A Method to Analyze the Potential of Optical Remote Sensing for Benthic Habitat Mapping
Abstract
:1. Introduction
| Acronym | Definition |
|---|---|
| BA | Brown Algae |
| CSE | Spectral covariance matrix pertaining to NEΔrrs |
| GA | Green algae |
| HDC | Hierarchical clustering using linear Discriminant Coordinates |
| HICO | Hyperspectral Imager for the Coastal Ocean |
| LDA | Linear Discriminant Analysis |
| NEΔrrs | Sensor-environmental noise equivalent perturbation of rrs |
| RMSE | Root mean square error |
| RA | Red algae |
| SA | Semianalytical shallow water forward model |
| SD | Sediment |
| SG | Seagrass |
| SRF | Spectral response function |
| WV2 | WorldView-2 |
| ZSD | Secchi Depth (m) |
| G | Absorption coefficient of colored dissolved and detrital matter at 440 nm |
| H | Geometric depth of the water column |
| κ | Attenuation coefficient |
| k | Number of benthic classes |
| n | Number of wavebands |
| ρb | Benthic irradiance reflectance |
| P | Absorption coefficient of phytoplankton at 440 nm |
| rrs | Subsurface remote sensing reflectance |
| s | Number of LD eigenvectors |
| τm | Proportion of misclassified Linear Discriminant Functions between a pair of classes |
| X | Backscattering coefficient of suspended particles at 550 nm |
2. Methods
2.1. Benthic Reflectance Library
| Genera | Species |
|---|---|
| Brown alga (BA) | Sargassum linearifolium Sargassum spinuligerum Ecklonia radiata Colpomenia sinuosa |
| Red alga (RA) | Asparagopsis armata Hypnea ramentacea Ballia sp. Amphiroa anceps Euptilota articulata Ballia callitrichia Metagoniolithon stelliferum on rubble |
| Green alga (GA) | Ulva australis Entermorpha sp. Codium duthieae Caulerpa germinata Caulerpa flexis Bryopsis vestita |
| Seagrass (SG) | Amphibolis antartica Posidonia sp. on sediment |
| Sand/sediment (SD) | Sediment Sediment/Rubble Rocks with encrusting red coralline algae |
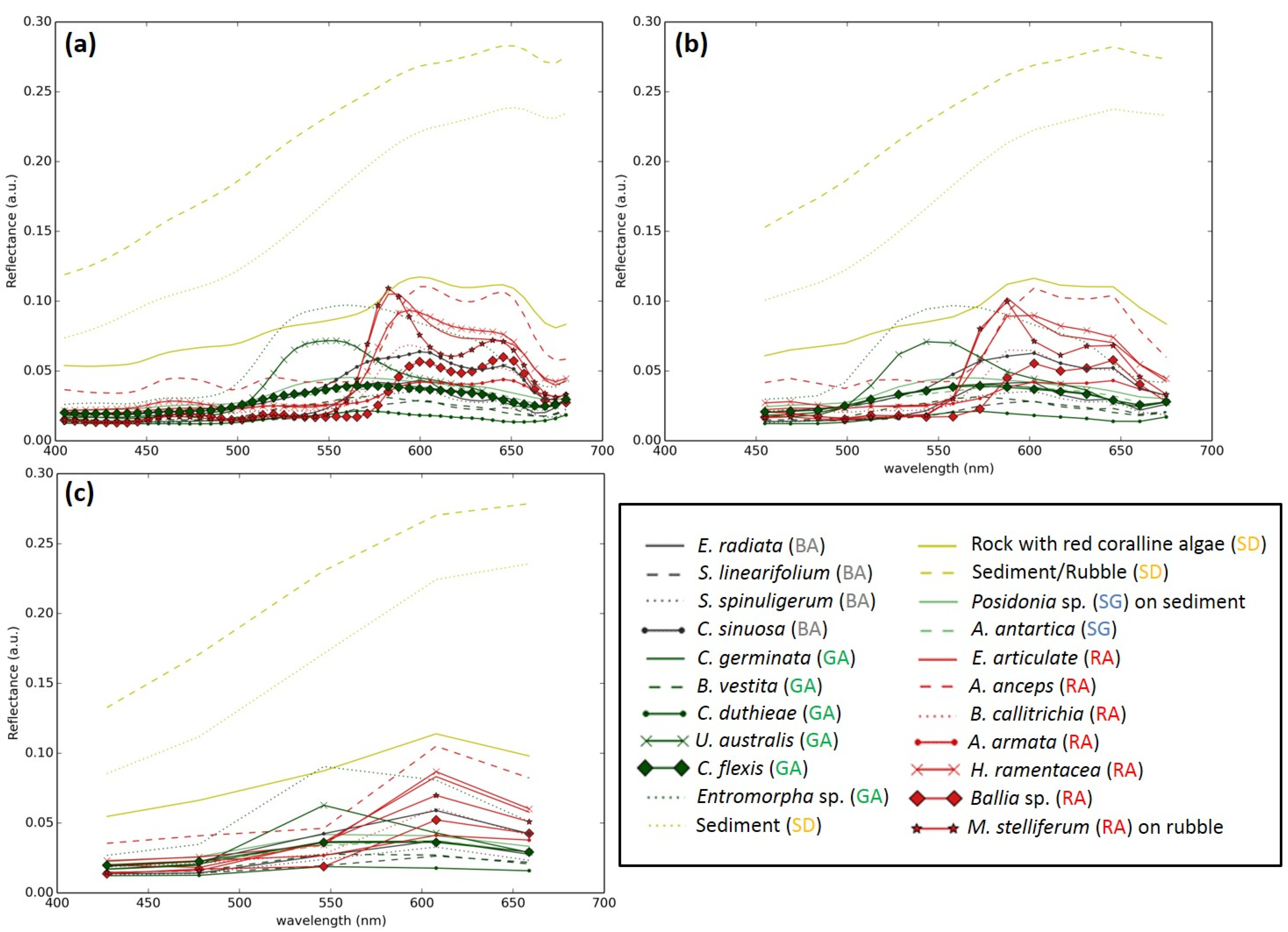
2.2. Clustering
2.2.1. Measure of Interclass Overlap
2.2.2. Hierarchical Clustering Using Linear Discriminant Coordinates (HDC)

2.2.3. Depth and Water Column Specific Spectral Libraries
3. Results and Discussion
3.1. Hierarchical Clustering of Benthic Irradiance Reflectance Spectra

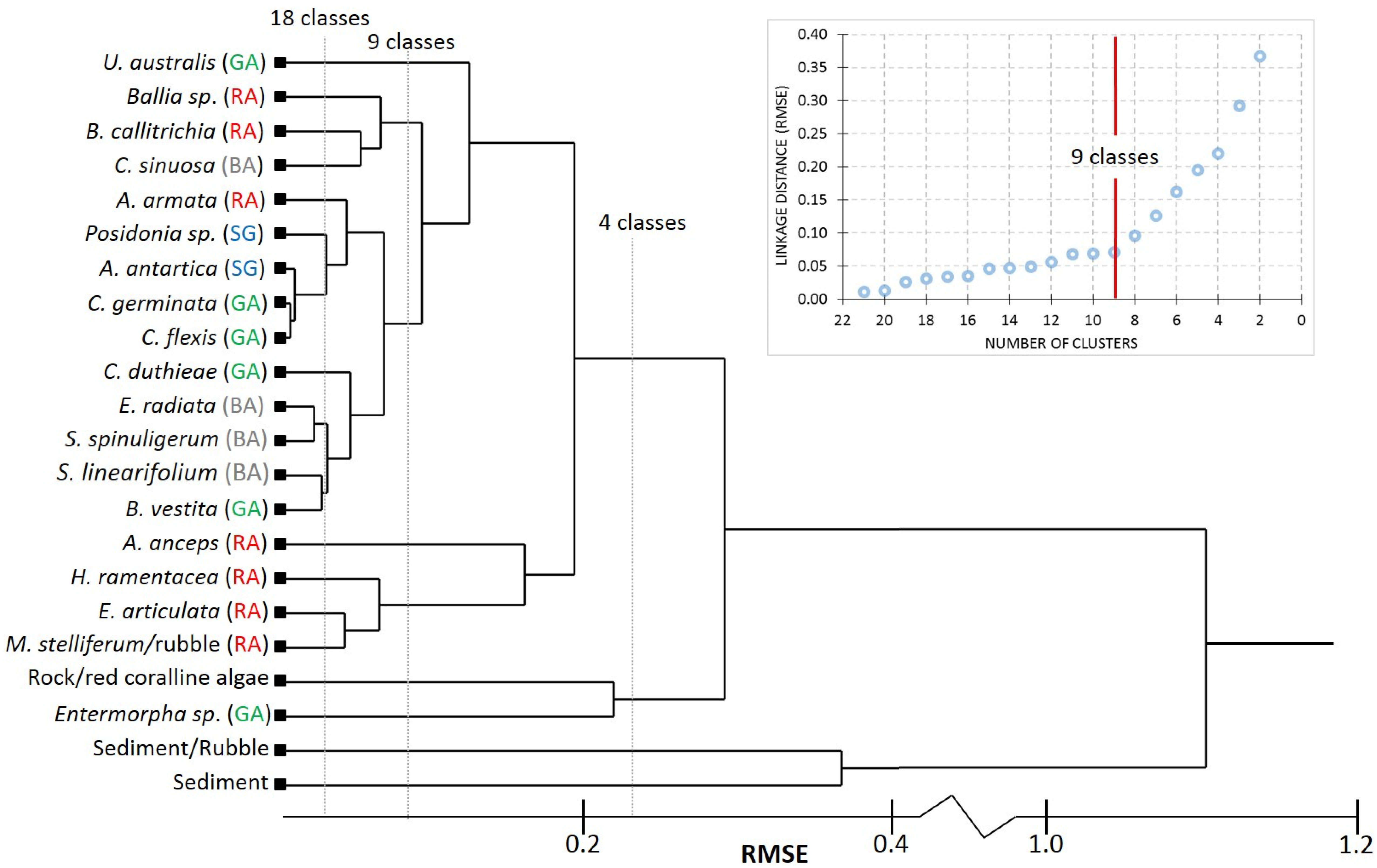


3.2. Water-Column Specific Benthic Spectral Libraries


 |
3.3. Resolving Seagrass Species from Algae

 |
3.4. Implications to Shallow Water Habitat Mapping
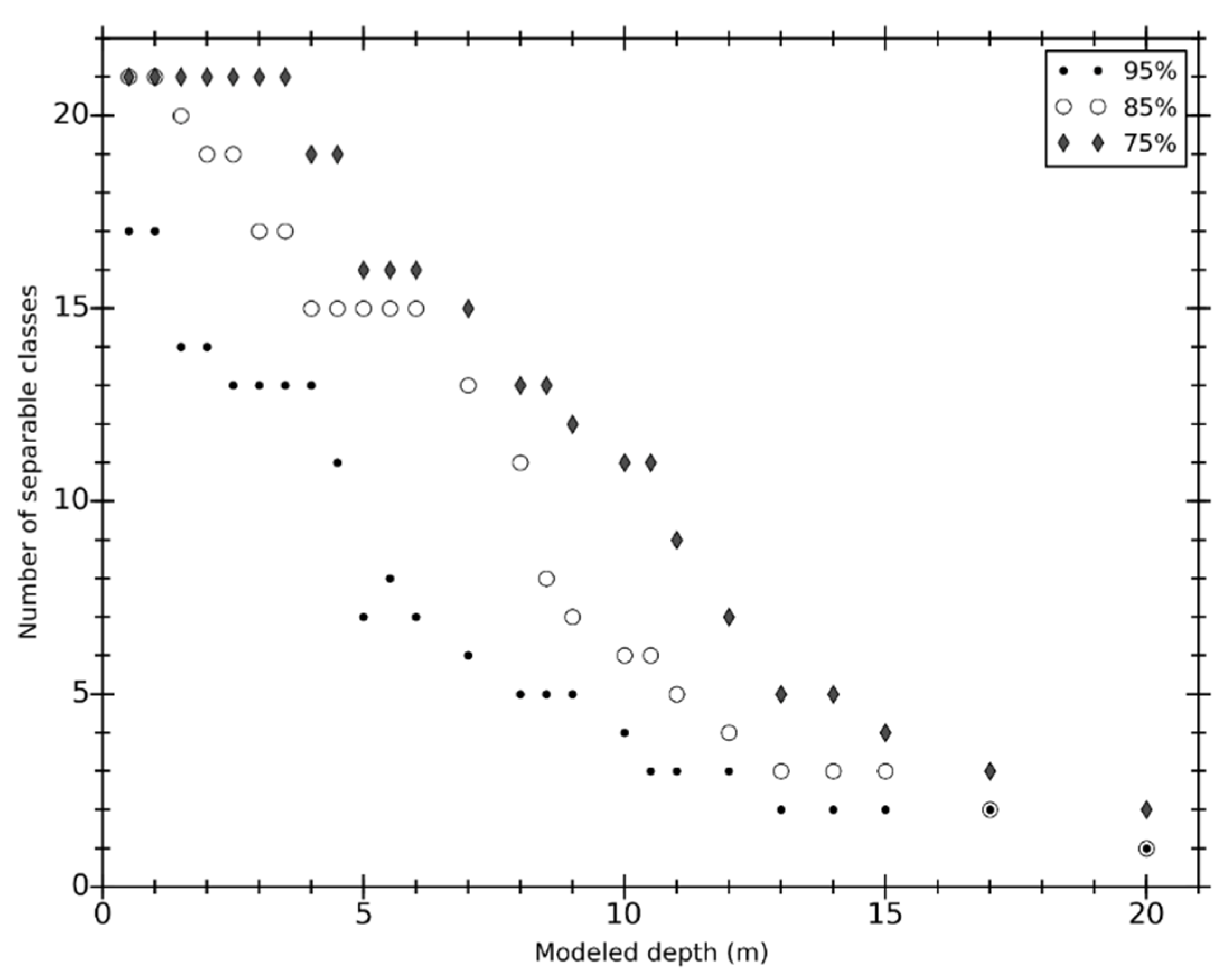
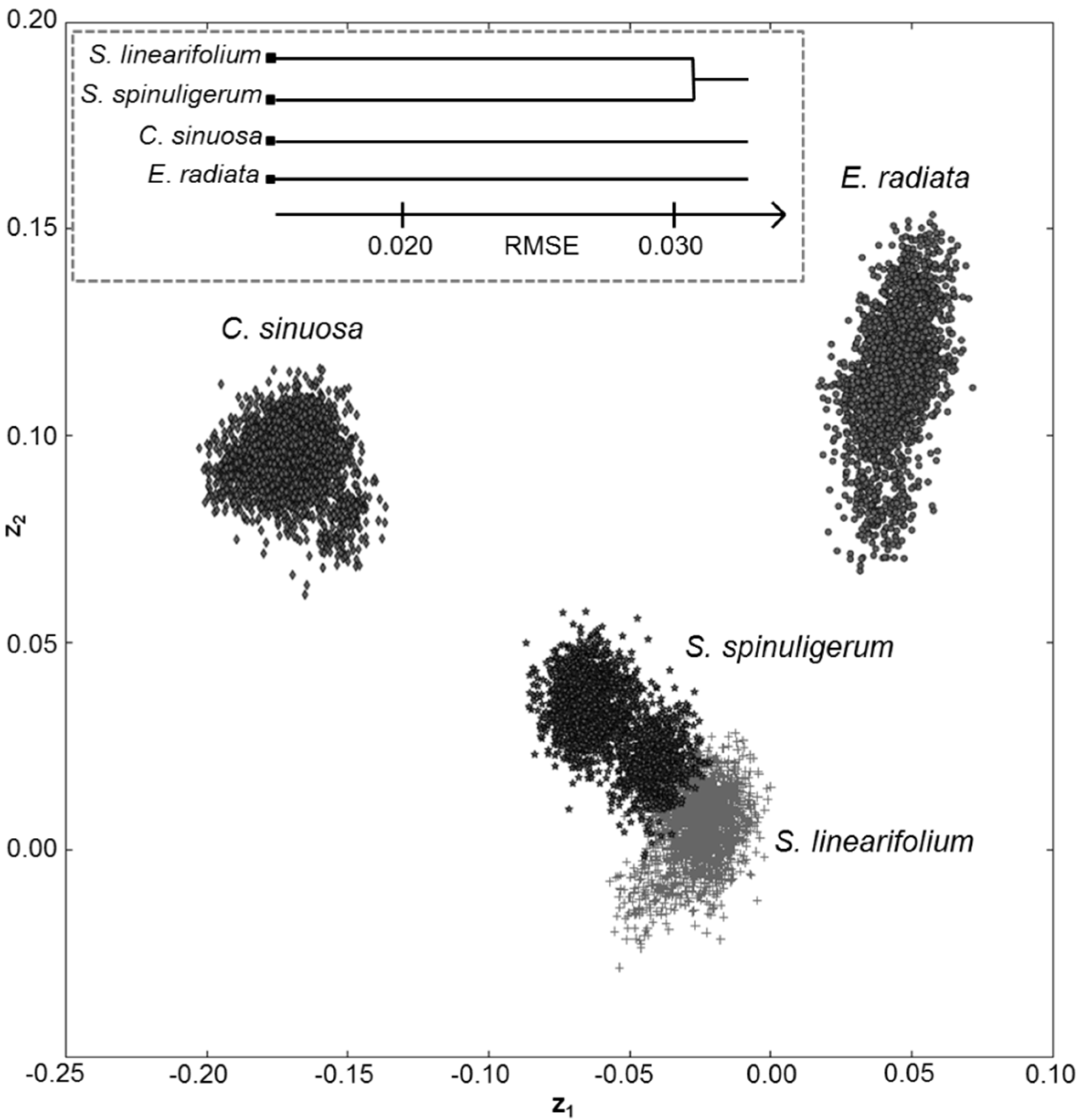
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Andrefouet, S.; Kramer, P.; Torres-Pulliza, D.; Joyce, K.E.; Hochberg, E.J.; Garcia-Perez, R.; Mumby, P.J.; Riegl, B.; Yamano, H.; White, W.H.; et al. Multi-site evaluation of IKONOS data for classification of tropical coral reef environments. Remote Sens. Environ. 2003, 88, 128–143. [Google Scholar]
- Mumby, P.J.; Harborne, A.R. Development of a systematic classification scheme of marine habitats to facilitate regional management and mapping of Caribbean coral reefs. Biol. Conserv. 1999, 88, 155–163. [Google Scholar] [CrossRef]
- Mumby, P.J.; Green, E.P.; Edwards, A.J.; Clark, C.D. Coral reef habitat mapping: How much detail can remote sensing provide. Mar. Biol. 1997, 130, 193–202. [Google Scholar] [CrossRef]
- Mumby, P.J.; Clark, C.D.; Green, E.P.; Edwards, A.J. Benefits of water column correction and contextual editing for mapping coral reefs. Int. J. Remote Sens. 1998, 19, 203–210. [Google Scholar] [CrossRef]
- Mumby, P.J.; Green, E.P.; Clark, C.D.; Edwards, A.J. Digital analysis of multispectral airborne imagery of coral reefs. Coral Reefs 1998, 17, 59–69. [Google Scholar] [CrossRef]
- Mumby, P.J.; Hedley, J.D.; Chisholm, J.R.M.; Clark, C.D.; Ripley, H.; Jaubert, J. The cover of living and dead corals from airborne remote sensing. Coral Reefs 2004, 23, 171–183. [Google Scholar] [CrossRef]
- Vahtmae, E.; Kutser, T. Mapping bottom type and water depth in shallow coastal waters with satellite remote sensing. J. Coast. Res. 2007, S50, 185–189. [Google Scholar]
- Mumby, P.J.; Edwards, A.J. Mapping marine environments with IKONOS imagery: Enhanced spatial resolution can deliver greater thematic accuracy. Remote Sens. Environ. 2002, 82, 248–257. [Google Scholar] [CrossRef]
- Vahtmae, E.; Kutser, T.; Kotta, J.; Parnoja, M. Detecting patterns and changes in a complex benthic environment of the Baltic Sea. J. Appl. Remote Sens. 2011, 5, 053559. [Google Scholar] [CrossRef]
- Purkis, S.; Kenter, J.A.M.; Oikonomou, E.K.; Robinson, I.S. High-resolution ground verification, cluster analysis and optical model of reef substrate coverage on Landsat TM imagery (Red Sea, Egypt). Int. J. Remote Sens. 2002, 23, 1677–1698. [Google Scholar] [CrossRef]
- Dekker, A.G.; Brando, V.E.; Anstee, J.M. Retrospective seagrass change detection in a shallow coastal tidal Australian lake. Remote Sens. Environ. 2005, 97, 415–433. [Google Scholar] [CrossRef]
- Gullstrom, M.; Lunden, B.; Bodin, M.; Kangwe, J.; Ohman, M.C.; Mtolera, M.S.P.; Bjork, M. Assessment of changes in the seagrass-dominated submerged vegetation of tropical Chwaka Bay (Zanzibar) using satellite remote sensing. Estuar. Coast. Shelf Sci. 2006, 67, 399–408. [Google Scholar] [CrossRef]
- Hedley, J.D.; Mumby, P.J.; Joyce, K.E.; Phinn, S.R. Spectral unmixing of coral reef benthos under ideal conditions. Coral Reefs 2004, 23, 60–73. [Google Scholar] [CrossRef]
- Mobley, C.D.; Sundman, L.K.; Davis, C.O.; Bowles, J.H.; Downes, T.V.; Leathers, R.A.; Montes, M.J.; Bissett, W.P.; Kohler, D.D.R.; Reid, R.P.; et al. Interpretation of hyperspectral remote-sensing imagery by spectrum matching and look-up-tables. Appl. Opt. 2005, 44, 3576–3592. [Google Scholar] [CrossRef] [PubMed]
- Hedley, J.; Roelfsema, C.; Phinn, S.R. Efficient radiative transfer model inversion for remote sensing applications. Remote Sens. Environ. 2009, 113, 2527–2532. [Google Scholar] [CrossRef]
- Lee, Z.P.; Carder, K.L.; Mobley, C.D.; Steward, R.G.; Patch, J.S. Hyperspectral remote sensing for shallow waters: 2. Deriving bottom depths and water properties by optimization. Appl. Opt. 1999, 38, 3831–3843. [Google Scholar] [CrossRef] [PubMed]
- Klonowski, W.M.; Fearns, P.R.C.S.; Lynch, M.J. Retrieving key benthic cover types and bathymetry from hyperspectral imagery. J. Appl. Remote Sens. 2007, 1, 011505. [Google Scholar] [CrossRef]
- Brando, V.E.; Anstee, J.M.; Wettle, M.; Dekker, A.G.; Phinn, S.R.; Roelfsema, C. A physics based retrieval and quality assessment of bathymetry from suboptimal hyperspectral data. Remote Sens. Environ. 2009, 49, 2972–2982. [Google Scholar] [CrossRef]
- Fearns, P.R.C.; Klonowski, W.; Babcock, R.C.; England, P.; Phillips, J. Shallow water substrate mapping using hyperspectral remote sensing. Cont. Shelf Res. 2011, 31, 1249–1259. [Google Scholar] [CrossRef]
- Dekker, A.G.; Phinn, S.R.; Anstee, J.; Bissett, P.; Brando, V.E.; Casey, B.; Fearns, P.; Hedley, J.; Klonowski, W.; Lee, Z.P.; et al. Intercomparison of shallow water bathymetry, hydro-optics, and benthos mapping techniques in Australian and Caribbean coastal environments. Limnol. Oceanogr. Methods 2011, 9, 396–425. [Google Scholar] [CrossRef]
- Kobryn, H.T.; Wouters, K.; Beckley, L.E.; Heege, T. Ningaloo Reef: Shallow marine habitats mapped using a hyperspectral sensor. PLoS ONE 2013, 8, e70105. [Google Scholar] [CrossRef] [PubMed]
- Harvey, M.J.; Kobryn, H.T.; Beckley, L.E.; Heege, T.; Hausknecht, P.; Pinnel, N. Mapping the shallow marine benthic habitats of Rottnest Island, Western Australia. In Proceedings of the 3rd EARSeL Workshop Remote Sensing of the Coastal Zone, Bolzano, Italy, 7–9 June 2007; Available online: http://researchrepository.murdoch.edu.au/6141/1/mapping_the_shallow_marine_benthic_habitats.pdf (accessed on 13 November 2014).
- Karpouzli, E.; Malthus, T.J.; Place, C.J. Hyperspectral discrimination of coral reef benthic communities in the western Caribbean. Coral Reefs 2004, 23, 141–151. [Google Scholar] [CrossRef]
- Hochberg, E.J.; Atkinson, M.J. Capabilities of remote sensors to classify coral, algae, and sand as pure and mixed spectra. Remote Sens. Environ. 2003, 85, 175–189. [Google Scholar] [CrossRef]
- Brando, V.E.; Dekker, A.G. Satellite hyperspectral remote sensing for estimating estuarine and coastal water quality. IEEE Trans. Geosci. Remote Sens. 2003, 41, 1378–1387. [Google Scholar] [CrossRef]
- Hedley, J.D.; Roelfsema, C.M.; Phinn, S.R.; Mumby, P.J. Environmental and sensor limitations in optical remote sensing of Coral Reefs: Implications for monitoring and sensor design. Remote Sens. 2012, 4, 271–302. [Google Scholar] [CrossRef]
- Hochberg, E.J.; Atkinson, M.J.; Apprill, A.; Andrefouet, S. Spectral reflectance of coral. Coral Reefs 2004, 23, 84–95. [Google Scholar] [CrossRef]
- Stambler, N.; Shashar, N. Variation in spectral reflectance of hermatypic corals, Stylophora pistillata and Pocillopora damicornis. J. Exp. Mar. Biol. Ecol. 2007, 351, 143–149. [Google Scholar] [CrossRef]
- Kruse, F.A.; Lefkoff, A.B.; Boardman, J.W.; Heidebrecht, K.B.; Shapiro, A.T.; Barloon, P.J.; Goetz, A.F.H. The spectral image processing system (SIPS)—Interactive visualization and analysis of imaging spectrometer data. Remote Sens. Environ. 1993, 44, 145–163. [Google Scholar] [CrossRef]
- Chang, C.I. An information-theoretic approach to spectral variability, similarity, and discrimination for hyperspectral image analysis. IEEE Trans. Inform. Theory 2000, 46, 1927–1932. [Google Scholar] [CrossRef]
- Sohn, Y.; Rebello, N.S. Supervised and unsupervised spectral angle classifiers. Photogramm. Eng. Remote Sens. 2002, 68, 1271–1280. [Google Scholar]
- Pu, R.; Bell, S.; Meyer, C.; Baggett, L.; Zhao, Y. Mapping and assessing seagrass along the western coast of Florida using Landsat TM and EO-1 ALI/Hyperion imagery. Estuar. Coast. Shelf Sci. 2012, 115, 234–245. [Google Scholar] [CrossRef]
- Maeder, J.; Narumalani, S.; Rundquist, D.C.; Perk, R.L.; Schalles, J.; Hutchins, K.; Keck, J. Classifying and mapping general coral-reef structure using IKONOS data. Photogramm. Eng. Remote Sens. 2002, 68, 1297–1305. [Google Scholar]
- Call, K.A.; Hardy, J.T.; Wallin, D.O. Coral reef habitat discrimination using multivariate spectral analysis and satellite remote sensing. Int. J. Remote Sens. 2003, 24, 2627–2639. [Google Scholar] [CrossRef]
- Minghelli-Roman, A.; Chisholm, J.R.M.; Marchioretti, M.; Ripley, H.; Jaubert, J.M. Discrimination of coral reflectance spectra in the Red Sea. Coral Reefs 2002, 21, 307–314. [Google Scholar] [CrossRef]
- Kutser, T.I.; Jupp, D.L.B. On the possibility of mapping living corals to the species level based on their optical signatures. Estuar. Coast. Shelf Sci. 2006, 69, 607–614. [Google Scholar] [CrossRef]
- Purkis, S.J.; Pasterkamp, R. Integrating in situ reef-top reflectance spectra with Landsat TM imagery to aid shallow-tropical benthic habitat mapping. Coral Reefs 2004, 23, 5–20. [Google Scholar] [CrossRef]
- Rencher, A.C.; Christensen, W.F. Methods of Multivariate Analysis, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 288–319. [Google Scholar]
- Holden, H.; LeDrew, E. Spectral discrimination of healthy and non-healthy corals based on cluster analysis, principal component analysis, and derivative spectroscopy. Remote Sens. Environ. 1998, 65, 217–224. [Google Scholar] [CrossRef]
- Anderberg, M.R. Cluster Analysis for Applications; Academic Press: New York, NY, USA, 1973; p. 15. [Google Scholar]
- Hedley, J.; Roelfsema, C.; Phinn, S.R. Propagating uncertainty through a shallow water mapping algorithm based on radiative transfer model inversion. In Proceedings of the Ocean Optics XX, Anchorage, AK, USA, 25 September–1 October 2010.
- Hedley, J.; Roelfsema, C.; Koetz, B.; Phinn, S. Capability of the Sentinel 2 mission for tropical coral reef mapping and coral bleaching detection. Remote Sens. Environ. 2012, 120, 145–155. [Google Scholar] [CrossRef]
- Garcia, R.A.; McKinna, L.I.W.; Fearns, P.R.C.S. Improving the optimization solution for a semi-analytical shallow water inversion model in the presence of spectrally correlated noise. Limnol. Oceanogr. Methods 2014, 12, 651–669. [Google Scholar]
- Garcia, R.A.; Fearns, P.R.C.S.; McKinna, L.I.W. Detecting trend and seasonal changes in bathymetry derived from HICO imagery: A case study of Shark Bay, Western Australia. Remote Sens. Environ. 2014, 147C, 186–205. [Google Scholar] [CrossRef]
- Everitt, B.S.; Landau, S.; Leese, M.; Stahl, D. Cluster Analysis, 5th ed.; John Wiley & Sons: Chichester, West Sussex, UK, 2011; p. 76. [Google Scholar]
- Salvador, S.; Chan, P. Determining the number of clusters/segments in hierarchical clustering/segmentation algorithms. IEEE Inter. Conf. Tools Artif. Intell. 2004, 16, 576–584. [Google Scholar]
- Torrecilla, E.; Stramski, D.; Reynolds, R.A.; Millan-Nunez, E.; Piera, J. Cluster analysis of hyperspectral optical data for discriminating phytoplankton pigment assemblages in the open ocean. Remote Sens. Environ. 2011, 115, 2578–2593. [Google Scholar] [CrossRef] [Green Version]
- Botha, E.J.; Brando, V.E.; Anstee, J.M.; Dekker, A.G.; Sagar, S. Increased spectral resolution enhances coral detection under varying water conditions. Remote Sens. Environ. 2013, 131, 247–261. [Google Scholar] [CrossRef]
- Holmes, R.W. The Secchi disk in turbid coastal waters. Limnol. Oceanogr. 1970, 15, 688–694. [Google Scholar] [CrossRef]
- Fyfe, S.K. Spatial and temporal variation in spectral reflectance: Are seagrass species spectrally distinct? Limnol. Oceanogr. 2003, 48, 464–479. [Google Scholar] [CrossRef]
- Kruse, K.A.; Broadman, J.W.; Lefkoff, A.B.; Young, J.M.; Kierein-Young, K.S. HyMap: An Australian hyperspectral sensor solving global problems—Results from USA HyMap data acquisitions. In Proceedings of the 10th Australasian Remote Sensing and Photogrammetry Conference, Adelaide, Australia, 21–25 August 2000; pp. 296–311.
- Clark, C.D.; Mumby, P.J.; Chisholm, J.R.M.; Jaubert, J.; Andrefouet, S. Spectral discrimination of coral mortality states following a severe bleaching event. Int. J. Remote Sens. 2000, 21, 2321–2327. [Google Scholar] [CrossRef]
- Hochberg, E.J.; Atkinson, M.J. Spectral discrimination of coral reef benthic communities. Coral Reefs 2000, 19, 164–171. [Google Scholar] [CrossRef]
- Roelfsema, C.; Kovacs, E.M.; Saunders, M.I.; Phinn, S.; Lyons, M.; Maxwell, P. Challenges of remote sensing for quantifying changes in large complex seagrass environments. Estuar. Coast. Shelf Sci. 2013, 133, 161–171. [Google Scholar] [CrossRef]
- Hedley, J.D. Hyperspectral applications. In Coral Reef Remote Sensing: A Guide for Mapping, Monitoring and Management; Goodman, J.A., Purkis, S.J., Phinn, S.R., Eds.; Springer: New York, NY, USA, 2013; pp. 79–112. [Google Scholar]
- Crebassol, P.; Ferrier, P.; Dedieu, G.; Hagolle, O.; Fougnie, B.; Tinto, F.; Yaniv, Y.; Herscovitz, J. VENμS (Vegetation and Environment Monitoring on a New Micro Satellite). In Small Satellite Missions for Earth Observation: New Development and Trends; Sandau, R., Roser, H.P., Valenzuela, A., Eds.; Springer: Heidelberg, Germany, 2010; pp. 47–65. [Google Scholar]
- Lee, Z.P.; Weidermann, A.; Arnone, R. Combined effect of reduced band number and increased bandwidth on shallow water remote sensing: The case of WorldView 2. IEEE Trans. Geosci. Remote Sens. 2013, 51, 2577–2586. [Google Scholar] [CrossRef]
- Hedley, J.D.; Harborne, A.; Mumby, P. Simple and robust removal of sun glint for mapping shallow-water benthois. Int. J. Remote Sens. 2005, 26, 2107–2112. [Google Scholar] [CrossRef]
- Lachenbruch, P.A.; Sneeringer, C.; Revo, L.T. Robustness of the linear and quadratic discriminant function to certain types of non-normality. Commun. Stat. 1973, 1, 39–56. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning: Data Mining, Inference and Prediction, 2nd ed.; Springer: New York, NY, USA, 2009; p. 110. Available online: http://web.stanford.edu/~hastie/local.ftp/Springer/OLD/ESLII_print4.pdf (accessed on 12 January 2015).
- Benfield, S.L.; Guzman, H.M.; Mair, J.M.; Young, J.A.T. Mapping the distribution of coral reefs and associated sublittoral habitats in Pacific Panama: A comparison of optical satellite sensors and classification methodologies. Int. J. Remote Sens. 2007, 28, 5047–5070. [Google Scholar] [CrossRef]
- Goodman, J.A.; Ustin, S.L. Classification of benthic composition in a coral reef environment using spectral unmixing. J. Appl. Remote Sens. 2007, 1, 011501. [Google Scholar] [CrossRef]
- Lee, Z.P.; Carder, K.L.; Chen, R.F.; Peacock, T.G. Properties of the water column and bottom derived from Airborne Visible Infrared Imaging Spectroradiometer (AVIRIS) data. J. Geophys. Res. 2001, 106, 11639–11651. [Google Scholar] [CrossRef]
- Lewis, D.; Gould, R.W., Jr.; Weidemann, A.; Ladner, S.; Lee, Z.P. Bathymetry estimations using vicariousy calibrated HICO data. Proc. SPIE 2013. [Google Scholar] [CrossRef]
- Richter, R. A fast atmospheric correction algorithm applied to Landsat TM images. Int. J. Remote Sens. 1990, 11, 159–166. [Google Scholar] [CrossRef]
- Jacobsen, A.; Heidebrecht, K.B.; Goetz, A.F.H. Assessing the quality of the radiometric calibration of casi data and retrieval of surface reflectance factors. Photogramm. Eng. Remote Sens. 2000, 66, 1083–1091. [Google Scholar]
- Moses, W.J.; Bowles, J.H.; Corson, M.R. Expected improvements in the quantitative remote sensing of optically complex waters with the use of an optically fast hyperspectral spectrometer—A modeling study. Sensors 2015, 15, 6152–6173. [Google Scholar] [CrossRef] [PubMed]
- Rand, W.M. Objective criteria for the evaluation of clustering methods. J. Am. Stat. Assoc. 1971, 66, 846–850. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. The comparison of dendrograms by objective methods. Taxon 1962, 11, 33–40. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, R.A.; Hedley, J.D.; Tin, H.C.; Fearns, P.R.C.S. A Method to Analyze the Potential of Optical Remote Sensing for Benthic Habitat Mapping. Remote Sens. 2015, 7, 13157-13189. https://doi.org/10.3390/rs71013157
Garcia RA, Hedley JD, Tin HC, Fearns PRCS. A Method to Analyze the Potential of Optical Remote Sensing for Benthic Habitat Mapping. Remote Sensing. 2015; 7(10):13157-13189. https://doi.org/10.3390/rs71013157
Chicago/Turabian StyleGarcia, Rodrigo A., John D. Hedley, Hoang C. Tin, and Peter R. C. S. Fearns. 2015. "A Method to Analyze the Potential of Optical Remote Sensing for Benthic Habitat Mapping" Remote Sensing 7, no. 10: 13157-13189. https://doi.org/10.3390/rs71013157






