the part of the leaf affected by the pathogen. Thus, the increase of temperature in affected areas of leaves is a local effect, not being sufficient to significantly increase the canopy temperature at early stages of disease development. This result is in agreement with the ones conducted by Lidenthal
et al. [
60] and Oerke
et al. [
61] with leaf infrared thermography in cucumber plants affected by downy mildew, showing a decrease in the transpiration rate and a consequent rise in temperature with the appearance of chlorotic and necrotic tissues. Leaf thermography was also similarly used for the detection of apple scab disease [
62] and downy mildew in grapevines [
63]. By contrast, chlorophyll a+b and carotenoid indices were able to detect the early effects of red leaf blotch here, while these indices only detected Verticillium wilt in olive in the more advanced stages [
19]. In addition, chlorophyll a+b and carotenoid indices were also demonstrated to detect citrus greening disease [
21]. In conclusion, the early detection of plant diseases is highly influenced by the type of symptoms induced, which leads to selecting different vegetation indices depending on the symptoms which, in turn, depend on the specific physiological responses induced by the pathogen infection.
Regarding the classification models developed, linear models (LDA and linear SVM) showed higher power to separate between asymptomatic trees and those affected by advanced stages of disease development while the non-linear SVM model was more effective in discriminating asymptomatic plants from those at early stages of red leaf blotch development. Rumpf
et al. [
15] also found that a non-linear SVM model conducted with the radial basis kernel could achieve a good separation between healthy sugar beet plants and plants with early infections of Cercospora leaf spot, sugar beet rust, and powdery mildew. Nevertheless, these results are opposite to the ones obtained by Calderón
et al. [
19] for Verticillium wilt quantification over large olive areas, where LDA was the more efficient than non-linear SVM in early detection.
In practice, the results obtained with linear and non-linear classification methods are different and can be applied in different scenarios depending on the objective pursued. In spring, when red leaf blotch starts, the non-linear SVM classification method, with an 80% of class recall for initially-symptomatic trees (
Table 7b), could be applied to detect field areas with initial symptoms, due to its efficiency in separating the trees at initial stages of disease development from the rest. In summer, when the disease is at its highest epidemic level, the main objective is to quantify the disease and assess possible consequent almond yield loss in the following year. In this case, linear classification models, which better separate trees at advanced stages of disease development, would be used.
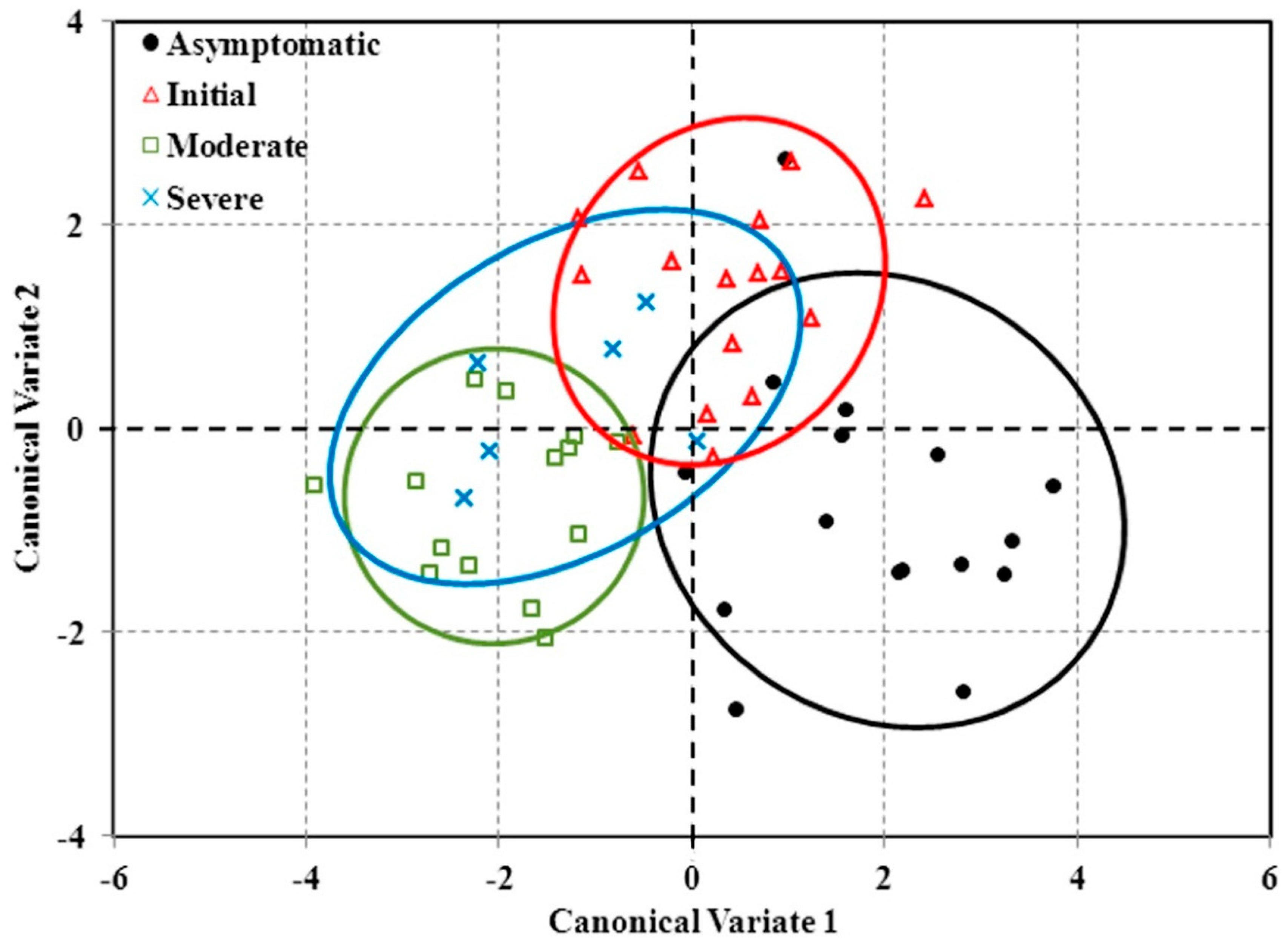
After LDA, a canonical discriminant analysis was conducted to reduce the dimensionality of the variables included in the model to two canonical variates. The first canonical variate allowed the discrimination between the asymptomatic and the moderate and severe levels of disease, while the second variate was able to distinguish between asymptomatic and initial stages of disease development. Pigment indices (
i.e., VOG, PSRI, PSNDc, GM1, CRI
550, and CRI
550_515) dominated both variates demonstrating its power to discriminate asymptomatic trees from symptomatic ones. Chlorophyll fluorescence (FLD2) dominated the second variate, proving its potential to distinguish between asymptomatic and initial symptomatic severity level. These results agree with the ones obtained when individual indices were studied for red leaf blotch detection using univariate statistical analyses. Analyses of variance demonstrated that pigment indices were very suitable to detect red leaf blotch at the canopy level at initial and advanced stages of disease development. Structural indices were found to be good indicators of moderate and severe
P. amygdalinum infection. Although severely affected and healthy trees overlap, it is just because of one single severely symptomatic tree (
Figure 8). Yet, this analysis is rather good in separating healthy from symptomatic trees and would, then, be useful in disease quantification.
This study demonstrated the difficulty of the quantification of red leaf blotch in almond orchards due to the local leaf effects caused by P. amygdalinum infection, so the necessity of high-resolution imagery for monitoring the disease is of higher importance.