An Alternative Quality Control Technique for Mineral Chemistry Analysis of Portland Cement-Grade Limestone Using Shortwave Infrared Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Carbonate Rock Samples
2.2. PXRF Geochemical Analysis
2.3. Carbonate Rock Spectral Measurements
2.4. Determination of Spectral Feature Parameters
3. Results
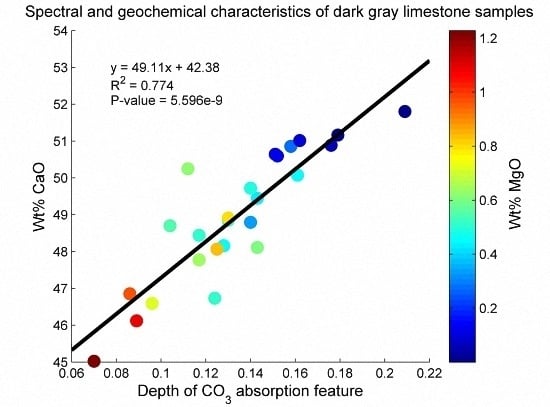
3.1. Geochemical Characterization of the Rock Samples
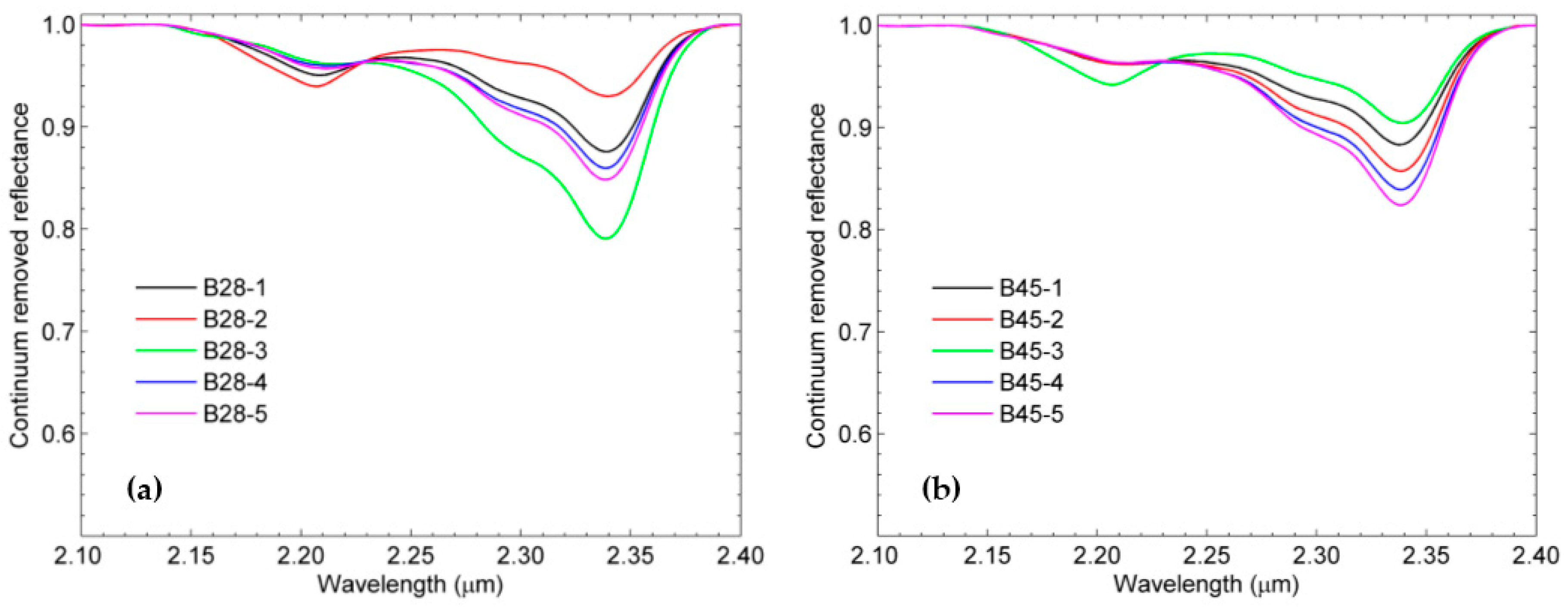
3.2. SWIR Reflectance Spectra of the Rock Samples
3.3. Estimation of Mineral Chemistry Abundance and Composition of the Rock Samples
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Meade, R.K. Raw materials. In Portland Cement: Its Composition, Raw Materials, Manufacture, Testing and Analysis, 3rd ed.; The Chemical Publishing Company: Easton, PA, USA, 1926; pp. 51–74. [Google Scholar]
- Ghosh, S.P. Raw materials: Geological characteristics, nomenclature, origin, occurrences and exploration. In Advances in Cement Technology; Ghosh, S.N., Ed.; Pergamon Press Ltd.: Exeter, UK, 1983; pp. 1–38. [Google Scholar]
- Chatterjee, A.K. Chemico-Physico-Mineralogical characteristics of raw materials of Portland cement. In Advances in Cement Technology; Ghosh, S.N., Ed.; Pergamon Press Ltd.: Exeter, UK, 1983; pp. 39–68. [Google Scholar]
- Taylor, H.F.W. The chemistry of Portland cement manufacture. In Cement Chemistry, 2nd ed.; Telford: London, UK, 1997; pp. 55–88. [Google Scholar]
- Pettijohn, F.J. Limestones and dolomite. In Sedimentary Rocks, 3rd ed.; Harper & Row: New York, NY, USA, 1975; pp. 316–391. [Google Scholar]
- Blatt, H.; Middleton, G.; Murray, R. Origin of limestones. In Origin of Sedimentary Rocks; Prentice-Hall: Englewood Cliffs, NJ, USA, 1972; pp. 409–455. [Google Scholar]
- Gineys, N.; Aouad, G.; Damidot, D. Managing trace elements in Portland cement—Part I: Interactions between cement paste and heavy metals added during mixing as soluble salts. Cem. Concr. Compos. 2010, 32, 563–570. [Google Scholar] [CrossRef]
- Gineys, N.; Aouad, G.; Sorrentino, F.; Damidot, D. Incorporation of trace elements in Portland cement clinker: Thresholds limits for Cu, Ni, Sn or Zn. Cem. Concr. Res. 2011, 41, 1177–1184. [Google Scholar] [CrossRef]
- Ichikawa, M.; Kanaya, M. Effects of minor components and heating rates on the fine textures of alite in Portland cement clinker. Cem. Concr. Res. 1997, 27, 1123–1129. [Google Scholar] [CrossRef]
- Li, X.R.; Xu, W.L.; Wang, S.P.; Tang, M.L.; Shen, X.D. Effect of SO3 and MgO on Portland cement clinker: Formation of clinker phases and alite polymorphism. Constr. Build. Mater. 2014, 58, 182–192. [Google Scholar] [CrossRef]
- Galan, I.; Perron, L.; Glasser, F.P. Impact of chloride-rich environments on cement paste mineralogy. Cem. Concr. Res. 2015, 68, 174–183. [Google Scholar] [CrossRef]
- Horkoss, S.; Lteif, R.; Rizk, T. Influence of the clinker SO3 on the cement characteristics. Cem. Concr. Res. 2011, 41, 913–919. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Distribution of sulfate between phases in Portland cement clinkers. Cem. Concr. Res. 1999, 29, 1173–1179. [Google Scholar] [CrossRef]
- Maki, I.; Fukuda, K.; Yoshida, H.; Kumaki, J. Effect of MgO and SO3 on the impurity concentration in alite in Portland-cement clinker. J. Am. Ceram. Soc. 1992, 75, 3163–3165. [Google Scholar] [CrossRef]
- Mazouzi, W.; Kacimi, L.; Cyr, M.; Clastres, P. Properties of low temperature belite cements made from aluminosilicate wastes by hydrothermal method. Cem. Concr. Compos. 2014, 53, 170–177. [Google Scholar] [CrossRef]
- Wu, K.; Shi, H.S.; De Schutter, G.; Guo, X.L.; Ye, G. Preparation of alinite cement from municipal solid waste incineration fly ash. Cem. Concr. Compos. 2012, 34, 322–327. [Google Scholar] [CrossRef]
- Fernandez, R.; Martirena, F.; Scrivener, K.L. The origin of the pozzolanic activity of calcined clay minerals: A comparison between kaolinite, illite and montmorillonite. Cem. Concr. Res. 2011, 41, 113–122. [Google Scholar] [CrossRef]
- Tosun, K.; Felekoglu, B.; Baradan, B.; Altun, I.A. Effects of limestone replacement ratio on the sulfate resistance of Portland limestone cement mortars exposed to extraordinary high sulfate concentrations. Constr. Build. Mater. 2009, 23, 2534–2544. [Google Scholar] [CrossRef]
- Pipilikaki, P.; Papageorgiou, D.; Teas, C.; Chaniotakis, E.; Katsioti, M. The effect of temperature on thaumasite formation. Cem. Concr. Compos. 2008, 30, 964–969. [Google Scholar] [CrossRef]
- De Weerdt, K.; Colombo, A.; Coppola, L.; Justnes, H.; Geiker, M.R. Impact of the associated cation on chloride binding of Portland cement paste. Cem. Concr. Res. 2015, 68, 196–202. [Google Scholar] [CrossRef]
- Irassar, E.F.; Bonavetti, V.L.; González, M. Microstructural study of sulfate attack on ordinary and limestone Portland cements at ambient temperature. Cem. Concr. Res. 2003, 33, 31–41. [Google Scholar] [CrossRef]
- Bertron, A.; Escadeillas, G.; de Parseval, P.; Duchesne, J. Processing of electron microprobe data from the analysis of altered cementitious materials. Cem. Concr. Res. 2009, 39, 929–935. [Google Scholar] [CrossRef]
- Kurokawa, D.; Honma, K.; Hirao, H.; Fukuda, K. Quality design of belite-melilite clinker. Cem. Concr. Res. 2013, 54, 126–132. [Google Scholar] [CrossRef]
- Ifka, T.; Palou, M.; Baracek, J.; Soukal, F.; Bohac, M. Evaluation of P2O5 distribution inside the main clinker minerals by the application of EPMA method. Cem. Concr. Res. 2014, 59, 147–154. [Google Scholar] [CrossRef]
- Taylor, H.F.W.; Newbury, D.E. An electron-microprobe study of a mature cement paste. Cem. Concr. Res. 1984, 14, 565–573. [Google Scholar] [CrossRef]
- Marjanovic, L.; McCrindle, R.I.; Botha, B.M.; Potgieter, J.H. Analysis of cement by inductively coupled plasma optical emission spectrometry using slurry nebulization. J. Anal. At. Spectrom. 2000, 15, 983–985. [Google Scholar] [CrossRef]
- Silva, C.S.; Blanco, T.; Nobrega, J.A. Analysis of cement slurries by inductively coupled plasma optical emission spectrometry with axial viewing. Spectrochim. Acta Part B 2002, 57, 29–33. [Google Scholar] [CrossRef]
- Potgieter, S.S.; Maljanovic, L. A further method for chloride analysis of cement and cementitious materials—ICP-OES. Cem. Concr. Res. 2007, 37, 1172–1175. [Google Scholar] [CrossRef]
- Frias, M.; Derojas, M.I.S.; Garcia, N.; Luxan, M.P. Contribution of toxic elements-hexavalent chromium in materials used in the manufacture of cement. Cem. Concr. Res. 1994, 24, 533–541. [Google Scholar] [CrossRef]
- Kruse, F.A.; Lefkoff, A.B.; Dietz, J.B. Expert system-based mineral mapping in Northern Death-Valley, California Nevada, using the airborne visible infrared imaging spectrometer (AVIRIS). Remote Sens. Environ. 1993, 44, 309–336. [Google Scholar] [CrossRef]
- Van der Meer, F.D. Analysis of spectral absorption features in hyperspectral imagery. Int. J. Appl. Earth Obs. 2004, 5, 55–68. [Google Scholar] [CrossRef]
- Perraki, T.; Kontori, E.; Tsivilis, S.; Kakali, G. The effect of zeolite on the properties and hydration of blended cements. Cem. Concr. Compos. 2010, 32, 128–133. [Google Scholar] [CrossRef]
- Kocak, Y.; Nas, S. The effect of using fly ash on the strength and hydration characteristics of blended cements. Constr. Build. Mater. 2014, 73, 25–32. [Google Scholar] [CrossRef]
- Ylmen, R.; Wadso, L.; Panas, I. Insights into early hydration of Portland limestone cement from infrared spectroscopy and isothermal calorimetry. Cem. Concr. Res. 2010, 40, 1541–1546. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Yu, W.H.; Schennach, R.; Cocke, D.L. A Fourier transform infrared spectroscopic investigation of the early hydration of Portland cement and the influence of sodium lignosulfonate. Cem. Concr. Res. 2000, 30, 267–273. [Google Scholar] [CrossRef]
- Haest, M.; Cudahy, T.; Laukamp, C.; Gregory, S. Quantitative mineralogy from infrared spectroscopic data. I. Validation of mineral abundance and composition scripts at the Rocklea channel iron deposit in Western Australia. Econ. Geol. 2012, 107, 209–228. [Google Scholar] [CrossRef]
- Magendran, T.; Sanjeevi, S. Hyperion image analysis and linear spectral unmixing to evaluate the grades of iron ores in parts of Noamundi, Eastern India. Int. J. Appl. Earth Obs. 2014, 26, 413–426. [Google Scholar] [CrossRef]
- Zaini, N.; van der Meer, F.; van der Werff, H. Determination of carbonate rock chemistry using laboratory-based hyperspectral imagery. Remote Sens. 2014, 6, 4149–4172. [Google Scholar] [CrossRef]
- Hunt, G.R.; Salisbury, J.W. Visible and near infrared spectra of minerals and rocks: II. Carbonates. Mod. Geol. 1971, 2, 23–30. [Google Scholar]
- Hunt, G.R. Spectral signatures of particulate minerals in the visible and near infrared. Geophysics 1977, 42, 501–513. [Google Scholar] [CrossRef] [Green Version]
- Clark, R.N.; King, T.V.V.; Klejwa, M.; Swayze, G.A.; Vergo, N. High spectral resolution reflectance spectroscopy of minerals. J. Geophys. Res. 1990, 95, 12653–12680. [Google Scholar] [CrossRef]
- Hunt, G.R.; Salisbury, J.W. Visible and near-infrared spectra of minerals and rocks: I. Silicate minerals. Mod. Geol. 1970, 1, 283–300. [Google Scholar]
- Clark, R.N. Spectroscopy of rocks and minerals, and principles of spectroscopy. In Remote Sensing for the Earth Sciences: Manual of Remote Sensing, 3rd ed.; Rencz, A.N., Ed.; John Wiley and Sons: New York, NY, USA, 1999; Volume 3, pp. 3–58. [Google Scholar]
- Povarennykh, A.S. Use of infrared-spectra for determination of minerals. Am. Mineral. 1978, 63, 956–959. [Google Scholar]
- Van der Meer, F.D. Spectral reflectance of carbonate mineral mixtures and bidirectional reflectance theory: Quantitative analysis techniques for application in remote sensing. Remote Sens. Rev. 1995, 13, 67–94. [Google Scholar] [CrossRef]
- Gaffey, S.J. Spectral reflectance of carbonate minerals in the visible and near infrared (0.35–2.55 microns): Calcite, aragonite, and dolomite. Am. Mineral. 1986, 71, 151–162. [Google Scholar]
- Zaini, N.; van der Meer, F.; van der Werff, H. Effect of grain size and mineral mixing on carbonate absorption features in the SWIR and TIR wavelength regions. Remote Sens. 2012, 4, 987–1003. [Google Scholar] [CrossRef]
- Crowley, J.K. Visible and near-infrared spectra of carbonate rocks-reflectance variations related to petrographic texture and impurities. J. Geophys. Res. Solid 1986, 91, 5001–5012. [Google Scholar] [CrossRef]
- Kurz, T.H.; Dewit, J.; Buckley, S.J.; Thurmond, J.B.; Hunt, D.W.; Swennen, R. Hyperspectral image analysis of different carbonate lithologies (limestone, karst and hydrothermal dolomites):The Pozalagua quarry case study (Cantabria, North-West Spain). Sedimentology 2012, 59, 623–645. [Google Scholar] [CrossRef]
- Bennett, J.D.; Bridge, D.M.; Cameron, N.R.; Djunuddin, A.; Ghazali, S.A.; Jeffery, D.H.; Kartawa, W.; Keats, W.; Rock, N.M.S.; Thomson, S.J.; et al. Geologic Map of the Banda Aceh Quadrangle, Sumatra; Indonesia Geological Research and Development Centre: Bandung, Indonesia, 1981. [Google Scholar]
- Barber, A.J.; Crow, M.J. Pre-tertiary stratigraphy. In Sumatra: Geology, Resources and Tectonic Evolution; Barber, A.J., Crow, M.J., Milsom, J.S., Eds.; Geological Society: London, UK, 2005; pp. 24–53. [Google Scholar]
- Bogdanoff, S.; Donnot, M.; Ellenberger, F. Carte Geologique de la France à 1:50.000 et Notice Explicative, Feuille Bedarieux; Edition du BRGM: Orléans, France, 1984. [Google Scholar]
- Murphy, R.J.; Schneider, S.; Monteiro, S.T. Consistency of measurements of wavelength position from hyperspectral imagery: Use of the ferric iron crystal field absorption at 900 nm as an indicator of mineralogy. IEEE Trans. Geosci. Remote Sens. 2014, 52, 2843–2857. [Google Scholar] [CrossRef]
- Clark, R.N.; Roush, T.L. Reflectance spectroscopy-quantitative analysis techniques for remote sensing applications. J. Geophys. Res. 1984, 89, 6329–6340. [Google Scholar] [CrossRef]
- Bakker, W. HypPy User Manual: Graphical User-Interface (GUI); Version 2.6; ITC: Enschede, The Netherlands, 2012. [Google Scholar]
- Rodger, A.; Laukamp, C.; Haest, M.; Cudahy, T. A simple quadratic method of absorption feature wavelength estimation in continuum removed spectra. Remote Sens. Environ. 2012, 118, 273–283. [Google Scholar] [CrossRef]
- Salisbury, J.W.; Hapke, B.; Eastes, J.W. Usefulness of weak bands in mid infrared remote sensing of particulate planetary surface. J. Geophys. Res. 1987, 92, 702–710. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Galan, E.; Theng, B.K.G. Structures and mineralogy of clay minerals. In Developments in Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 19–86. [Google Scholar]
- Post, J.L.; Noble, P.N. The near-infrared combination band frequencies of dioctahedral smectites, micas, and illites. Clays Clay Miner. 1993, 41, 639–644. [Google Scholar] [CrossRef]
- Younis, M.T.; Gilabert, M.A.; Melia, J.; Bastida, J. Weathering process effects on spectral reflectance of rocks in a semi-arid environment. Int. J. Remote Sens. 1997, 18, 3361–3377. [Google Scholar] [CrossRef]
- Yue, Y.; Zhang, B.; Wang, K.; Liu, B.; Li, R.; Jiao, Q.; Yang, Q.; Zhang, M. Spectral indices for estimating ecological indicators of karst rocky desertification. Int. J. Remote Sens. 2010, 31, 2115–2122. [Google Scholar] [CrossRef]






| PXRF Spots Code | CaO | MgO | Al2O3 | SiO2 | Fe2O3 | K2O | SO3 | TiO2 | Sample Quality |
|---|---|---|---|---|---|---|---|---|---|
| B28-1 | 46.736 | 0.501 | 2.984 | 4.463 | 0.345 | 0.373 | 0.197 | 0.148 | Good [3,4] |
| B28-2 | 45.023 | 1.228 | 8.674 | 14.289 | 0.440 | 1.166 | 0.230 | 0.572 | |
| B28-3 | 51.804 | 0 | 0.897 | 2.939 | 0.276 | 0.218 | 0.220 | 0.113 | |
| B28-4 | 48.798 | 0.342 | 1.759 | 5.808 | 0.785 | 0.436 | 1.026 | 0.322 | |
| B28-5 | 50.595 | 0.142 | 1.960 | 3.260 | 0.222 | 0.317 | 0.095 | 0.127 | |
| B41-1 | 52.313 | 0 | 0.295 | 1.074 | 0.067 | 0.070 | 0.172 | 0.060 | Good [3,4] |
| B41-2 | 51.388 | 0 | 0.395 | 1.196 | 0.059 | 0.045 | 0.225 | 0.045 | |
| B41-3 | 50.967 | 0.342 | 1.240 | 2.390 | 0.076 | 0.039 | 0 | 0.053 | |
| B41-4 | 50.360 | 0.511 | 1.578 | 2.846 | 0.060 | 0.069 | 0 | 0.050 | |
| B41-5 | 50.525 | 0 | 1.265 | 1.862 | 0.069 | 0.052 | 0 | 0.053 | |
| F20-1 | 27.635 | 21.941 | 0 | 0.169 | 0 | 0 | 0.172 | 0 | Bad [3,4] |
| F20-2 | 29.132 | 21.239 | 0.079 | 0.182 | 0.034 | 0 | 0.192 | 0 | |
| F20-3 | 28.606 | 18.086 | 0.711 | 0.958 | 0.107 | 0.023 | 0.162 | 0.020 | |
| F20-4 | 26.877 | 17.728 | 0.832 | 1.535 | 0.113 | 0 | 0.157 | 0.022 | |
| F20-5 | 29.442 | 20.174 | 0 | 0.237 | 0 | 0 | 0.140 | 0 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaini, N.; Van der Meer, F.; Van Ruitenbeek, F.; De Smeth, B.; Amri, F.; Lievens, C. An Alternative Quality Control Technique for Mineral Chemistry Analysis of Portland Cement-Grade Limestone Using Shortwave Infrared Spectroscopy. Remote Sens. 2016, 8, 950. https://doi.org/10.3390/rs8110950
Zaini N, Van der Meer F, Van Ruitenbeek F, De Smeth B, Amri F, Lievens C. An Alternative Quality Control Technique for Mineral Chemistry Analysis of Portland Cement-Grade Limestone Using Shortwave Infrared Spectroscopy. Remote Sensing. 2016; 8(11):950. https://doi.org/10.3390/rs8110950
Chicago/Turabian StyleZaini, Nasrullah, Freek Van der Meer, Frank Van Ruitenbeek, Boudewijn De Smeth, Fadli Amri, and Caroline Lievens. 2016. "An Alternative Quality Control Technique for Mineral Chemistry Analysis of Portland Cement-Grade Limestone Using Shortwave Infrared Spectroscopy" Remote Sensing 8, no. 11: 950. https://doi.org/10.3390/rs8110950
APA StyleZaini, N., Van der Meer, F., Van Ruitenbeek, F., De Smeth, B., Amri, F., & Lievens, C. (2016). An Alternative Quality Control Technique for Mineral Chemistry Analysis of Portland Cement-Grade Limestone Using Shortwave Infrared Spectroscopy. Remote Sensing, 8(11), 950. https://doi.org/10.3390/rs8110950