A New Fluorescence Quantum Yield Efficiency Retrieval Method to Simulate Chlorophyll Fluorescence under Natural Conditions
Abstract
1. Introduction
2. Methodology
2.1. Study Area
2.2. Data Acquisition
- (1)
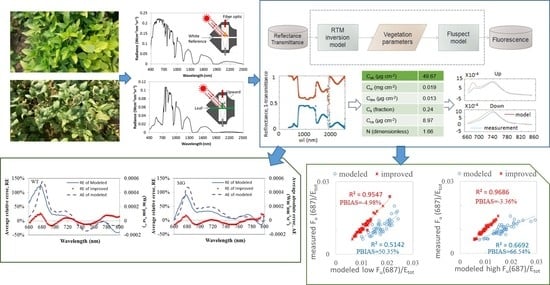
- The unfiltered radiance (Figure 2A1) is the radiance reflected by a standard white reference board; the reference board can reflect the total light without absorbing energy. The unfiltered radiance is the total incident light.
- (2)
- The filtered (cut off at ~650 nm) radiance (Figure 2A2) is the light that is cut off from the incident light between 650 nm and 800 nm.
- (3)
- Leaf up (Figure 2B1) is the radiance from the sun on the ventral side of the leaf (adaxial side up). The fiber optics focus on the top first and contain the reflectance and emissions of the leaf.
- (4)
- Leaf up with filter (Figure 2B2) is the radiance from the leaf in the upward direction, with filtered upwelling radiance. From 650 nm to 800 nm, this radiance only includes the emissions from the leaf; in other words, the incident light in the filter wavelength is cut off.
- (5)
- Leaf down (Figure 2C1) is the radiance in the downward direction. It contains the transmittance of light and the emissions from the leaf.
- (6)
- Leaf down with filter (Figure 2C2) is the radiance in the downward direction with filtered downwelling radiance (dorsal side of the leaf), and the fiber optics are focused at the bottom. The transmittance from the incident light is cut off by the filter from 650 nm to 800 nm.
- (7)
- The unfiltered radiance is the same as in Figure 2A1. It is used to ensure that the characteristics of sunlight do not change during the measurement.
- (8)
- The filtered radiance has the same meaning as that in Figure 2A2.
2.3. Fluspect-B Model
2.3.1. Pre-Processing
2.3.2. Model Generation
2.4. New FQE Retrieval Method
2.5. Model Performance Evaluation
3. Results
3.1. Simulation Results by the Fluspect Model with/without the FQE Inversion Code
3.2. Validation of the FQE Retrieval Method
3.3. FQE Parameter Comparison
3.4. Absolute and Relative Errors for All Wavelength
3.5. Relative Error at Peak Value
4. Discussion
4.1. Measurement Considerations
4.2. Efficiency of the Photosystems
5. Conclusions
Author Contributions
Funding
Data Availability
Acknowledgments
Conflicts of Interest
References
- Krause, G.H.; Weis, E. Chlorophyll Fluorescence and Photosynthesis: The Basics. Annu. Rev. Plant Physiol. 1991, 42, 313–349. [Google Scholar] [CrossRef]
- Romero, J.M.; Cordon, G.B.; Lagorio, M.G. Modeling re-absorption of fluorescence from leaf to the canopy level. Remote Sens. Environ. 2017, 204, 138–146. [Google Scholar] [CrossRef]
- Frankenberg, C.; Fisher, J.B.; Worden, J.; Badgley, G.; Saatchi, S.S.; Lee, J.E.; Toon, G.C.; Butz, A.; Jung, M.; Kuze, A.; et al. New global observations of the terrestrial carbon cycle from GOSAT: Patterns of plant fluorescence with gross primary productivity. Geophys. Res. Lett. 2011, 38, 351–365. [Google Scholar] [CrossRef]
- Wagle, P.; Zhang, Y.G.; Jin, C.; Xiao, X.M. Comparison of solar-induced chlorophyll fluorescence, light-use efficiency, and process-based GPP models in maize. Ecol. Appl. 2016, 26, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Frankenberg, C.; Wood, J.D.; Schimel, D.S.; Jung, M.; Guanter, L.; Drewry, D.T.; Verma, M.; Porcar-Castell, A.; Griffis, T.J.; et al. OCO-2 advances photosynthesis observation from space via solar-induced chlorophyll fluorescence. Science 2017, 358, 6360. [Google Scholar] [CrossRef]
- Méthy, M.; Olioso, A.; Trabaud, L. Chlorophyll fluorescence as a tool for management of plant resources. Remote Sens. Environ. 1994, 47, 2–9. [Google Scholar] [CrossRef]
- Verhoef, A.; Allen, S.J. A SVAT scheme describing energy and CO2 fluxes for multi-component vegetation: Calibration and test for a Sahelian savannah. Ecol. Model. 2000, 127, 245–267. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Berni, J.A.J.; Suarez, L.; Sepulcre-Canto, G.; Morales, F.; Miller, J.R. Imaging chlorophyll fluorescence with an airborne narrow-band multispectral camera for vegetation stress detection. Remote Sens. Environ. 2009, 113, 1262–1275. [Google Scholar] [CrossRef]
- Ritchie, N.W.M. Efficient Simulation of Secondary Fluorescence via NIST DTSA-II Monte Carlo. Microsc. Microanal. 2017, 23, 618–633. [Google Scholar] [CrossRef]
- Sytarab, O.; Zivcaka, M.; Neugartcd, S.; Brestica, M. Assessment of hyperspectral indicators related to the content of phenolic compounds and multispectral fluorescence records in chicory leaves exposed to various light environments. Plant Physiol. Biochem. 2020, 154, 429–438. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C.; Rinderle, U.; Schmuck, G. Application of chlorophyll fluorescence in ecophysiology. Radiat. Environ. Bioph. 1986, 25, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Duysens, L.N.M.; Amesz, J.; Kamp, B.M. Two photochemical systems in photosynthesis. Nature 1961, 190, 510–511. [Google Scholar] [CrossRef] [PubMed]
- Fromme, P.; Mathis, P. Unraveling the photosystem I reaction center: A history, or the sum of many efforts. Photosynth. Res. 2004, 80, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N.; Yocum, C.F. Structure and function of photosystems I and II. Annu. Rev. Plant Biol. 2006, 57, 521–565. [Google Scholar] [CrossRef]
- Dubreuil, C.; Jin, X.; Barajas-López, J.; Hewitt, T.C.; Tanz, S.K.; Dobrenel, T.; Schroder, W.P.; Hanson, J.; Pesquet, E.; Gronlund, A.; et al. Establishment of Photosynthesis through Chloroplast Development Is Controlled by Two Distinct Regulatory Phases. Plant Physiol. 2018, 176, 1199–1214. [Google Scholar] [CrossRef]
- Lysenko, E.A.; Klausa, A.A.; Kartashova, A.V.; Kusnetsova, V.V. Specificity of Cd, Cu, and Fe effects on barley growth, metal contents in leaves and chloroplasts, and activities of photosystem I and photosystem II. Plant Physiol. Biochem. 2020, 147, 191–204. [Google Scholar] [CrossRef]
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 1986, 10, 51–62. [Google Scholar] [CrossRef]
- Harbinson, J.B.; Genty, N.R. Baker Relationship between the Quantum Efficiencies of Photosystems I and II in Pea Leaves. Plant Physiol. 1989, 90, 1029–1034. [Google Scholar] [CrossRef]
- Strand, D.D.; Kramer, D.M. Control of Non-Photochemical Exciton Quenching by the Proton Circuit of Photosynthesis. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria; Demmig-Adams, B., Garab, G., Adams, W., III, Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 387–408. [Google Scholar]
- Corcuera, L.; Notivol, E. Differences in photosynthetic activity might explain the large-scale shifts in pine recruitment in favour of oaks in continental Mediterranean climates. Forestry 2015, 88, 248–256. [Google Scholar] [CrossRef][Green Version]
- Tan, S.; Yang, Y.J.; Liu, T.; Zhang, S.B.; Huang, W. Responses of photosystem I compared with photosystem II to combination of heat stress and fluctuating light in tobacco leaves. Plant Sci. 2020, 292, 110371. [Google Scholar] [CrossRef]
- Magney, T.S.; Frankenberg, C.; Köhler, P.; North, G.; Davis, T.S.; Dold, C.; Dutta, D.; Fisher, J.B.; Grossmann, K.; Harrington, A.; et al. Disentangling changes in the spectral shape of chlorophyll fluorescence: Implications for remote sensing of photosynthesis. J. Geophys. Res. Biogeo. 2019, 124, 1491–1507. [Google Scholar] [CrossRef]
- Lork, W.; Fukshansky, L. The influence of chlorophyll fluorescence on the light gradients and the phytochrome state in a green model leaf under natural conditions. Plant Cell Environ. 1985, 8, 33–39. [Google Scholar] [CrossRef]
- Miller, J.R.; Berger, M.; Alonso, L.; Cerovic, Z.; Goulas, Y.; Jacquemoud, S.; Louis, J.; Mohammed, G.; Moya, I.; Pedros, R.; et al. Progress on the development of an integrated canopy fluorescence model. In Proceedings of the IEEE International Geoscience & Remote Sensing Symposium 1, Toulouse, France, 21–25 July 2003; pp. 601–603. [Google Scholar]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Porcar-Castell, A.; Tyystjärvi, E.; Atherton, J.; van der Tol, C.; Flexas, J.; Pfündel, E.E.; Moreno, J.; Frankenberg, C.; Berry, J.A. CORRIGENDUM: Linking chlorophyll a fluorescence to photosynthesis for remote sensing applications: Mechanisms and challenges. J. Exp. Bot. 2014, 65, 4065–4095. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Singh, H.; Raj, S.; Soni, V. Chlorophyll a fluorescence kinetics of mung bean (Vigna radiata L.) grown under artificial continuous light. Biochem. Biophys. Rep. 2020, 24, 100813. [Google Scholar] [CrossRef] [PubMed]
- Pedrós, R.; Goulas, Y.; Jacquemoud, S.; Louis, J.; Moya, I. FluorMODleaf: A new leaf fluorescence emission model based on the PROSPECT model. Remote Sens. Environ. 2010, 114, 155–167. [Google Scholar] [CrossRef]
- Van der Tol, C.; Verhoef, W.; Timmermans, J.; Verhoef, A.; Su, Z. An integrated model of soil-canopy spectral radiances, photosynthesis, fluorescence, temperature and energy balance. Biogeosciences 2009, 6, 3109–3129. [Google Scholar] [CrossRef]
- Vilfan, N.; Van der Tol, C.; Muller, O.; Rascher, U.; Verhoef, W. Fluspect-B: A model for leaf fluorescence, reflectance and transmittance spectra. Remote Sens. Environ. 2016, 186, 596–615. [Google Scholar] [CrossRef]
- Zhao, F.; Ni, Q. A Model to Simulate the Radiative Transfer of Fluorescence in a Leaf. ISPAr 2018, XLII-3, 2347–2351. [Google Scholar] [CrossRef]
- Kallel, A. FluLCVRT: Reflectance and fluorescence of leaf and canopy modeling based on Monte Carlo vector radiative transfer simulation. J. Quant. Spectrosc. Radiat. Transf. 2020, 253, 107183. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, S.H.; Yan, J.X.; Jiang, D.S.; Li, J.Z. Application of laser induced fluorescence technique on detection of pesticide leftover. Laser Infrared 2003, 33, 417–418. [Google Scholar]
- Gameiro, C.; Utkin, A.B.; Cartaxana, P.; Da Silva, J.M.; Matos, A.R. The use of laser induced chlorophyll fluorescence (LIF) as a fast and non-destructive method to investigate water deficit in Arabidopsis. Agr. Water Manag. 2016, 164, 127–136. [Google Scholar] [CrossRef]
- Takahashi, K.; Nakamura, T.; Emori, Y.; Kimura, A. Laser-Induced Fluorescence of In-Vivo Chlorophyll of a Rice Plant-a Technique for the Remote Detection of Plant Growth. Spectr. Signat. Objects Remote Sens. 1988, 287, 255–258. [Google Scholar]
- Zarco-Tejada, P.J.; Miller, J.R.; Mohammed, G.H.; Noland, T.L.; Sampson, P.H. Chlorophyll Fluorescence Effects on Vegetation Apparent Reflectance II. Laboratory and Airborne Canopy-Level Measurements with Hyperspectral Data. Remote Sens. Environ. 2000, 74, 596–608. [Google Scholar] [CrossRef]
- Pandey, J.K.; Gopal, R. Laser-Induced Chlorophyll Fluorescence: A Technique for Detection of Dimethoate Effect on Chlorophyll Content and Photosynthetic Activity of Wheat Plant. J. Fluoresc. 2011, 21, 785–791. [Google Scholar] [CrossRef]
- Rosema, A.; Zahn, H. Laser pulse energy requirements for remote sensing of chlorophyll fluorescence. Remote Sens. Environ. 1997, 62, 101–108. [Google Scholar] [CrossRef]
- Migliavacca, M.; Perez-Priego, O.; Rossini, M.; El-Madany, T.S.; Moreno, G.; van der Tol, C.; Rascher, U.; Berninger, A.; Bessenbacher, V.; Burkart, A.; et al. Plant functional traits and canopy structure control the relationship between photosynthetic CO2 uptake and far-red sun-induced fluorescence in a Mediterranean grassland under different nutrient availability. New Phytol. 2017, 214, 1078–1091. [Google Scholar] [CrossRef]
- Liu, X.J.; Guanter, L.; Liu, L.Y.; Damm, A.; Malenovský, Z.; Rascher, U.; Peng, D.L.; Du, S.S.; Gastellu-Etchegorry, J.P. Downscaling of solar-induced chlorophyll fluorescence from canopy level to photosystem level using a random forest model. Remote Sens. Environ. 2019, 231, 110772. [Google Scholar] [CrossRef]
- Bandopadhyay, S.; Rastogi, A.; Juszczak, R. Review of Top-of-Canopy Sun-Induced Fluorescence (SIF) Studies from Ground, UAV, Airborne to Spaceborne Observations. Sensors 2020, 20, 1144. [Google Scholar] [CrossRef]
- Hall, F.G.; Huemmrich, K.F.; Goetz, S.J.; Sellers, P.J.; Nickeson, J.E. Satellite remote sensing of surface energy balance: Success, failures, and unresolved issues in FIFE. J. Geophys. Res. Atmos 1992, 97, 19061. [Google Scholar] [CrossRef]
- Stuckens, J.; Verstraeten, W.W.; Delalieux, S.; Swennen, R.; Coppin, P. A dorsiventral leaf radiative transfer model: Development, validation and improved model inversion techniques. Remote Sens. Environ. 2009, 113, 2560–2573. [Google Scholar] [CrossRef]
- Vilfan, N.; Van der Tol, C.; Yang, P.Q.; Wyber, R.; Malenovky, Z.; Robinson, S.A.; Verhoef, W. Extending Fluspect to simulate xanthophyll driven leaf reflectance dynamics. Remote Sens. Environ. 2018, 211, 345–356. [Google Scholar] [CrossRef]
- Yang, P.Q.; Van der Tol, C. Linking canopy scattering of far-red sun-induced chlorophyll fluorescence with reflectance. Remote Sens. Environ. 2018, 209, 456–467. [Google Scholar] [CrossRef]
- Van der Tol, C.; Vilfan, N.; Dauwe, D.; Cendrero-Mateo, M.P.; Yang, P.Q. The scattering and re-absorption of red and near-infrared chlorophyll fluorescence in the models Fluspect and SCOPE. Remote Sens. Environ. 2019, 232, 111292. [Google Scholar] [CrossRef]
- Alonso, L.; Gomez-Chova, L.; Vila-Frances, J.; Amoros-Lopez, J.; Guanter, L.; Calpe, J.; Moreno, J. Sensitivity analysis of the Fraunhofer line discrimination method for the measurement of chlorophyll fluorescence using a field spectroradiometer. In Proceedings of the IEEE International Geoscience and Remote Sensing Symposium, Barcelona, Spain, 23–28 July 2007; pp. 3756–3759. [Google Scholar]
- Van Wittenberghe, S.; Alonso, L.; Verrelst, J.; Moreno, J.; Samson, R. Bidirectional sun-induced chlorophyll fluorescence emission is influenced by leaf structure and light scattering properties-A bottom-up approach. Remote Sens. Environ. 2015, 158, 169–179. [Google Scholar] [CrossRef]
- Zou, T.Y. Simulating the Fluorescence under Natural Conditions by Fluspect Model and Comparing Simulated Fluorescence Spectra to FluoWat Measurements. Master’s Thesis, University of Twente, Enschede, The Netherlands, 2016. [Google Scholar]
- Schickling, A.; Matveeva, M.; Damm, A.; Schween, J.; Wahner, A.; Graf, A.; Crewell, S.; Rascher, U. Combining Sun-Induced Chlorophyll Fluorescence and Photochemical Reflectance Index Improves Diurnal Modeling of Gross Primary Productivity. Remote Sens. 2016, 8, 574. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence-a practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Franck, F.; Juneau, P.; Popovic, R. Resolution of the photosystem I and photosystem II contributions to chlorophyll fluorescence of intact leaves at room temperature. Biochim. Biophys. Acta Bioenerg. 2002, 1556, 239–246. [Google Scholar] [CrossRef]











| Parameters | Explanation | Unit | Range | Default Value |
|---|---|---|---|---|
| Cab | Chlorophyll a + b content | [μg cm−2] | 0–100 | 40 |
| Cdm | Dry matter content | [g cm−2] | 0–0.5 | 0.012 |
| Cw | Water equivalent thickness, an indicator of leaf water content | [cm] | 0–0.4 | 0.009 |
| Cs | Senescent material | [-] | 0–0.6 | 0 |
| Cca | Carotenoid | [μg cm−2] | 0–30 | 5 |
| N | Leaf mesophyll structure parameter | [-] | 0–4 | 1.5 |
| FQE II | Fluorescence quantum efficiency for Photosystem II. | [-] | 0–0.2 | 0.01 |
| FQE I | Fluorescence quantum efficiency for Photosystem I. | [-] | 0–0.2 | 0.002 |
| Sample Setup | RMSE ×10−4 | R2 | Pearson | RMSE Improved × 10−1 | R2 Improved | Pearson Improved |
|---|---|---|---|---|---|---|
| HT Up-A | 1.73 | 0.774 | 0.881 | 0.73 | 0.949 | 0.974 |
| HT Up-B | 2.28 | 0.813 | 0.901 | 0.52 | 0.966 | 0.983 |
| HT Up-C | 1.63 | 0.821 | 0.906 | 0.86 | 0.969 | 0.985 |
| HT Up-D | 1.54 | 0.786 | 0.887 | 0.53 | 0.962 | 0.981 |
| HT Down-A | 0.99 | 0.870 | 0.933 | 0.65 | 0.962 | 0.981 |
| HT Down-B | 1.49 | 0.953 | 0.976 | 0.43 | 0.977 | 0.988 |
| HT Down-C | 0.85 | 0.975 | 0.987 | 0.85 | 0.982 | 0.991 |
| HT Down-D | 0.78 | 0.923 | 0.961 | 0.42 | 0.974 | 0.987 |
| HM Up-A | 2.49 | 0.813 | 0.902 | 0.36 | 0.969 | 0.985 |
| HM Up-B | 1.53 | 0.846 | 0.920 | 0.60 | 0.964 | 0.982 |
| HM Up-C | 1.79 | 0.827 | 0.909 | 0.48 | 0.960 | 0.980 |
| HM Up-D | 1.76 | 0.724 | 0.851 | 0.72 | 0.957 | 0.978 |
| HM Down-A | 1.68 | 0.943 | 0.971 | 0.28 | 0.975 | 0.987 |
| HM Down-B | 0.98 | 0.930 | 0.964 | 0.51 | 0.971 | 0.986 |
| HM Down-C | 1.40 | 0.938 | 0.969 | 0.38 | 0.973 | 0.986 |
| HM Down-D | 0.55 | 0.943 | 0.971 | 0.50 | 0.970 | 0.985 |
| LT Up-A | 3.00 | 0.922 | 0.960 | 0.31 | 0.980 | 0.990 |
| LT Up-B | 2.19 | 0.918 | 0.958 | 0.41 | 0.980 | 0.990 |
| LT Up-C | 1.88 | 0.740 | 0.860 | 1.00 | 0.904 | 0.946 |
| LT Up-D | 2.22 | 0.966 | 0.983 | 0.23 | 0.974 | 0.987 |
| LT Down-A | 2.81 | 0.868 | 0.932 | 0.34 | 0.967 | 0.983 |
| LT Down-B | 2.34 | 0.871 | 0.933 | 0.43 | 0.970 | 0.985 |
| LT Down-C | 2.41 | 0.942 | 0.971 | 0.98 | 0.966 | 0.983 |
| LT Down-D | 2.03 | 0.943 | 0.971 | 0.24 | 0.966 | 0.983 |
| LM Up-A | 1.56 | 0.934 | 0.966 | 0.47 | 0.979 | 0.990 |
| LM Up-B | 1.09 | 0.907 | 0.952 | 0.69 | 0.975 | 0.988 |
| LM Up-C | 1.23 | 0.872 | 0.934 | 0.85 | 0.971 | 0.986 |
| LM Up-D | 2.97 | 0.978 | 0.989 | 0.37 | 0.984 | 0.992 |
| LM Down-A | 1.79 | 0.893 | 0.945 | 0.49 | 0.971 | 0.985 |
| LM Down-B | 1.31 | 0.917 | 0.958 | 0.68 | 0.978 | 0.989 |
| LM Down-C | 1.78 | 0.855 | 0.925 | 0.82 | 0.973 | 0.987 |
| LM Down-D | 2.72 | 0.948 | 0.973 | 0.30 | 0.969 | 0.984 |
| Sample Setup | Cab (μg cm−2) | Cw (mg m−2) | Cdm (mg cm−2) | Cs | Cca (μg cm−2) | N | FQE I | FQE II |
|---|---|---|---|---|---|---|---|---|
| HT leaf A | 46.43 | 0.02 | 0.005 | 0.32 | 6.35 | 1.43 | 0.002 | 0.01 |
| (46.40) | (-) | (-) | (0.33) | (5.99) | (-) | (0.004) | (0.005) | |
| HT leaf B | 55.65 | 0.02 | 0.004 | 0.19 | 7.47 | 1.82 | 0.002 | 0.01 |
| (55.63) | (-) | (-) | (-) | (7.21) | (-) | (0.003) | (0.004) | |
| HT leaf C | 75.19 | 0.02 | 0.003 | 0.05 | 6.67 | 1.86 | 0.002 | 0.01 |
| (-) | (-) | (0.004) | (-) | (6.47) | (-) | (0.004) | (0.005) | |
| HT leaf D | 53.69 | 0.02 | 0.011 | 0.32 | 9.07 | 1.79 | 0.002 | 0.01 |
| (-) | (-) | (0.012) | (-) | (-) | (-) | (0.004) | (0.005) | |
| HM leaf A | 50.46 | 0.02 | 0.02 | 0.08 | 8.63 | 1.47 | 0.002 | 0.01 |
| (50.45) | (-) | (-) | (-) | (8.34) | (-) | (0.003) | (0.004) | |
| HM leaf B | 51.44 | 0.02 | 0.003 | 0.03 | 8.01 | 1.64 | 0.002 | 0.01 |
| (51.43) | (-) | (-) | (-) | (7.65) | (-) | (0.003) | (0.005) | |
| HM leaf C | 38.33 | 0.01 | 0.02 | 0.12 | 8.34 | 1.56 | 0.002 | 0.01 |
| (38.32) | (-) | (-) | (0.13) | (8.16) | (-) | (0.003) | (0.005) | |
| HM leaf D | 61.44 | 0.02 | 0.02 | 0.26 | 7.68 | 1.87 | 0.002 | 0.01 |
| (61.45) | (-) | (-) | (-) | (7.69) | (-) | (0.004) | (0.005) | |
| LT leaf A | 20.96 | 0.01 | 0.01 | 0 | 6.02 | 1.42 | 0.002 | 0.01 |
| (-) | (-) | (-) | (-) | (-) | (-) | (-) | (0.005) | |
| LT leaf B | 22.26 | 0.01 | 0.01 | 0.014 | 5.25 | 1.32 | 0.002 | 0.01 |
| (22.29) | (-) | (0.02) | (0.012) | (5.42) | (-) | (-) | (0.005) | |
| LT leaf C | 17.74 | 0.01 | 0.01 | 0 | 6.03 | 1.52 | 0.002 | 0.01 |
| (17.73) | (-) | (-) | (-) | (6.13) | (-) | (0.003) | (0.005) | |
| LT leaf D | 16.63 | 0.01 | 0.01 | 0 | 7.57 | 1.41 | 0.002 | 0.01 |
| (16.62) | (-) | (-) | (-) | (7.69) | (-) | (-) | (0.005) | |
| LM leaf A | 23.18 | 0.01 | 0.01 | 0.02 | 5.52 | 1.51 | 0.002 | 0.01 |
| (-) | (-) | (-) | (-) | (5.56) | (-) | (-) | (0.006) | |
| LM leaf B | 27.04 | 0.01 | 0.01 | 0.01 | 5.7 | 1.59 | 0.002 | 0.01 |
| (26.99) | (-) | (-) | (-) | (5.54) | (-) | (0.003) | (0.007) | |
| LM leaf C | 28.35 | 0.01 | 0.01 | 0.08 | 4.55 | 1.26 | 0.002 | 0.01 |
| (28.36) | (-) | (-) | (-) | (4.66) | (-) | (0.003) | (0.006) | |
| LM leaf D | 19.23 | 0.02 | 0.01 | 0.05 | 5.92 | 1.64 | 0.002 | 0.01 |
| (-) | (-) | (-) | (0.04) | (6.00) | (-) | (0.001) | (0.005) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, T.; Zhang, J. A New Fluorescence Quantum Yield Efficiency Retrieval Method to Simulate Chlorophyll Fluorescence under Natural Conditions. Remote Sens. 2020, 12, 4053. https://doi.org/10.3390/rs12244053
Zou T, Zhang J. A New Fluorescence Quantum Yield Efficiency Retrieval Method to Simulate Chlorophyll Fluorescence under Natural Conditions. Remote Sensing. 2020; 12(24):4053. https://doi.org/10.3390/rs12244053
Chicago/Turabian StyleZou, Tianyuan, and Jing Zhang. 2020. "A New Fluorescence Quantum Yield Efficiency Retrieval Method to Simulate Chlorophyll Fluorescence under Natural Conditions" Remote Sensing 12, no. 24: 4053. https://doi.org/10.3390/rs12244053
APA StyleZou, T., & Zhang, J. (2020). A New Fluorescence Quantum Yield Efficiency Retrieval Method to Simulate Chlorophyll Fluorescence under Natural Conditions. Remote Sensing, 12(24), 4053. https://doi.org/10.3390/rs12244053