Spatial Association of Shrubs and Their Interrelation to Burrowing Site Preference of Subterranean Rodents on Dune Slope in the Otindag Sandy Land, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Site
2.3. Data Collection
2.4. Data Analysis
2.4.1. Spatial Association of Shrubs
2.4.2. Burrowing Site Preference of Subterranean Rodents to Shrubs
3. Results
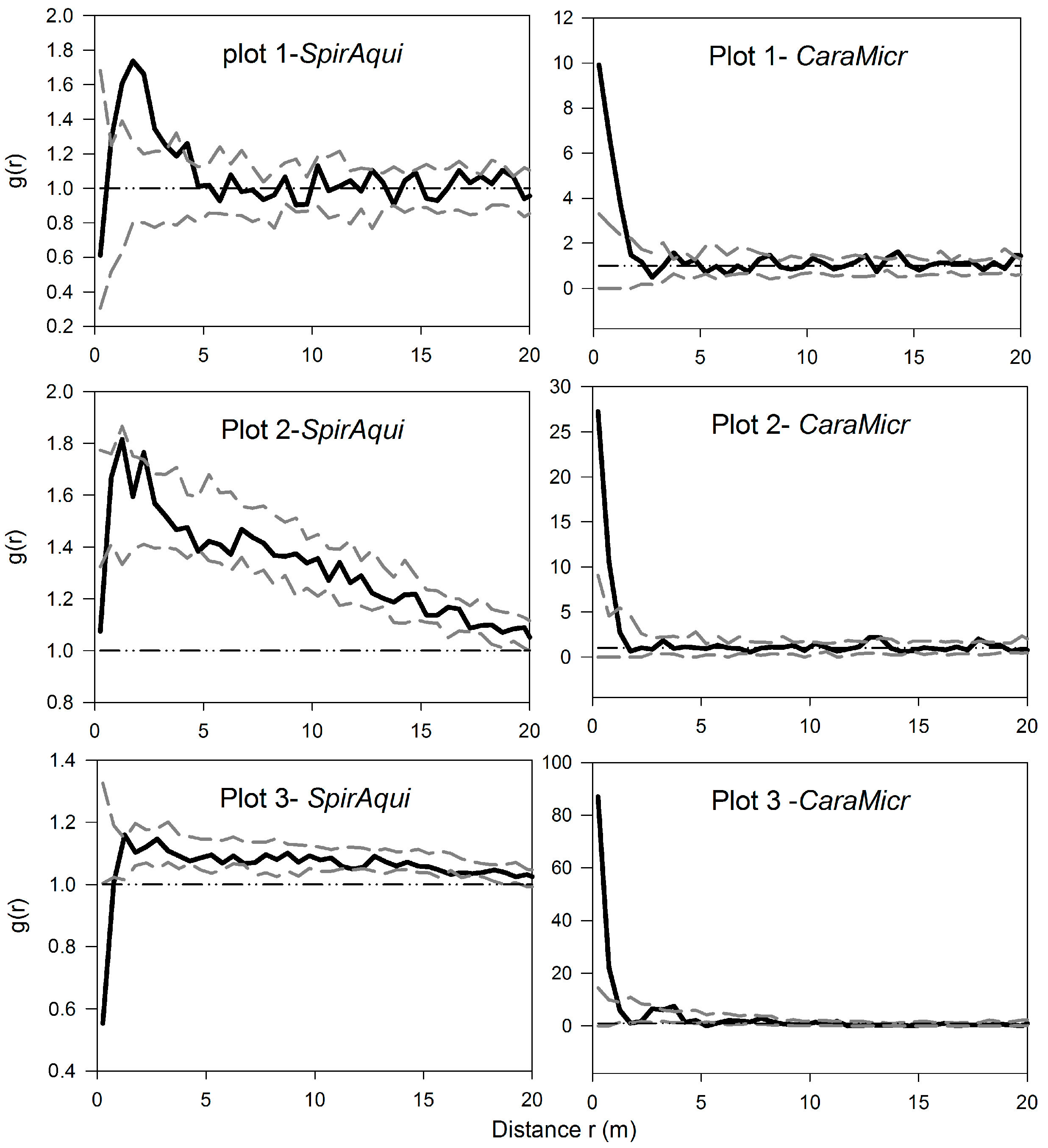
3.1. Univariate Analysis of Two Shrub Species
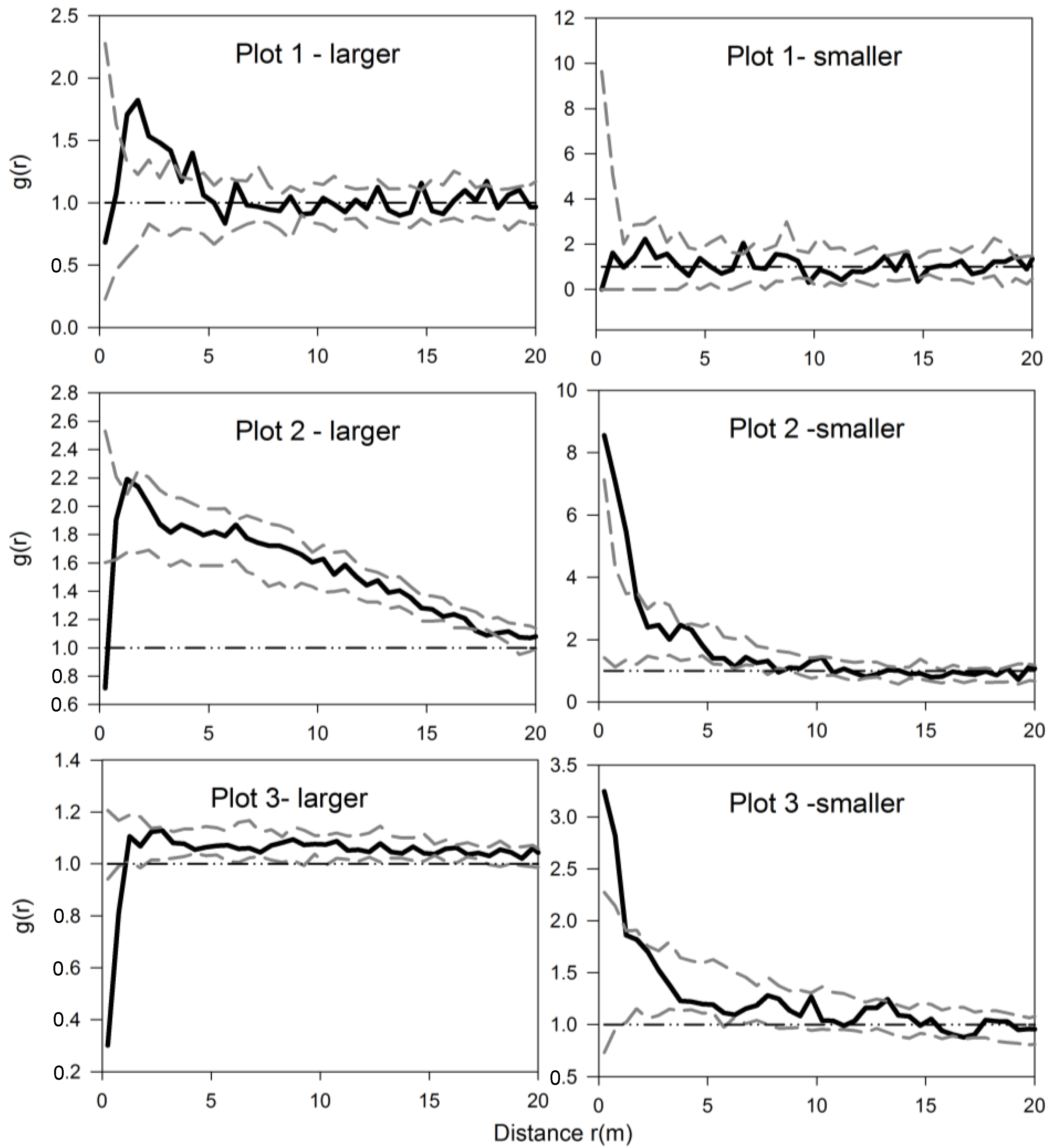
3.2. Univariate Analysis of S. aquilegifolia
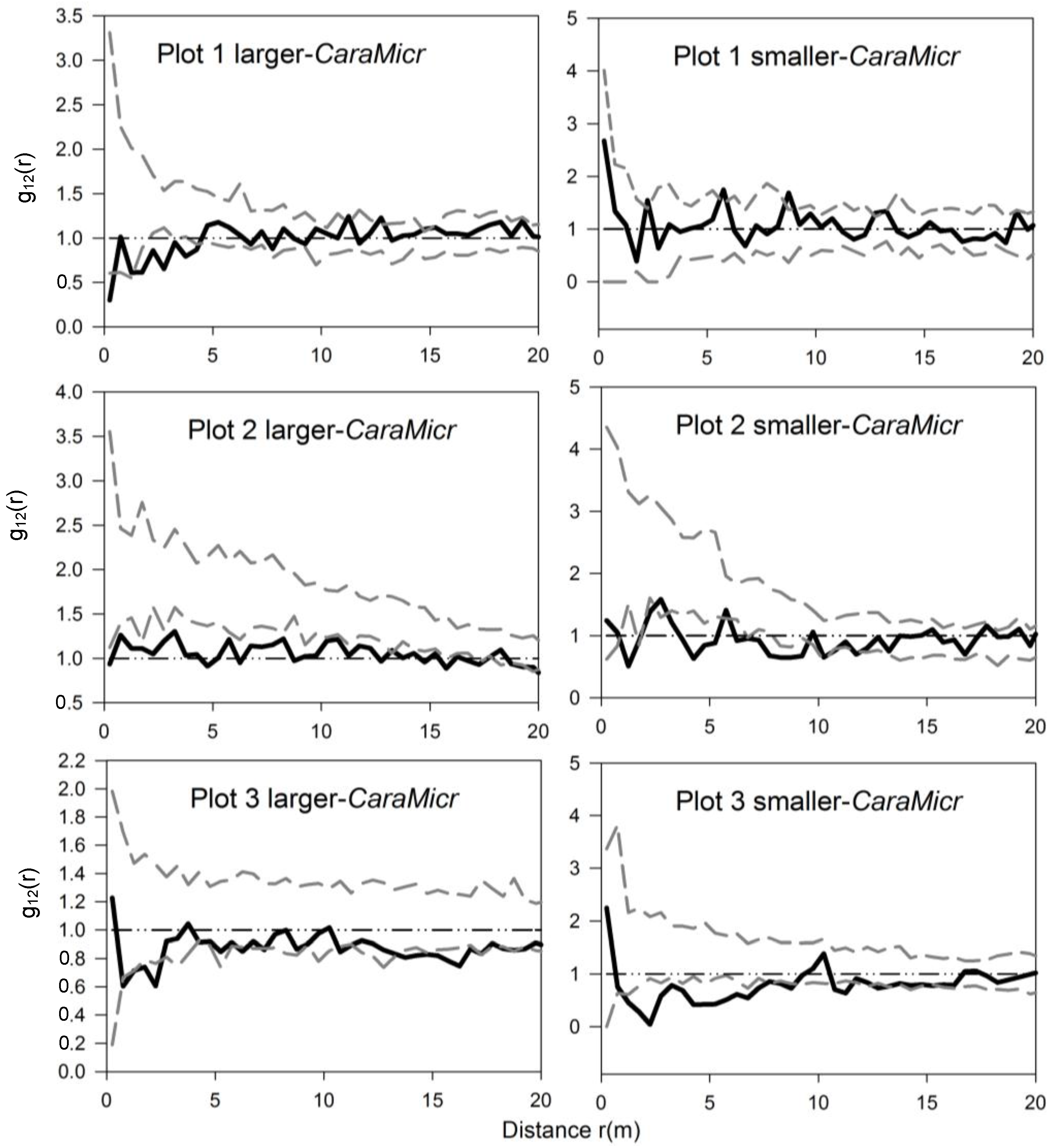
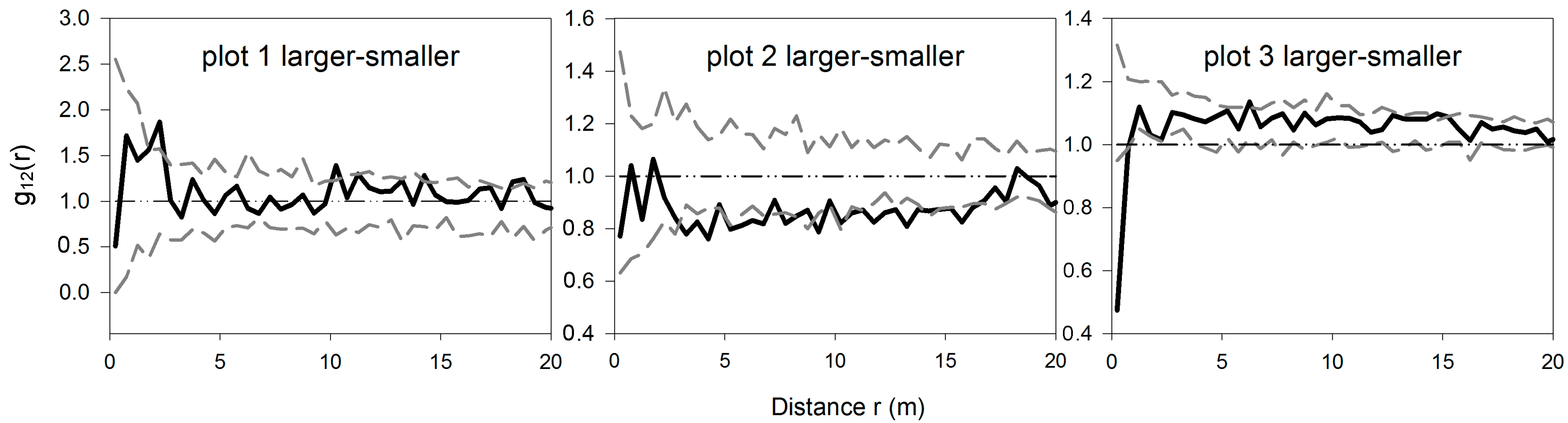
3.3. Bivariate Analysis of Intra- and Inter-Specific Shrub Distribution
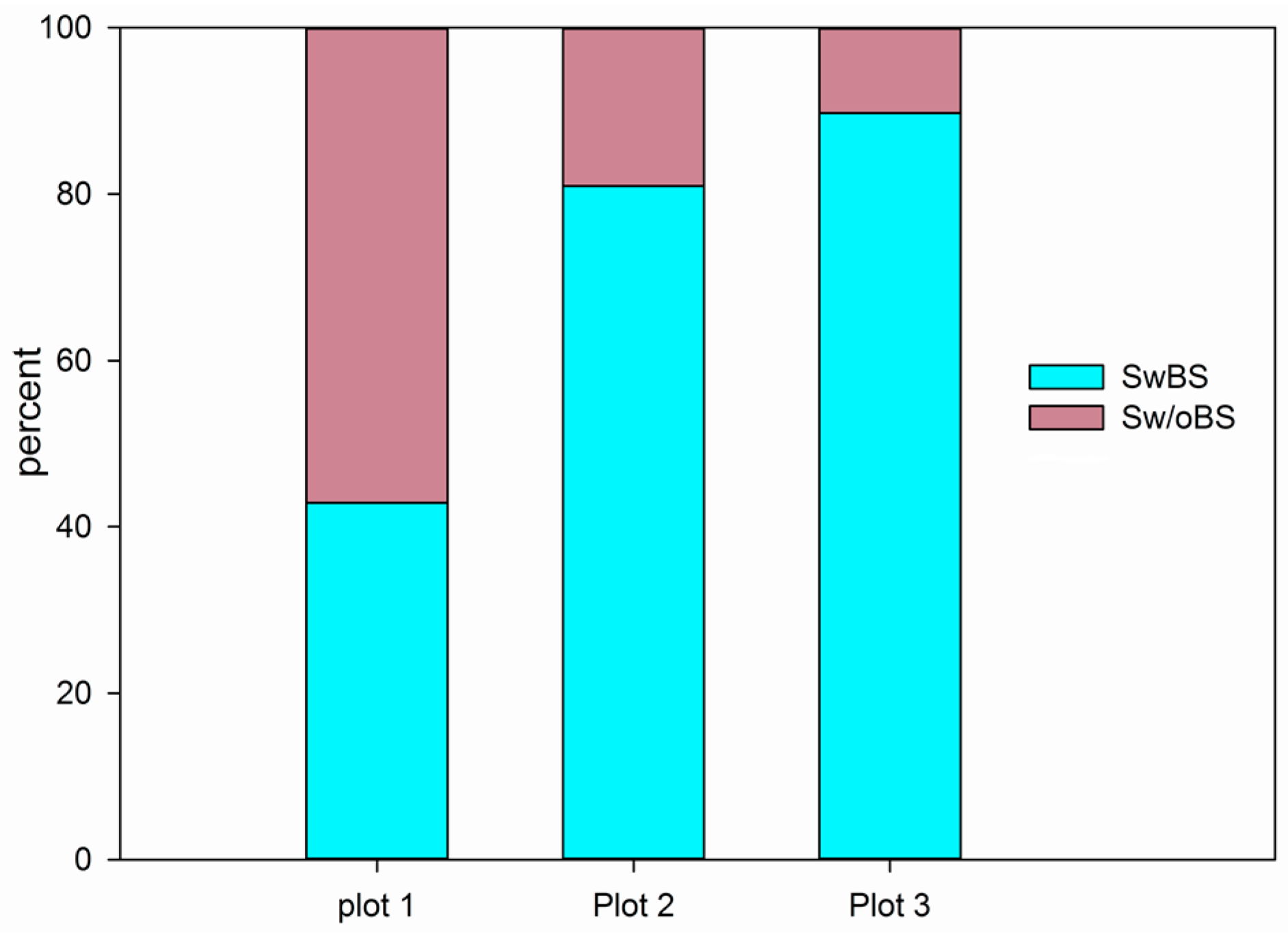
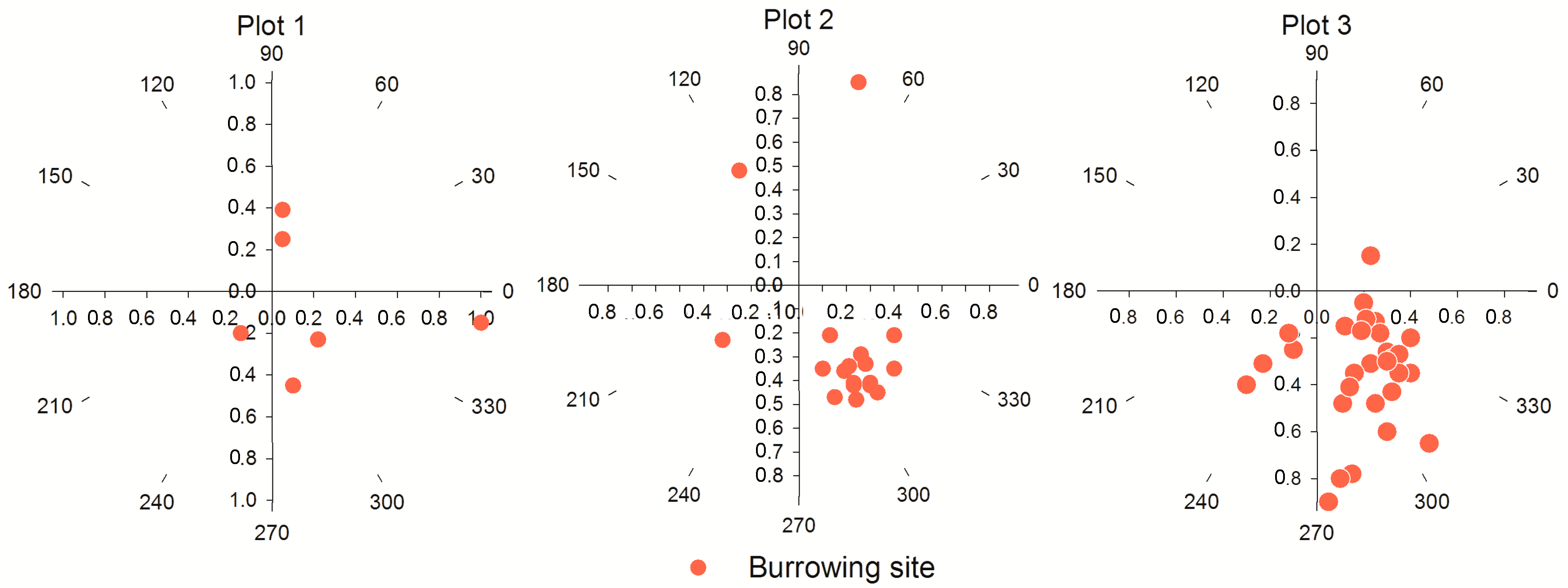
3.4. The Analysis of Burrowing Site Preference of Subterranean Rodents
4. Discussion
4.1. Alternative Stable State Formed by Spatial Self-Organization of Shrub Species
4.2. The Nurse–Protégé Interactions between Shrubs and Rodents
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Garner, W.; Steinberger, Y. A proposed mechanism for the formation of fertile islands in the desert ecosystem. J. Arid Environ. 1989, 16, 257–262. [Google Scholar]
- Li, X. Study on shrub community diversity of Ordos Plateau, Inner Mongolia, northern China. J. Arid Environ. 2001, 47, 271–279. [Google Scholar]
- Wang, Y.; Yang, X.; Shi, Z. The formation of the patterns of desert shrub communities on the western Ordos Plateau, China: The roles of seed dispersal and sand burial. PLoS ONE 2013, 8, e69970. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, W.H.; Reynolds, J.; Cunningham, G.L.; Huenneke, L.F.; Jarrell, W.M.; Virginia, R.A.; Whitford, W.G. Biological feedbacks in global desertification. Science 1990, 247, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Van Auken, O.W. Shrub invasions of North American semiarid grasslands. Annu. Rev. Ecol. Syst. 2000, 31, 197–215. [Google Scholar] [CrossRef]
- Jackson, R.B.; Banner, J.L.; Jobbágy, E.G.; Pockman, W.T.; Wall, D.H. Ecosystem carbon loss with woody plant invasion of grasslands. Nature 2002, 418, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Ravi, S.; D’Odorico, P.; Collins, S.L.; Huxman, T.E. Can biological invasions induce desertification? New Phytol. 2009, 181, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, Z.; Nippert, J.B.; Collins, S.L. Woody encroachment decreases diversity across North American grasslands and savannas. Ecology 2012, 93, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Sankey, J.B.; Ravi, S.; Wallace, C.S.; Webb, R.H.; Huxman, T.E. Quantifying soil surface change in degraded drylands: Shrub encroachment and effects of fire and vegetation removal in a desert grassland. J. Geophys. Res. Biogeosci. 2012, 117. [Google Scholar] [CrossRef]
- Maestre, F.T.; Bowker, M.A.; Puche, M.D.; Hinojosa, M.B.; Martínez, I.; Palacios, P.G.; Castillo, A.P.; Soliveres, S.; Luzuriaga, A.L.; Sa´nchez, A.M.; et al. Shrub encroachment can reverse desertification in semi-arid Mediterranean grasslands. Ecol. Lett. 2009, 12, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, D.J.; Bowker, M.A.; Maestre, F.T.; Roger, E.; Reynolds, J.F.; Whitford, W.G. Impacts of shrub encroachment on ecosystem structure and functioning: Towards a global synthesis. Ecol. Lett. 2011, 14, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Maestre, F.T.; Puche, M.D.; Guerrero, C.; Escudero, A. Shrub encroachment does not reduce the activity of some soil enzymes in Mediterranean semiarid grasslands. Soil Biol. Biochem. 2011, 43, 1746–1749. [Google Scholar] [CrossRef]
- D’Odorico, P.; Okin, G.S.; Bestelmeyer, B.T. A synthetic review of feedbacks and drivers of shrub encroachment in arid grasslands. Ecohydrology 2012, 5, 520–530. [Google Scholar] [CrossRef]
- Van Auken, O.W.; Bush, J. Invasion of Woody Legumes; Springer: Berlin, Germany, 2013. [Google Scholar]
- Li, Y.; Chen, J.; Cui, J.; Zhao, X.; Zhang, T. Nutrient resorption in, C. microphylla along a chronosequence of plantations: Implications for desertified land restoration in North China. Ecol. Eng. 2013, 53, 299–305. [Google Scholar] [CrossRef]
- Peng, H.Y.; Li, X.Y.; Li, G.Y.; Zhang, Z.H.; Zhang, S.Y.; Liu, L.; Zhao, G.Q.; Jiang, Z.Y.; Ma, Y.J. Shrub encroachment with increasing anthropogenic disturbance in the semiarid Inner Mongolian grasslands of China. Catena 2013, 109, 39–48. [Google Scholar] [CrossRef]
- Hu, X.; Li, Z.C.; Li, X.Y.; Liu, Y. Influence of shrub encroachment on CT-measured soil macropore characteristics in the Inner Mongolia grassland of northern China. Soil Tillage Res. 2015, 150, 1–9. [Google Scholar] [CrossRef]
- Zheng, Y.; Xie, Z.; Robert, C.; Jiang, L.; Shimizu, H. Did climate drive ecosystem change and induce desertification in Otindag sandy land, China over the past 40 years? J. Arid Environ. 2006, 64, 523–541. [Google Scholar] [CrossRef]
- Liu, S.; Wang, T. Aeolian desertification from the mid-1970s to 2005 in Otindag Sandy Land, Northern China. Environ. Geol. 2007, 51, 1057–1064. [Google Scholar] [CrossRef]
- Yang, X.; Ding, Z.; Fan, X.; Zhou, Z.; Ma, N. Processes and mechanisms of desertification in northern China during the last 30 years, with a special reference to the Hunshandake Sandy Land, eastern Inner Mongolia. Catena 2007, 71, 2–12. [Google Scholar] [CrossRef]
- Yang, X.; Scuderi, L.A.; Wang, X.; Scuderi, L.J.; Zhang, D.; Li, H.W.; Formane, S.; Xu, Q.H.; Wang, R.C.; Huang, W.; et al. Groundwater sapping as the cause of irreversible desertification of Hunshandake Sandy Lands, Inner Mongolia, northern China. Proc. Natl. Acad. Sci. USA 2015, 112, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Li, S.H.; Sun, J.; Xue, L. Environmental changes in Hunshandake (Otindag) sandy land revealed by optical dating and multi-proxy study of dune sands. J. Asian Earth Sci. 2013, 76, 30–36. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Liu, Z.; Li, H.; Ren, X.; Zhang, D.; Ma, Z.; Riouala, P.; Jina, X.; Scuderic, L. Initiation and variation of the dune fields in semi-arid China–with a special reference to the Hunshandake Sandy Land, Inner Mongolia. Quat. Sci. Rev. 2013, 78, 369–380. [Google Scholar] [CrossRef]
- Wu, X.; Li, P.; Jiang, C.; Liu, P.; He, J.; Hou, X.Y. Climate changes during the past 31 years and their contribution to the changes in the productivity of rangeland vegetation in the Inner Mongolian typical steppe. Rangel. J. 2014, 36, 519–526. [Google Scholar]
- Yang, T.; Li, P.; Wu, X.; Hou, X.; Liu, P.; Yao, G.Z. Assessment of vulnerability to climate change in the Inner Mongolia steppe at a county scale from 1980 to 2009. Rangel. J. 2014, 36, 545–555. [Google Scholar] [CrossRef]
- Ci, L.; Yang, X. Desertification and Its Control in China; Higher Education Press: Beijing, China; Springer: Berlin, Germany, 2010. [Google Scholar]
- Yang, L.; Wu, J. Knowledge-driven institutional change: An empirical study on combating desertification in northern china from 1949 to 2004. J. Environ. Manag. 2012, 110, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, G.A.; Squires, V.R. Combating Desertification in Asia, Africa and the Middle East; Springer: Berlin, Germany, 2013. [Google Scholar]
- Zhang, T.H.; Su, Y.Z.; Cui, J.Y.; Zhang, Z.H.; Chang, X.X. A leguminous shrub (C. microphylla) in semiarid sandy soils of north China. Pedosphere 2006, 16, 319–325. [Google Scholar] [CrossRef]
- Pasternak, D.; Schlissel, A. Combating Desertification with Plants; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Flores, J.; Jurado, E. Are nurse-protégé interactions more common among plants from arid environments? J. Veg. Sci. 2003, 14, 911–916. [Google Scholar] [CrossRef]
- Smit, C.; Ruifrok, J.L. From protege to nurse plant: Establishment of thorny shrubs in grazed temperate woodlands. J. Veg. Sci. 2011, 22, 377–386. [Google Scholar] [CrossRef]
- Zhao, H.L.; Zhou, R.L.; Su, Y.Z.; Zhang, H.; Zhao, L.Y.; Drake, S. Shrub facilitation of desert land restoration in the Horqin Sand Land of Inner Mongolia. Ecol. Eng. 2007, 31, 1–8. [Google Scholar] [CrossRef]
- Wolff, J.O.; Sherman, P.W. Rodent Societies: An Ecological and Evolutionary Perspective; University of Chicago Press: Chicago, IL, USA, 2008. [Google Scholar]
- Brown, J.H.; Fox, B.J.; Kelt, D.A. Assembly rules: Desert rodent communities are structured at scales from local to continental. Am. Nat. 2000, 156, 314–321. [Google Scholar] [CrossRef]
- Price, M.V.; Joyner, J.W. What resources are available to desert granivores: Seed rain or soil seed bank? Ecology 1997, 78, 764–773. [Google Scholar] [CrossRef]
- Brown, J.H.; Heske, E.J. Control of a desert-grassland transition by a keystone rodent guild. Science 1990, 250, 1705–1707. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Thompson, D.B.; Valone, T.J.; Brown, J.H. The effects of vertebrate granivores and folivores on plant community structure in the Chihuahuan Desert. Oikos 1995, 73, 251–259. [Google Scholar] [CrossRef]
- Montiel, S.; Montaña, C. Seed bank dynamics of the desert cactus Opuntia rastrera in two habitats from the Chihuahuan Desert. Plant Ecol. 2003, 166, 241–248. [Google Scholar] [CrossRef]
- Longland, W.S. Desert rodents reduce seedling recruitment of Salsola paulsenii. West. N. Am. Nat. 2007, 67, 378–383. [Google Scholar] [CrossRef]
- Davidson, A.; Lightfoot, D. Burrowing rodents increase landscape heterogeneity in a desert grassland. J. Arid Environ. 2008, 72, 1133–1145. [Google Scholar] [CrossRef]
- Eldridge, D.J.; Whitford, W.G. Disturbances by desert rodents are more strongly associated with spatial changes in soil texture than woody encroachment. Plant Soil 2014, 381, 395–404. [Google Scholar] [CrossRef]
- Price, M.V. The role of microhabitat in structuring desert rodent communities. Ecology 1978, 59, 910–921. [Google Scholar] [CrossRef]
- Abramsky, Z.; Rosenzweig, M.; Pinshow, B.; Brown, J.; Kotler, B.; Mitchell, W. Habitat selection: An experimental field test with two gerbil species. Ecology 1990, 71, 2358–2369. [Google Scholar] [CrossRef]
- Hughes, J.J.; Ward, D.; Perrin, M.R. Predation risk and competition affect habitat selection and activity of Namib Desert gerbils. Ecology 1994, 75, 1397–1405. [Google Scholar] [CrossRef]
- Ziv, Y.; Kotler, B.P.; Abramsky, Z.; Rosenzweig, M.L. Foraging efficiencies of competing rodents: Why do gerbils exhibit shared-preference habitat selection? Oikos 1995, 73, 260–268. [Google Scholar] [CrossRef]
- Shenbrot, G. Habitat selection in a seasonally variable environment: Test of the isodar theory with the fat sand rat, Psammomys obesus, in the Negev Desert, Israel. Oikos 2004, 106, 359–365. [Google Scholar] [CrossRef]
- Wu, R.; Chai, Q.; Zhang, J.; Zhong, M.; Liu, Y.; Wei, X.T.; Pan, D.; Shao, X.Q. Impacts of burrows and mounds formed by plateau rodents on plant species diversity on the Qinghai-Tibetan Plateau. Rangel. J. 2015, 37, 117–123. [Google Scholar] [CrossRef]
- Komonen, M.; Komonen, A.; Otgonsuren, A. Daurian pikas (Ochotona daurica) and grassland condition in eastern Mongolia. J. Zool. 2003, 259, 281–288. [Google Scholar] [CrossRef]
- Fang, J.; Wang, Z.; Tang, Z. Atlas of Woody Plants in China: Distribution and Climate; Springer Science & Business Media: Berlin, Germany, 2011. [Google Scholar]
- Wu, B.; Yang, H. Spatial patterns and natural recruitment of native shrubs in a semi-arid sandy land. PLoS ONE 2013, 8, e58331. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.T.; Xie, Y. Mammals of China; Princeton University Press: Princeton, NJ, USA, 2013. [Google Scholar]
- Wiegand, T.; Moloney, K.A. Rings, circles, and null-models for point pattern analysis in ecology. Oikos 2004, 104, 209–229. [Google Scholar] [CrossRef]
- Zhang, J.; Song, B.; Li, B.; Ye, J.; Wang, X.; Hao, Z. Spatial patterns and associations of six congeneric species in an old-growth temperate forest. Acta Oecol. 2010, 36, 29–38. [Google Scholar] [CrossRef]
- Condit, R.; Ashton, P.S.; Baker, P.; Bunyavejchewin, S.; Gunatilleke, S.; Hubbell, S.P.; Foster, R.B.; Itoh, A.; LaFrankie, J.V.; Lee, H.S. Spatial patterns in the distribution of tropical tree species. Science 2000, 288, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Stoyan, D.; Stoyan, H. Fractals, Random Shapes, and Point Fields: Methods of Geometrical Statistics; Wiley: Chichester, UK; Hoboken, NJ, USA, 1994. [Google Scholar]
- Illian, J.; Penttinen, A.; Stoyan, H.; Stoyan, D. Statistical Analysis and Modelling of Spatial Point Patterns; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Wiegand, T.; Moloney, K.A. Handbook of Spatial Point-Pattern Analysis in Ecology; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Fernández-Aláez, C.; Fernández-Aláez, M.; Garcáa-Criado, F. Spatial distribution pattern of the riparian vegetation in a basin in the NW Spain. Plant Ecol. 2005, 179, 31–42. [Google Scholar]
- Coop, J.D.; Massatti, R.T.; Schoettle, A.W. Subalpine vegetation pattern three decades afterstand-replacing fire: Effects of landscape context and topography on plant community composition, tree regeneration, and diversity. J. Veg. Sci. 2010, 21, 472–487. [Google Scholar] [CrossRef]
- Ostendorf, B.; Hilbert, D.W.; Hopkins, M.S. The effect of climate change on tropical rainforest vegetation pattern. Ecol. Model. 2001, 145, 211–224. [Google Scholar] [CrossRef]
- Yu, L.; Cao, M.; Li, K. Climate-induced changes in the vegetation pattern of China in the 21st century. Ecol. Res. 2006, 21, 912–919. [Google Scholar] [CrossRef]
- Liu, H.; Wang, L.; Yang, J.; Nakagoshi, N.; Liang, C.; Wang, W.; Lv, Y.M. Predictive modeling of the potential natural vegetation pattern in northeast China. Ecol. Res. 2009, 24, 1313–1321. [Google Scholar] [CrossRef]
- Levin, S.A. The problem of pattern and scale in ecology: The Robert, H. MacArthur award lecture. Ecology 1992, 73, 1943–1967. [Google Scholar]
- Kikuchi, T. Vegetation and Landforms; University of Tokyo Press: Tokyo, Japan, 2001. [Google Scholar]
- Yang, Y. Vegetation structure in relation to micro-landform in Tiantong National Forest Park, Zhejiang, China. Acta Ecol. Sin. 2005, 25, 2830–2840. (In Chinese) [Google Scholar]
- Whittaker, R.H.; Niering, W.A. Vegetation of the Santa Catalina Mountains, Arizona. V. Biomass, production, and diversity along the elevation gradient. Ecology 1975, 56, 771–790. [Google Scholar]
- Reed, R.A.; Peet, R.K.; Palmer, M.W.; White, P.S. Scale dependence of vegetation-environment correlations: A case study of a North Carolina piedmont woodland. J. Veg. Sci. 1993, 4, 329–340. [Google Scholar] [CrossRef]
- Sakai, A.; Ohsawa, M. Topographical pattern of the forest vegetation on a river basin in a warm-temperate hilly region, central Japan. Ecol. Res. 1994, 9, 269–280. [Google Scholar] [CrossRef]
- Hara, M.; Hirata, K.; Fujihara, M.; Oono, K. Vegetation structure in relation to micro-landform in an evergreen broad-leaved forest on Amami Ohshima Island, south-west Japan. Ecol. Res. 1996, 11, 325–337. [Google Scholar] [CrossRef]
- Nagamatsu, D.; Hirabuki, Y.; Mochida, Y. Influence of micro-landforms on forest structure, tree death and recruitment in a Japanese temperate mixed forest. Ecol. Res. 2003, 18, 533–547. [Google Scholar] [CrossRef]
- Hassler, S.; Kreyling, J.; Beierkuhnlein, C.; Eisold, J.; Samimi, C.; Wagenseil, H.; Jentsch, A. Vegetation pattern divergence between dry and wet season in a semiarid savanna–Spatio-temporal dynamics of plant diversity in northwest Namibia. J. Arid Environ. 2010, 74, 1516–1524. [Google Scholar] [CrossRef]
- Zelený, D.; Li, C.F.; Chytrý, M. Pattern of local plant species richness along a gradient of landscape topographical heterogeneity: Result of spatial mass effect or environmental shift? Ecography 2010, 33, 578–589. [Google Scholar] [CrossRef]
- Parker, A.J. The topographic relative moisture index: An approach to soil-moisture assessment in mountain terrain. Phys. Geogr. 1982, 3, 160–168. [Google Scholar]
- Tamura, T. Landform-soil features of the humid temperate hills. Pedologist 1987, 31, 135–146. [Google Scholar]
- McDonald, D.; Cowling, R.; Boucher, C. Vegetation-environment relationships on a species-rich coastal mountain range in the fynbos biome (South Africa). Vegetation 1996, 123, 165–182. [Google Scholar] [CrossRef]
- Del Barrio, G.; Alvera, B.; Puigdefabregas, J.; Diez, C. Response of high mountain landscape to topographic variables: Central Pyrenees. Landsc. Ecol. 1997, 12, 95–115. [Google Scholar] [CrossRef]
- Fang, J.; Li, Y.; Zhu, B.; Liu, G.; Zhou, G. Community structures and species richness in the montane rain forest of Jianfengling, Hainan Island, China. Biodivers. Sci. 2003, 12, 29–43. (In Chinese) [Google Scholar]
- Sieben, E.; Mucina, L.; Boucher, C. Scaling hierarchy of factors controlling riparian vegetation patterns of the Fynbos Biome at the Western Cape, South Africa. J. Veg. Sci. 2009, 20, 17–26. [Google Scholar] [CrossRef]
- Tatian, M.; Arzani, H.; Reihan, M.K.; Bahmanyar, M.A.; Jalilvand, H. Effect of soil and physiographic factors on ecological plant groups in the eastern Elborz mountain rangeland of Iran. Grassl. Sci. 2010, 56, 77–86. [Google Scholar] [CrossRef]
- Kikuchi, T.; Miura, O. Vegetation patterns in relation to micro-scale landforms in hilly land regions. Vegetation 1993, 106, 147–154. [Google Scholar]
- Nagamatsu, D.; Miura, O. Soil disturbance regime in relation to micro-scale landforms and its effects on vegetation structure in a hilly area in Japan. Plant Ecol. 1997, 133, 191–200. [Google Scholar] [CrossRef]
- Pye, K.; Tsoar, H. Aeolian Sand and Sand Dunes; Springer Science & Business Media: Berlin, Germany, 2008. [Google Scholar]
- Lancaster, N. Geomorphology of Desert Dunes; Routledge: London, UK, 2013. [Google Scholar]
- Bergkamp, G. A hierarchical view of the interactions of runoff and infiltration with vegetation and microtopography in semiarid shrublands. Catena 1998, 33, 201–220. [Google Scholar] [CrossRef]
- Rietkerk, M.; Boerlijst, M.C.; van Langevelde, F.; HilleRisLambers, R.; van de Koppel, J.; Kumar, L.; Prins, H.H.; de Roos, A.M. Self-organization of vegetation in arid ecosystems. Am. Nat. 2002, 160, 524–530. [Google Scholar] [PubMed]
- McGrath, G.S.; Paik, K.; Hinz, C. Microtopography alters self-organized vegetation patterns in water-limited ecosystems. J. Geophys. Res. Biogeosci. 2012, 117. [Google Scholar] [CrossRef]
- Lawley, V.; Parrott, L.; Lewis, M.; Sinclair, R.; Ostendorf, B. Self-organization and complex dynamics of regenerating vegetation in an arid ecosystem: 82 years of recovery after grazing. J. Arid Environ. 2013, 88, 156–164. [Google Scholar] [CrossRef]
- Tongway, D.J.; Valentin, C.; Seghieri, J. Banded Vegetation Patterning in Arid and Semiarid Environments: Ecological Processes and Consequences for Management; Springer Science & Business Media: Berlin, Germany, 2001. [Google Scholar]
- Barbier, N.; Couteron, P.; Lejoly, J.; Deblauwe, V.; Lejeune, O. Self-organized vegetation patterning as a fingerprint of climate and human impact on semi-arid ecosystems. J. Ecol. 2006, 94, 537–547. [Google Scholar] [CrossRef]
- Chen, L.; Li, H.; Zhang, P.; Zhao, X.; Zhou, L.; Liu, T.; Hu, H.; Bai, Y.; Shen, H.; Fang, J.; et al. Climate and native grassland vegetation as drivers of the community structures of shrub-encroached grasslands in Inner Mongolia, China. Landsc. Ecol. 2014. [Google Scholar] [CrossRef]
- Beisner, B.E.; Haydon, D.T.; Cuddington, K. Alternative stable states in ecology. Front. Ecol. Environ. 2003, 1, 376–382. [Google Scholar] [CrossRef]
- Suding, K.N.; Gross, K.L.; Houseman, G.R. Alternative states and positive feedbacks in restoration ecology. Trends Ecol. Evol. 2004, 19, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Pugnaire, F. Positive Plant Interactions and Community Dynamics; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Valiente-Banuet, A.; Ezcurra, E. Shade as a cause of the association between the cactus Neobuxbaumia tetetzo and the nurse plant Mimosa luisana in the Tehuacan Valley, Mexico. J. Ecol. 1991, 79, 961–971. [Google Scholar] [CrossRef]
- Munguía-Rosas, M.A.; Sosa, V.J. Nurse plants vs. nurse objects: Effects of woody plants and rocky cavities on the recruitment of the Pilosocereus leucocephalus columnar cactus. Ann. Bot. 2008, 101, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.M.; Martorell, C.; Ezcurra, E. Nurse rocks are more important than nurse plants in determining the distribution and establishment of globose cacti (Mammillaria) in the Tehuacán Valley, Mexico. J. Arid Environ. 2008, 72, 593–601. [Google Scholar] [CrossRef]
- Cody, M.L. Do Cholla Cacti (Opuntia spp., Subgenus Cylindropuntia) use or need nurse plants in the Mojave Desert? J. Arid Environ. 1993, 24, 139–154. [Google Scholar] [CrossRef]
- Ernest, S.M.; Brown, J.H.; Parmenter, R.R. Rodents, plants, and precipitation: Spatial and temporal dynamics of consumers and resources. Oikos 2000, 88, 470–482. [Google Scholar] [CrossRef]
- Brown, J.H.; Ernest, S.M. Rain and Rodents: Complex Dynamics of Desert Consumers although water is the primary limiting resource in desert ecosystems, the relationship between rodent population dynamics and precipitation is complex and nonlinear. BioScience 2002, 52, 979–987. [Google Scholar] [CrossRef]
- Shenbrot, G.; Krasnov, B.; Burdelov, S. Long-term study of population dynamics and habitat selection of rodents in the Negev Desert. J. Mamm. 2010, 91, 776–786. [Google Scholar] [CrossRef]
- Prakash, I.; Ghosh, P.K. Rodents in Desert Environments; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Taraborelli, P.; Corbalan, V.; Giannoni, S. Locomotion and escape modes in rodents of the Monte Desert (Argentina). Ethology 2003, 109, 475–485. [Google Scholar] [CrossRef]
- Corbalán, V.; Ojeda, R. Spatial and temporal organisation of small mammal communities in the Monte desert, Argentina. Mammalia 2004, 68, 5–14. [Google Scholar] [CrossRef]
- Haim, A.; Izhaki, I. Changes in rodent community during recovery from fire: Relevance to conservation. Biodivers. Conserv. 1994, 3, 573–585. [Google Scholar] [CrossRef]
- Lima, M.; Marquet, P.A.; Jaksic, F.M. El Nino events, precipitation patterns, and rodent outbreaks are statistically associated in semiarid Chile. Ecography 1999, 22, 213–218. [Google Scholar] [CrossRef]
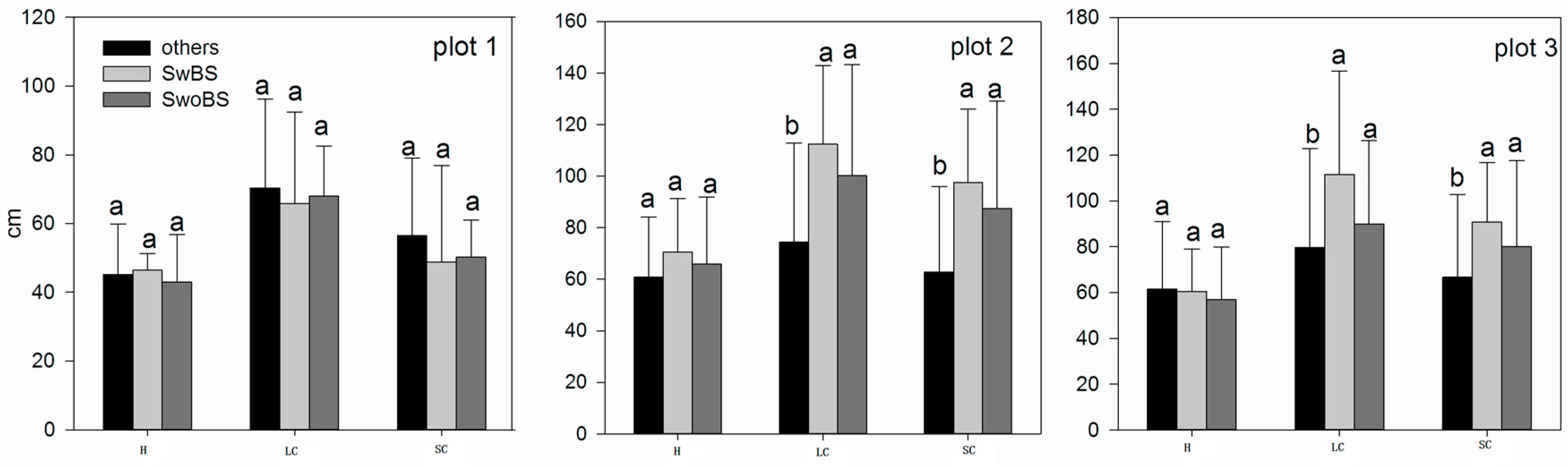
| PLOT | Parameters | SpirAqui | CaraMicr | BS | |
|---|---|---|---|---|---|
| Larger | Smaller | ||||
| Plot 1 | N | 150 | 33 | 56 | 14 |
| H | 48.8 ± 13.14 | 29.1 ± 9.06 | 35.8 ± 12.08 | ||
| LC | 77.6 ± 22.65 | 37.9 ± 7.22 | 46.9 ± 22.01 | ||
| SC | 62.6 ± 19.99 | 28.8 ± 7.05 | 35.5 ± 14.76 | ||
| Plot 2 | N | 348 | 104 | 39 | 21 |
| H | 71.3 ± 25.91 | 29.0 ± 11.74 | 44.9 ± 17.24 | ||
| LC | 94.7 ± 37.60 | 29.3 ± 10.16 | 63.1 ± 36.98 | ||
| SC | 79.5 ± 30.93 | 24.0 ± 7.72 | 48.6 ± 28.83 | ||
| Plot 3 | N | 747 | 251 | 30 | 39 |
| H | 65.2 ± 18.45 | 28.1 ± 10.88 | 31.6 ± 9.82 | ||
| LC | 90.1 ± 30.03 | 26.9 ± 10.75 | 34.9 ± 14.82 | ||
| SC | 76.3 ± 27.04 | 22.7 ± 7.63 | 29.16 ± 11.02 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Wang, X.; Li, L.; Shi, Z.; Yang, X. Spatial Association of Shrubs and Their Interrelation to Burrowing Site Preference of Subterranean Rodents on Dune Slope in the Otindag Sandy Land, China. Sustainability 2017, 9, 1729. https://doi.org/10.3390/su9101729
Jiang L, Wang X, Li L, Shi Z, Yang X. Spatial Association of Shrubs and Their Interrelation to Burrowing Site Preference of Subterranean Rodents on Dune Slope in the Otindag Sandy Land, China. Sustainability. 2017; 9(10):1729. https://doi.org/10.3390/su9101729
Chicago/Turabian StyleJiang, Lina, Xiao Wang, Long Li, Zhongjie Shi, and Xiaohui Yang. 2017. "Spatial Association of Shrubs and Their Interrelation to Burrowing Site Preference of Subterranean Rodents on Dune Slope in the Otindag Sandy Land, China" Sustainability 9, no. 10: 1729. https://doi.org/10.3390/su9101729




