4. Discussion
Numerous studies on remote-sensing–based mapping of mangroves have been published over the last two decades. In this paper, they were divided into five sensor categories: airborne photography, optical medium-resolution, optical high-resolution, hyperspectral, and radar studies. The selection of the appropriate sensor depends mainly on the purpose of the investigation, the attainable final map scale, the discrimination level required, the time frame to be covered, special characteristics of the geographic region, and the funds and training level of personnel available for the envisioned study.
Table 3,
Table 4,
Table 5,
Table 6 and
Table 7 compare the benefits and limitations of the five groups of remote-sensing data types for mangrove mapping: aerial photography, medium-resolution spaceborne multispectral imagery, high-resolution spaceborne multispectral imagery, hyperspectral imagery, and radar imagery.
Table 3.
Benefits and limitations of aerial photography for mangrove mapping.
Table 3.
Benefits and limitations of aerial photography for mangrove mapping.
| Aerial photography | Benefits | Limitations |
|---|
| 1. Spectral resolution | Red–near-infrared spectral information with red-edge slope | None at all or very low (R,G,B; near-infrared) |
| 2. Spatial resolution | Very high (centimeter to meter range) | Only small area is covered |
| 3. Temporal resolution | Always available on demand | Complex acquisition of equipment and flight campaign planning is needed |
| 4. Costs | Low costs for small areas | Increasing costs with increasing spatial coverage; high costs if professional flight campaign planning and multispectral camera |
| 5. Long-term monitoring | Data available for >50 years | |
| 6. Purposes | Local maps of mangrove ecosystems, parametrization, change detection | Only local-scale studies |
| 7. Discrimination level | Species communities, density parameters | Sometimes too much detail (hampering unbiased image processing) |
| 8. Methods | Visual interpretation with on-screen digitizing and object-oriented approaches | Automatization usually not possible; considerable analyst bias and, thus, hampered transferability or comparability |
| 9. Other | Valuable additional information source to support field survey, image interpretation, or accuracy assessments. If overlapping pictures are acquired (stereo pairs), it is possible to derive canopy-elevation model | |
Although aerial imagery and high-resolution multispectral, hyperspectral, and radar data partially provide information with high spatial detail, which is suitable for detecting subtle changes in species composition and distribution, extent of subcanopy flooding, health condition, growth pattern, and more, which is of the utmost importance for local or regional agencies responsible for the protection and management of mangroves [
157], some national agencies are more interested in updated overview information on a regional or even a country-wide scale, for their spatial planning and conservation-planning tasks, and the reporting of status and trends [
157]. The advantages of using medium-resolution imagery, for example, are that it delivers appropriate coverage and information depth (see
Table 4) in a cost-effective manner [
9,
12,
75,
97,
123].
Table 4.
Benefits and limitations of medium-resolution imagery for mangrove mapping.
Table 4.
Benefits and limitations of medium-resolution imagery for mangrove mapping.
| Medium-resolution imagery | Benefits | Limitations |
|---|
| 1. Spectral resolution | Several multispectral bands, always including R,G,B; near-infrared; and oftentimes even mid-infrared; and thermal bands | Skilled trained personnel are required to best exploit the information content of the multiple bands (considering transformations, etc.) |
| 2. Spatial resolution | Ideal for mapping on a large regional scale | Too coarse for local observations requiring in-depth species differentiation and parameterization |
| 3. Temporal resolution | Frequent mapping (e.g., rainy season and dry season within 1 year; or repeated annual mapping) is possible | Repetition rate may be too low to record impact of extreme events (e.g., cyclones, floods, tsunamis); furthermore, very weather dependent (clouds) = critical in subtropical and tropical regions |
| 4. Costs | Depending on sensor, freely available (e.g., Landsat), very cost efficient (ASTER), or expensive (e.g., SPOT); but all are cost efficient compared with field surveys and airborne campaigns | Software for image processing needed (common software, such as Erdas, ENVI, and ArcGIS, have high license fees), but usually not a real limitation |
| 5. Long-term monitoring | Data availability over three decades | Depending on the future duration of the systems and subsequent comparable sensors |
| 6. Purposes | Inventory and status maps; change detection, such as assessment of impact damages; assessment of reforestation and conservation success | For some species-oriented botany-focused studies, resolution may already be too coarse |
| 7. Discrimination level | Mangrove–non-mangrove, density variations, condition status, mangrove zonation, in rare cases also species discrimination | High regional differences; classification Result depends highly on the ecosystem conditions, such as biodiversity, heterogeneity of forests, adjacent targets; species identification is rarely possible |
| 8. Methods | Visual interpretation with on-screen digitizing, pixel-based, object based, and hybrid classification approaches; image transformation and analyses (PCA, TCT, IHS indices, etc.) | To exploit the full potential of the data skilled analysts needed |
| 9. Other | Data easy to access or order; best explored data type and, thus, most literature available; long-term monitoring granted | |
Table 5.
Benefits and limitations of high-resolution imagery for mangrove mapping.
Table 5.
Benefits and limitations of high-resolution imagery for mangrove mapping.
| High-resolution imagery | Benefits | Limitations |
|---|
| 1. Spectral resolution | Red–near-infrared spectral information with red-edge slope; usually panchromatic band allowing image fusion (pan-sharpening) | Relatively few spectral bands |
| 2. Spatial resolution | High resolution (0.5–4 m range) for mapping on a local scale | Only a small area is covered |
| 3. Temporal resolution | Regular mapping is possible on demand | Weather dependent (clouds); cost intensive if repeated monitoring is requested |
| 4. Costs | Moderate costs for single acquisitions (usually 2,000–10,000 Euro, depending on area) | Very high costs if repeated monitoring is requested. Also, high costs of object-oriented image-processing software (e.g., Ecognition) |
| 5. Long-term monitoring | Theoretically possible, but usually not used because of expense. Sensors, such as IKONOS, QuickBird, etc., available since late 1990s/2000. | Depending on the future duration of the systems and subsequent comparable sensors. Only back to the late 1990s. |
| 6. Purposes | Discrimination of mangrove species, spatial distribution and variability, health status, parameterization | Single-tree species discrimination usually not possible |
| 7. Discrimination level | Down to species communities; detailed parameterization | Regional differences; classification result depends highly on the ecosystem conditions, such as biodiversity, heterogeneity of forests, adjacent targets |
| 8. Methods | Visual interpretation with on-screen digitizing, pixel-based, object-based, and hybrid-classification approaches | Skilled analysts needed to exploit the full potential of the data |
| 9. Other | Valuable information source to support field survey and accuracy assessment. Easy to close the scale gap to in situ investigations | In some (developing/emerging) countries, data of the relevant sensors very difficult to purchase; few studies published based on the data type |
In contrast to medium-resolution data, high-resolution imagery is very cost intensive. This requires a careful consideration of the reasons to apply such datasets. A further point concerns the data availability, because these data are collected according to customer-defined areas of interest. This may lead to difficulties, because the data provider might have different over-flight priorities than does the customer. Mumby
et al. [
14] pointed out that, for example, that IKONOS-based studies need to fulfill the following prerequirements to be cost-effective: extensive and detailed field data are available, the area of interest must be <500 km
2 (only 22 × 22 km), and the monitoring of the habitat dynamics is requested at a scale <10 m.
Table 6.
Benefits and limitations of hyperspectral imagery for mangrove mapping.
Table 6.
Benefits and limitations of hyperspectral imagery for mangrove mapping.
| Hyperspectral imagery | Benefits | Limitations |
|---|
| 1. Spectral resolution | Very high, covering a broad range with narrow bandwidths | High data volume, bands with redundant information |
| 2. Spatial resolution | Usually very high (centimeter to meter range) | Very small area covered |
| 3. Temporal resolution | Spaceborne: because of few sensors without long-term acquisition, maximum monthly; airborne: on demand if costs do not play a role | Weather dependent (clouds); complex acquisition of equipment is needed; very cost intensive |
| 4. Costs | None | Very high costs for airborne campaigns and sensor operation; very high costs for personnel working in airborne or spaceborne data |
| 5. Long-term monitoring | Theoretically possible; practically not feasible | Unsuitable because of small areas covered and very high costs; will only be possible with a reliable spaceborne, operational sensor |
| 6. Purposes | Maps of mangroves on species level; highly detailed parameterization; detailed analyses of status (vigor, health, etc.) | No major limitations |
| 7. Discrimination level | Species communities | No major limitations |
| 8. Methods | Typical hyperspectral data-analysis methods (spectral unmixing, SAM, MTMF, etc.); partially also paired with object-oriented analyses | Specialized knowledge is needed for data analysis; experience in sound hyperspectral data processing often not available; hyperspectral analyses often lead to only seemingly quantitative results (e.g., end member-fraction images) |
| 9. Other | Detailed mapping of non-mangrove constituents also probably beneficial (e.g., different water classes, depending on sediment load, algae, etc.; or soil types) | Relatively few studies have been conducted; still in a testing phase; very few spaceborne sensors available (Hyperion with questionable SNR, Sebas, etc.). See Table 3 for airborne sensor limitations |
Hyperspectral imagery has similar limitations with regard to data availability. In general, it is airborne generated and, therefore, requires the coordination of a flight campaign, appropriate sensor equipment, and trained personnel. Costs are high. This challenge can be overcome in the future when spaceborne hyperspectral data of the planned sensor EnMap become available in 2014.
The wide variety of application options, available sensors, and processing methods used/performed globally on ecologically varying ecosystems is enormous. The large number of parameters involved leads to enormous difficulties in comparing data, methods, and results. Some well-accepted standards or definitions might be helpful to simplify the applied approaches for mangrove mapping and to allow better comparisons. Such standards are likely to facilitate a better understanding of the ecosystem processes and the assessment of technical investigations in a global context. The aspects suggested below might be considered an initial contribution toward simplifying the aforementioned difficulties.
Table 7.
Benefits and limitations of radar imagery for mangrove mapping.
Table 7.
Benefits and limitations of radar imagery for mangrove mapping.
| Radar (SAR) imagery | Benefits | Limitations |
|---|
| 1. Spectral resolution | Active microwave radiation; delivers alternative information about the surface structure; various wavelengths and polarizations are selectable | No spectral information |
| 2. Spatial resolution | Varies | Usually low, except TerraSAR-X |
| 3. Temporal resolution | High; weather independent | None |
| 4. Costs | Many data types available at low cost in the context of science proposals (ESA, JAXA, DLR, etc.) | Restricted access to data (certain number of scenes; also some data not sharable with certain developing countries (e.g., TSX) |
| 5. Long-term monitoring | Good; long-duration systems | None |
| 6. Purposes | Mangrove extent, condition, canopy properties, deforestation, biomass estimation | No information derivable from typical spectra (species differentiation not possible unless species vary in their structural appearance) |
| 7. Discrimination level | Age structure, forest parameters, biomass estimation | No discrimination between mangroves and other vegetation forms without a priori knowledge; no separation among species |
| 8. Methods | Analyses of the backscatter signals using advanced image-processing techniques; very quantitative physics-based manner of image analysis | Extremely skilled analysts with experience in radar-image processing needed (availability, costs) |
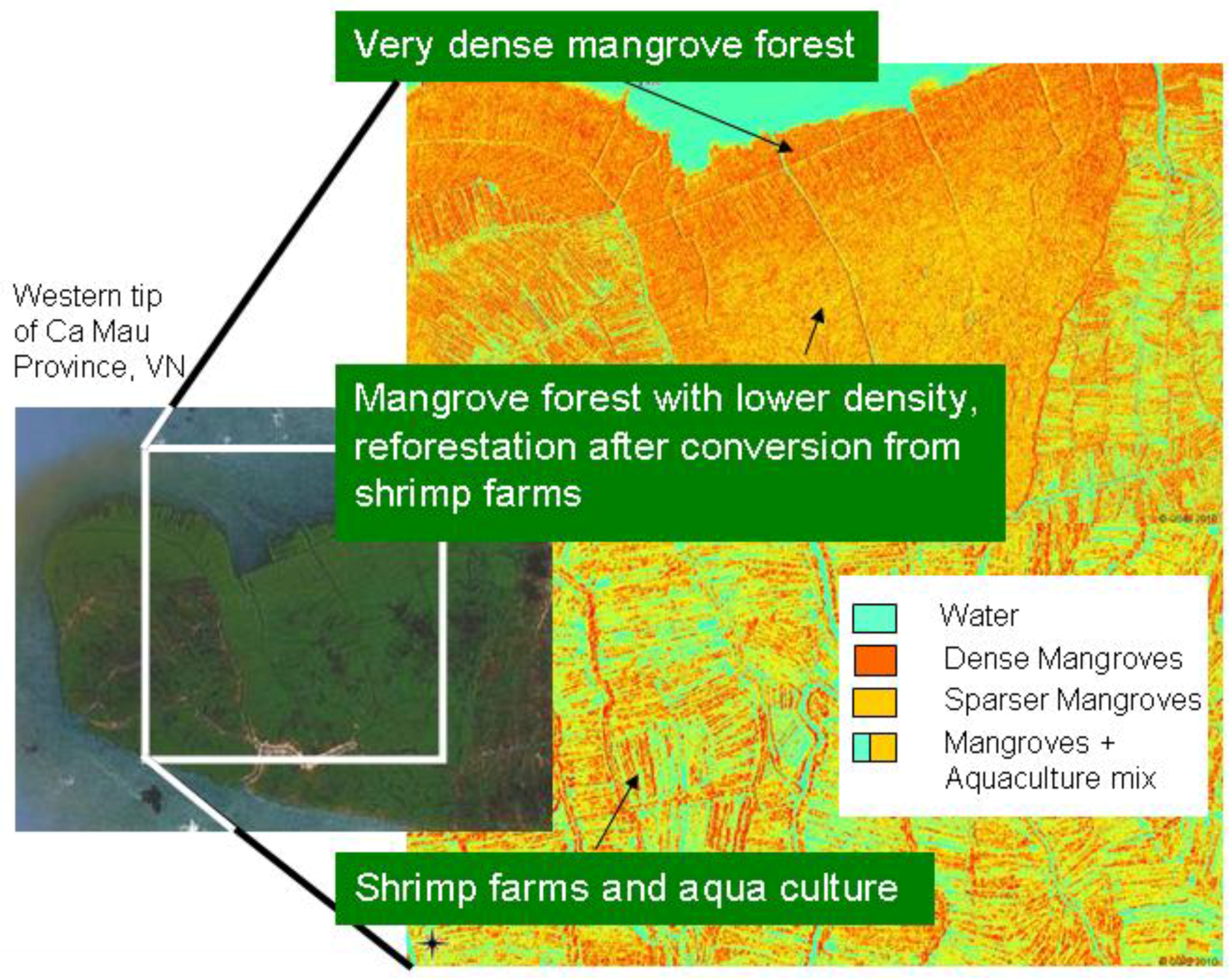
| 9. Other | Most promising results when SAR data combined with optical imagery (e.g., Figure 5) | Relatively few studies have been conducted; special software or modules are needed for radar-image processing |
Need for a Homogeneous Definition of the Term “Mangrove”
In remote-sensing literature, the term “mangrove” covers a broad range of meanings. For example, it is used to describe:
The mangrove ecosystem, with mangroves as leading plant families, but also containing other vegetation, open water surfaces, rivers, creeks, and open muddy soil surfaces;
An evergreen woody formation of shrubs or trees belonging to the mangrove family; or
A single mangrove tree
This lack of precision for the term “mangrove” was underscored >10 years ago by Blasco
et al. [
10], and it remains a current matter of discussion. Gaining a better understanding and comparison of international research studies through the use of an established definition for the term “mangrove” would be highly desirable.
Need for Homogenized Classification Schemes
Varying criteria were used to define the classification scheme in most of the studies. For example, in practice, this means that density growth can be classified as sparse or dense mangroves, as defined by the percentage of canopy closure. The percentages used for discrimination of different classes are usually chosen by the analysts individually, which leads to very inconsistent results that are very difficult to compare. It should be noted that the same classification scheme is applied consistently, at least for multitemporal mapping and monitoring tasks in the same area. However, depending on the knowledge of the analysts (physicists, geographers, environmentalists, biologists, local community experts) who perform the classification, completely different maps will result. Therefore, an urgent need exists for a uniform mangrove-classification scheme (or intersectable schemes) based on species differentiation, stand density, background surface, and additional criteria (e.g., biomass and vigor).
Need for Standardized Data-Processing Methods
The application of standardized techniques and analyses as routine tools is a future challenge for the consistent monitoring and mapping of mangroves [
18]. Standardization would enhance the comparison of the suitability of methods for certain purposes on different datasets and locations. A greater transparency with regard to the processing steps (and preprocessing steps, including georectification and atmospheric correction, as well as image transformations) would also be beneficial for the transferability of a specific method to another research site. The development of techniques for the assessment of changes in mangrove areas requires a standardization of methods for the application to time-series of datasets [
18], granting optimal comparability. However, the differences in environmental and atmospheric conditions and the high variability of mangrove ecosystems hinder the transferability of image-processing methods and analyses [
21,
137,
158].
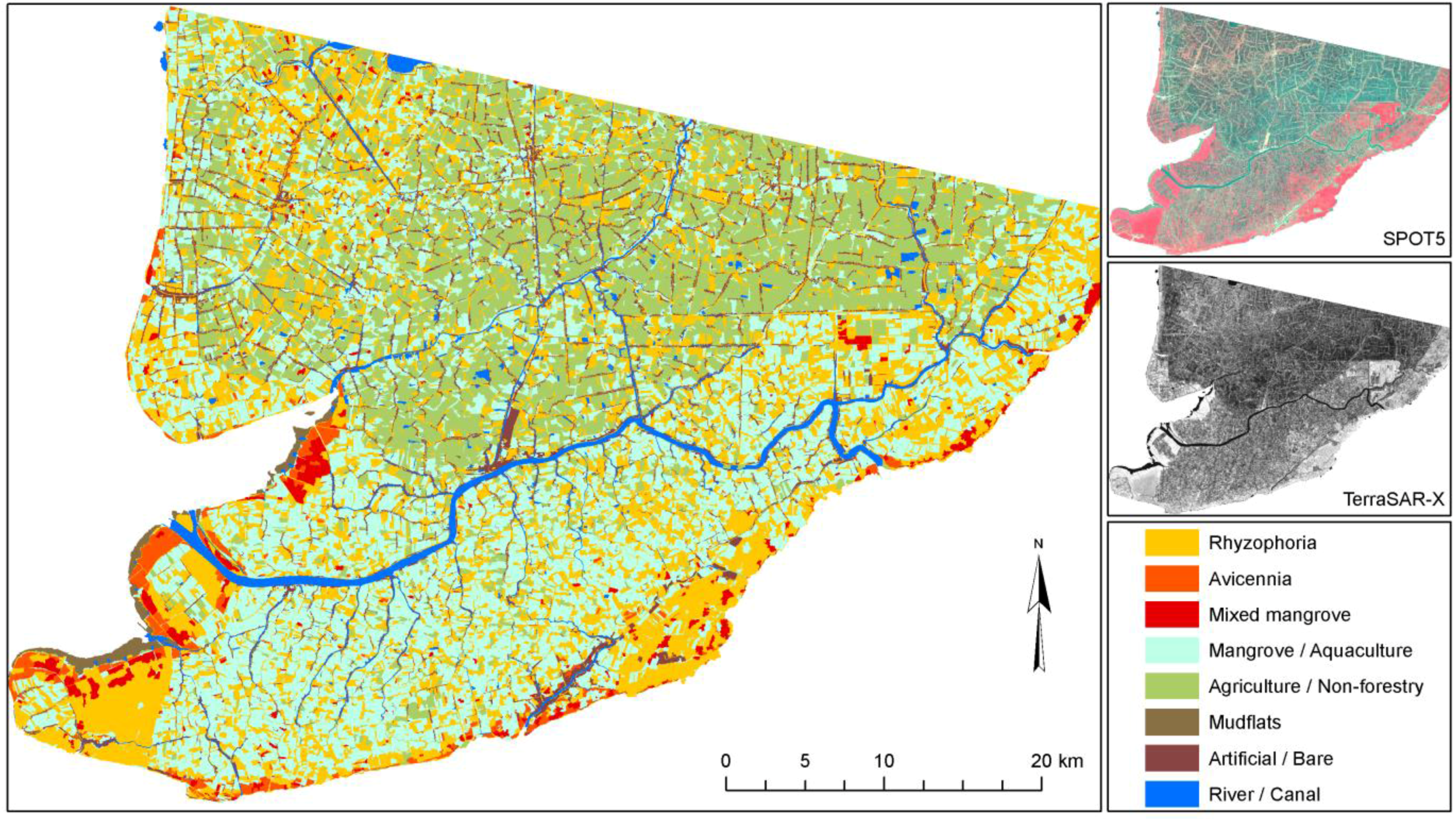
However, even in similar areas where transfer would theoretically be possible, the transferability and standardization of environmental projects are usually hindered by the lack of communication and homogenization efforts among different research groups. For example, three mangrove-mapping projects are ongoing in three coastal provinces (Ca Mau, Soc Trang, and Bac Lieu) of the Mekong Delta in Vietnam. All are being undertaken by the German Society for Technical Cooperation and have the goal to map current mangrove cover in the Mekong Delta. However, although one local project is simply digitizing the mangroves on screen, based on Google Earth QuickBird imagery (older data), another project is analyzing up-to-date SPOT scenes, and the third project is relying exclusively on field surveys by local untrained experts. The resulting mangrove maps created for the different provinces will be neither comparable nor complementary. Within the context of the current project RICEMAN, funded by the German Ministry of Education and Research, we aim to produce a homogenized mangrove map covering all mangrove areas of the Mekong Delta in Vietnam. For this purpose, SPOT data, Envisat ASAR data, TerraSAR-X data, and extensive field survey data are being used, and all data will be analyzed in a standardized repeatable way, with as little analyst influence as possible. The goal is an up-to-date Mekong Delta mangrove map representing the year 2009. The results of this mapping project (a first glimpse is
Figure 5) will be reported in another paper.
Need for Homogenized, Transparent Accuracy Assessment
Scientists and users all over the world have different goals and requirements for their investigations. In many studies, accuracy assessment was not performed or was not considered necessary. Furthermore, accuracy assessments can be carried out using different methods and relying on different quality measures [
18]. This is an additional factor that diminishes the comparability among studies. If management decisions depend on researchers’ results, an accuracy assessment is essential; otherwise, the findings could lead to inappropriate and cost-intensive actions for the user [
17]. As for a standardized classification scheme, as well as for accuracy assessment, it is of the utmost importance that all steps are well documented.
Need for Further Investigations on Synergetic Data Use
Much remote sensing data exist that are promising for remote-sensing research, but they have not been exploited (i.e., no publications are available). In our opinion, this especially includes the joint analyses of multispectral and radar data, such as combined analyses based on high-resolution TerraSAR-X and QuickBird data, combined analyses based on TerraSAR-X and Rapid Eye data, combined analyses based on TerraSAR-X and SPOT data (ongoing), and combined analyses of Envisat ASAR and ASTER data, to name a few. Additional datasets that will greatly improve mangrove-mapping activities in the future include the TerraSAR-Tandem DEM dataset at 1-m resolution, which is foreseen to be available from 2013 onward (the Tandem Mission [the second Terra-SAR-X] is already in orbit); spaceborne hyperspectral 200 band; 30-m EnMAP data expected from 2014 onward; and technologies currently moving from airborne to spaceborne platforms, such as LIDAR-based mapping.
Need for Ecosystem Service Assessment
Numerous investigators have attempted to assess the monetary value of mangrove ecosystems worldwide, by trying to relate the services and values of natural ecosystems to economic parameters (e.g., de Groot and colleagues [
159,
160], Pearce [
161], Turner
et al. [
162], Bingham
et al. [
163], Daily [
164], Costanza
et al. [
57], Limburg and Folke [
165], Wilson and Carpenter [
166], Daily
et al. [
167], and Lal [
179]). Recently, multinational gatherings, including the Convention on Biological Diversity, the Ramsar Convention on Wetlands and Migratory Species, and the Convention to Combat Desertification, have incorporated the concept of ecosystem services into their discussions and meetings. Also, major non-governmental organizations, including the Nature Conservancy, the World Wildlife Fund, and the World Resource Institute, have begun piloting ecosystem services programs, as have major intergovernmental agencies, including the United Nation Development Program and the World Bank [
168]. The total economic value of the different ecosystem functions of mangroves (
i.e., regulation functions, production functions, habitat functions, and information functions [
57,
160,
167]) have been assessed by numerous investigators, including Lal [
169], Ruitenbeck [
170], Barbier [
171], and Sathirathai [
172]. Sathirathai and Barbier [
173] concluded that the economic value for mangroves in a local community in Thailand ranges between US $27,264 and $35,921/ha. Especially in Thailand, it was demonstrated that the economic value estimation of an ecosystem in hard currency is much more eye opening to regional and national governments than is underscoring the threatening decline of a certain species. If governments learn to appreciate the ecosystem service functions and their real economic value, the willingness for protection (which costs money) usually increases [
55]. In Thailand, the demonstration that several tens of thousands of US dollars are lost with each degraded hectare of mangroves has led to drastic reforestation programs and protection measures. However, for science to meet such a “real-world demand,” it is of utmost importance that, at least within a country, mapping procedures are homogenized, transferable, well-documented, conducted with local trained staff, and relatively cost efficient.
Need for Interdisciplinary and Well-Networked Research Teams
A broad range of experts in the field of mangrove mapping exists globally. However, when reviewing the articles, it became obvious that the topic of mangrove mapping is basically addressed by investigators with two or three different research backgrounds: biologists and ecologists with excellent biologic/botanic knowledge, environmentalists with excellent local knowledge of the ecosystem setting and its role in the local community, and remote sensors/physicists with excellent data-processing knowledge. Obviously, all three groups can have sound expertise in the other related fields; however, some papers revealed that this is not always the case. Thus, the optimal mangrove ecosystem-research group should include biologists focusing on mangrove botany, local environmental experts (ideally living in the area of investigation and speaking the local language), sound physicists, geographers and remote sensors, and socio-economists who can the transfer the research results to local decision- and policy-makers and planners. Research groups within the field should focus on international exchange and cooperation, rather than on isolation and competition.
5. Conclusions
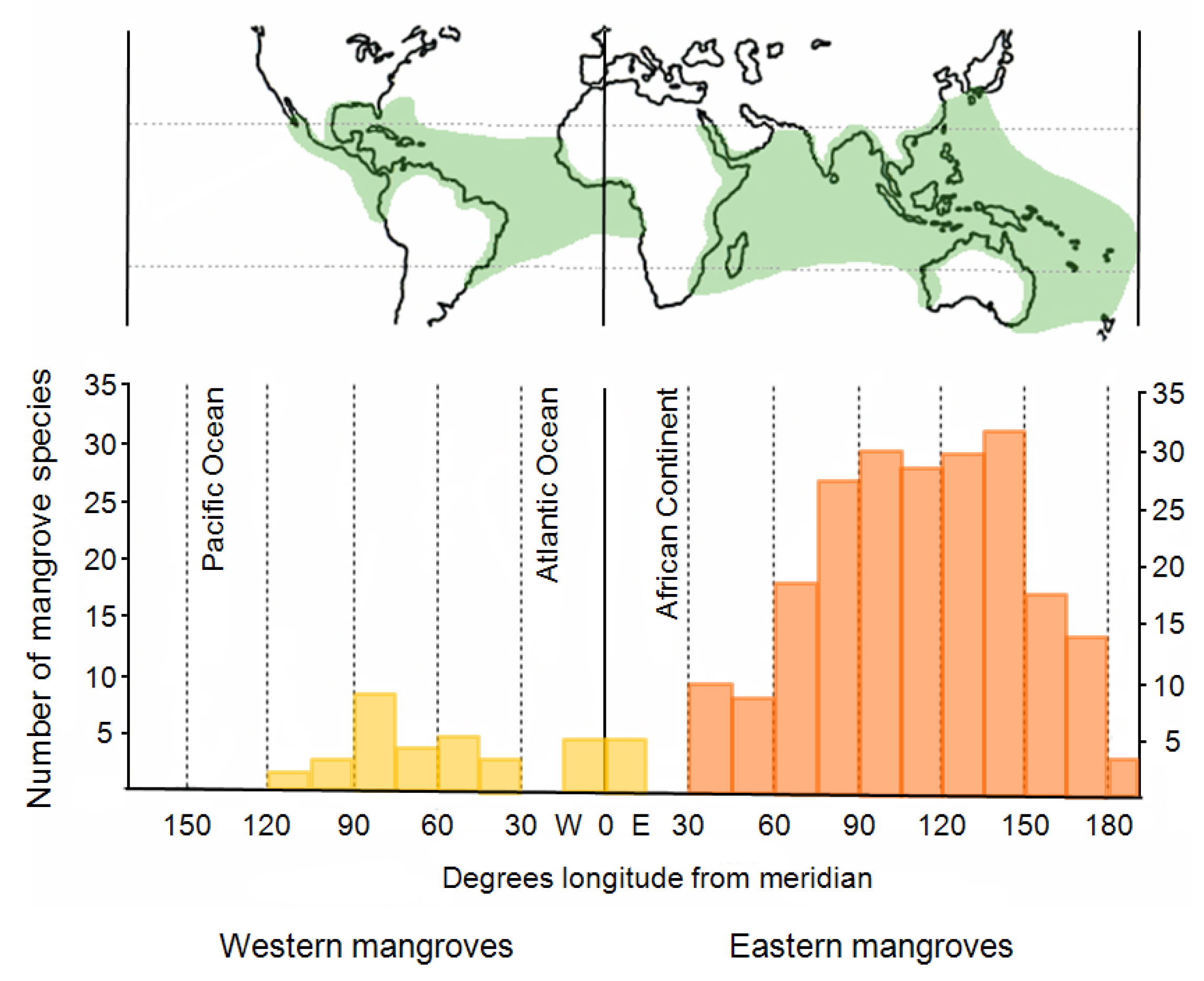
The aim of this review paper was to provide a comprehensive overview of remote-sensing–based mangrove-mapping studies undertaken during last two decades and including studies in different regions of the world using different sensor data, emphasizing different research foci, and using different image-processing methods. Well over 100 studies were published during the last two decades, all focusing on the remote-sensing–based mapping of mangroves; the number of studies reflects the growing scientific interest in the topic. The majority of studies were conducted in Asia (Bangladesh, India, Thailand, Vietnam, Sri Lanka, Taiwan, and Malaysia); followed by Australia (including New Zealand); North, Central, and South America (Florida, Texas, Mexico, Brazil, Panama, French Guiana, British West Indies, and Belize); and Africa (Gabon, Kenya, Tanzania, Senegal, and Madagascar).
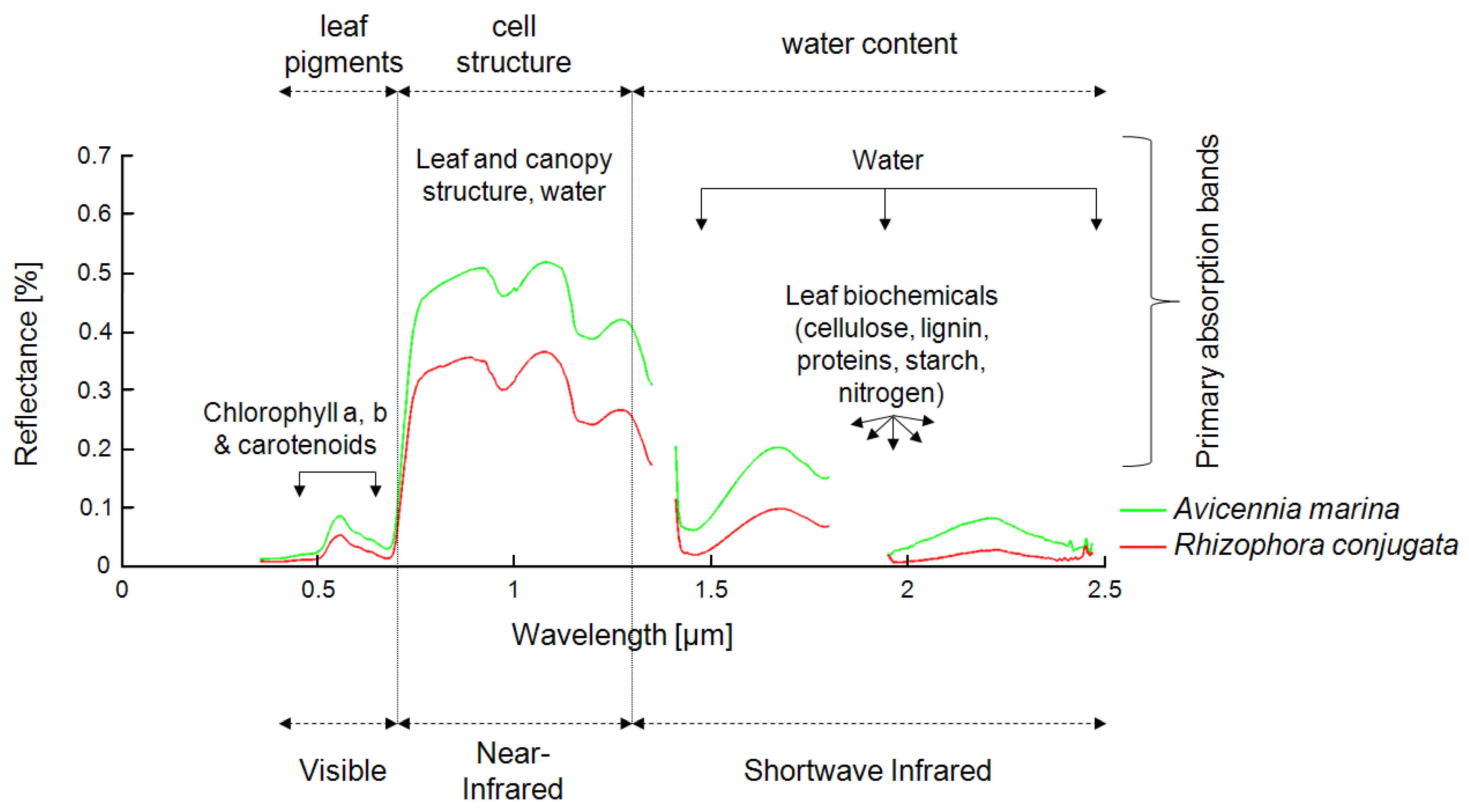
Mangrove mapping is one of the most demanding tasks in remote sensing, because the remotely sensed signal from mangrove ecosystems is composed of several components and is influenced by a large number of other parameters. In optical data, the spectrum of a pixel containing “mangrove” is usually influenced by pixel fractions of mangrove leaves, stems, and branches; underlying mudflats; soils; and water surfaces. All of these components differ depending on mangrove species, vigor, age, and season, as well as soil type and water turbidity and quality, among others. Other parameters influencing the spectral signal include plant and leaf geometry, LAI, stand density, and atmospheric conditions, to name a few. Furthermore, the spectral signal, its “mixing,” and its distinctiveness in optical data vary, depending on the spatial and spectral resolution of the sensors used, ranging from aerial photography (pixels in centimeter to meter range) to highest-resolution spaceborne multispectral data (pixel size in meter range) to multispectral data of medium resolution (10–30 m) to airborne or spaceborne hyperspectral data (pixel size in 1–30-m range, but up to 200 spectral bands).
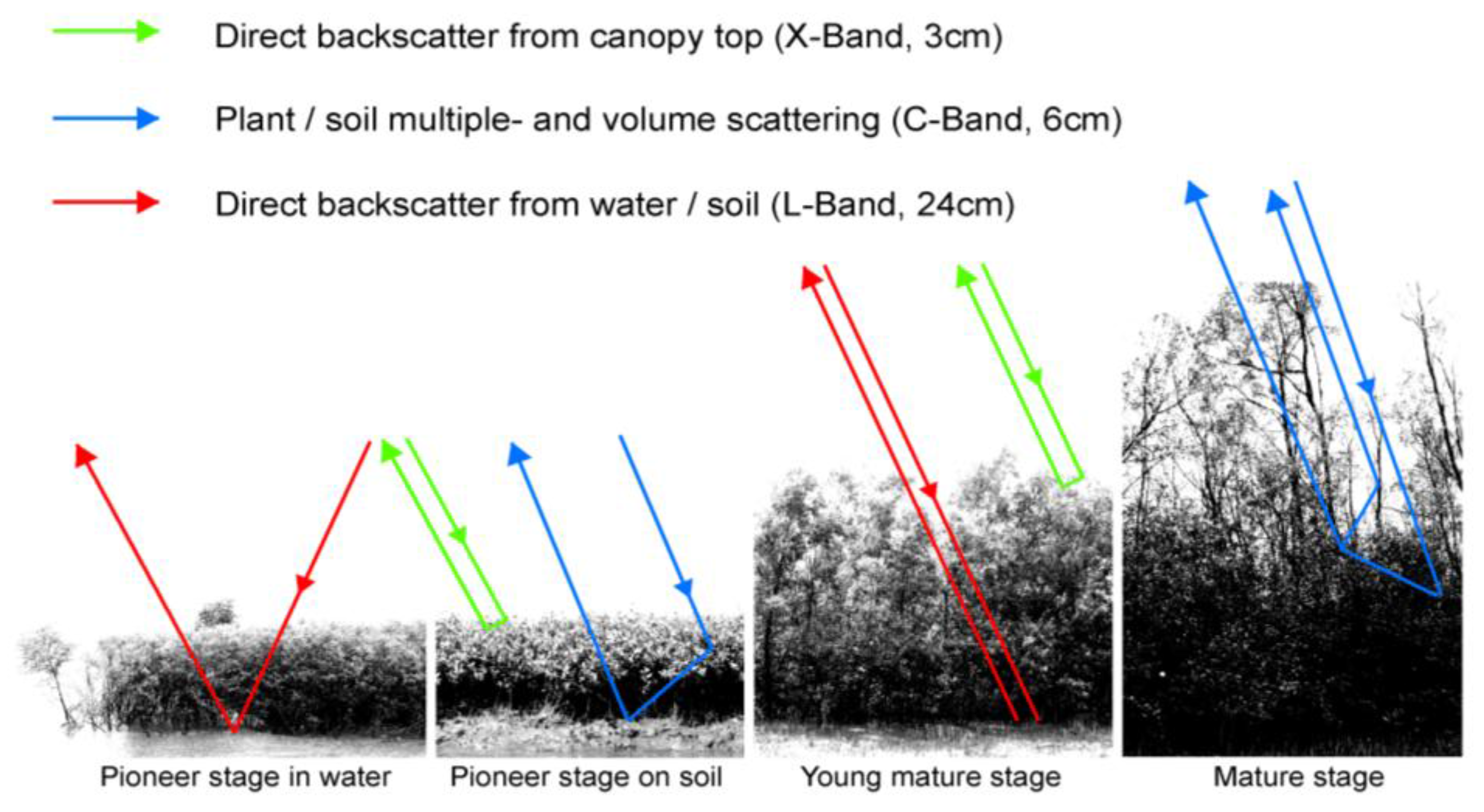
In SAR data, the backscatter signal of mangrove ecosystems is influenced by the geometric properties of the stand (canopy closure, canopy geometry, leaf structure, cell structure, stem structure, and the underlying surface component and its roughness: soil mudflats water) and dielectric properties, which vary, depending on the soil moisture, plant moisture, and underlying water surfaces. The responses of these different conditions vary, depending on the incidence wavelength (e.g., C-band, L-band, P-band), the polarization of the radar beams (HH, VV, HV, etc.), and the incidence angle of the radar waves, which makes the interpretation of radar data over mangrove ecosystems very complex. Furthermore, different sensors map different ground fractions, depending mainly on spatial resolution, varying from very high (airborne or TerraSAR-X [e.g., in the meter range]) to medium resolution (e.g., Envisat ASAR, ERS).
The data used for a mangrove-mapping campaign and the methods used for data analyses also depend on a variety of factors, such as goals and focus of the study (many purposes possible, such as mangrove versus non-mangrove mapping, species discrimination, stand vigor, density and age estimation, biomass retrieval, and change-detection studies, to name a few), size of the area to be mapped and available budget (defining the choice of sensor and the quantity of data), staff expertise needed for the mapping procedure (defining the complexity of image-analyses steps), mangrove-ecosystem accessibility (defining the amount of ground-truth data available), availability of additional GIS data on related ecosystem components (e.g., soil maps, in situ data), local labor prices (influencing the amount of manual interpretation and possible on-screen digitization), governmental restrictions (e.g., possibly hindering flight campaigns), and many more.
Thus, it is obvious that is it very difficult to compare the studies reviewed, because each research group faces different starting conditions. However, some similarities were found.
For >50 years, high spatial-resolution airborne data have been very valuable for mapping small coastal fringing areas of mangroves. Such images are usually classified by visual interpretation using on-screen digitization. The typical image information consists of tonality, surface texture, and structural arrangement. Species communities with dissimilar density and age structures can be easily differentiated; as a result of the small spatial coverage, aerial photography often plays a minor part in remote-sensing applications aiming at national or regional investigations. However, aerial survey is still the first choice for local-mapping campaigns. Aerial data is the only data source allowing time-series observation back to the 1950s and, compared with high-resolution spaceborne data, has the advantage that it can be acquired below the cloud cover, which often prevails in subtropical and tropical mangrove regions.
More than 40 published papers underscore the importance of medium-resolution imagery for mangrove-habitat mapping. Landsat TM and SPOT data have been used extensively, but Landsat MSS, ETM+, IRS, and ASTER data have also been analyzed. Medium-resolution imagery is best suited for applications on a national or regional scale. Visual interpretation followed by on-screen digitizing, as well as pixel-based classification approaches, are the most frequently applied methods. The Maximum Likelihood Classification algorithm has proven to be a particularly useful and robust classifier. Some investigators used hybrid-classification techniques combining pixel- and object-based approaches. This demonstrates that even at this (lower-resolution) level, mangrove-ecosystem mapping is a highly interactive, analyst-biased task. Medium-resolution techniques are excellent for the mapping of ecosystems (however, usually not at the species level), the monitoring of large-scale changes, the analyses of regional environmental relationships, and the assessment of the condition of mangroves (vigor, age, density, etc.). Global mangrove loss numbers have been derived solely from the analysis of medium-resolution data.
The highest-resolution sensors, such as IKONOS and QuickBird, offer the ability cover larger areas at high spatial resolution < 4 m, which is especially suitable for local mapping applications. Results from the few investigations performed to date showed that pixel-based, object-based, and neural network analytical approaches alone, as well as in combination, seem to be promising methods for diverse purposes. A strong degree of interactive, analyst-biased interpretation remains, which usually hinders the temporal and spatial transferability of results. In contrast to medium-resolution imagery, an increase in the level of detail, which can be discriminated, can be observed, and mapping approaches at the species level are feasible. Nevertheless, the suitability and limitations of image analyses based on these sensors (method wise and with respect to cost-benefit analyses) must be investigated more intensively to fill the current knowledge gap. Furthermore, new high-resolution sensors, such as the commercial GeoEye-1 (launched in 2008 with 0.41-m spatial resolution in the panchromatic band and 1.65-m resolution in the multispectral channels), need to be investigated. Based on these data, pilot studies have been undertaken in Belize, but the results have not been published. Also, data from the sensor Rapid Eye (5-m spatial resolution) should be exploited for mangrove mapping. The good spectral resolution and the frequent overpass of this new sensor make it an ideal tool that will probably enable analyses down to the species level. Last, but not least, most of the above-mentioned optical sensors (airborne and medium and high resolution) also have the potential for canopy DEM generation based on stereo data, but this has not been exploited fully. However, the soon-to-be-available TerraSAR Tandem DEM at 1-m resolution will be an invaluable asset for canopy investigations.
Studies based on hyperspectral imagery are rare, although the large number of bands enable very detailed mapping tasks, down to species discrimination and plant vigor assessment. Although the reported results (mainly derived based from airborne hyperspectral data) look very promising, it is difficult to evaluate the applied processing techniques, such as SAM, spectral unmixing, MTMF, and additional pixel- and object-based approaches, because of the limited availability of comparable studies. In general, hyperspectral flight campaigns are relatively expensive, and the analytical skills of image-processing personnel need to be profound. Thus, airborne hyperspectral analyses are only applied locally and are not an option for national or regional mapping endeavors or mapping campaigns with a limited budget. It is foreseen that upcoming spaceborne hyperspectral sensors will improve this situation.
About 25 publications have addressed the usefulness of SAR imagery for the mapping of mangroves. Several investigators carried out important fundamental research work to assess the relationship between the backscatter signals and mangrove structure components and stand parameters. These findings are important because the interpretation of microwave-derived vegetation information is much more complex to interpret than is that based on the visual-reflectance spectrum. SAR data are available at different resolutions (from airborne at meter scale to medium-resolution scale), and they are usually easily acquirable for a low cost (e.g., research proposals for Envisat ASAR data, ALOS Palsar data, or even TerraSAR-X data). However, trained image analysts and special software are required to exploit the full potential of these data. The benefit of weather-independent SAR data for mapping mangrove ecosystems is greatest when jointly analyzed with optical data.
For all of the above data sources, detailed in situ knowledge and field data are usually needed to correlate spectral signals or backscatter signals with geophysical parameters. Without any ground knowledge, it is not possible to differentiate mangroves at the species level or to derive quantitative parameters of the stand.
The biggest challenges for mangrove remote sensing lie in the still too-high degree of interactivity when analyzing data, which makes the comparison, as well as the temporal and spatial transferability, of study results nearly impossible. First, the mangrove remote-sensing community needs a common, well-defined understanding of the term “mangrove,” which is used to refer to an ecosystem, mangrove-related plant species in general, or single trees. Second, there is a high demand for homogenized classification schemes and standardized data-processing approaches (at least on a national level), which are indispensable if aiming at strongly needed ecosystem service (and economic ecosystem function) evaluation. These must be accompanied by thorough documentation and comparable, standardized accuracy assessment. Third, the exploitation of new sensors and new synergistic approaches will enable further in-depth mapping and allow a deeper understanding of the complex interactions between mangrove ecosystems and electromagnetic radiation. Finally, a strong research community, focusing on cooperation, exchange, and mutual support, can result in a more rapid advancement of the field. The goal of all remote-sensing–based mangrove mapping and monitoring activities should be the protection of these unique ecosystems, whose value cannot be overestimated. This is especially true with respect to climate change-related sea level rise scenarios globally. Sea level rise would have a severe impact on coastal communities in the tropics and subtropics. Natural mangrove ecosystems are a productive, extremely valuable shield against this threat.