Phytoremediation Opportunities with Alimurgic Species in Metal-Contaminated Environments
Abstract
:1. Introduction
2. Experimental Section
2.1. Pot Trial Set-Up
2.2. Soil and Leachate Analyses
2.3. Plant Analysis
2.4. Plant Sampling in Critical Sites
2.5. Statistical Analysis
3. Results
3.1. Soil Contamination
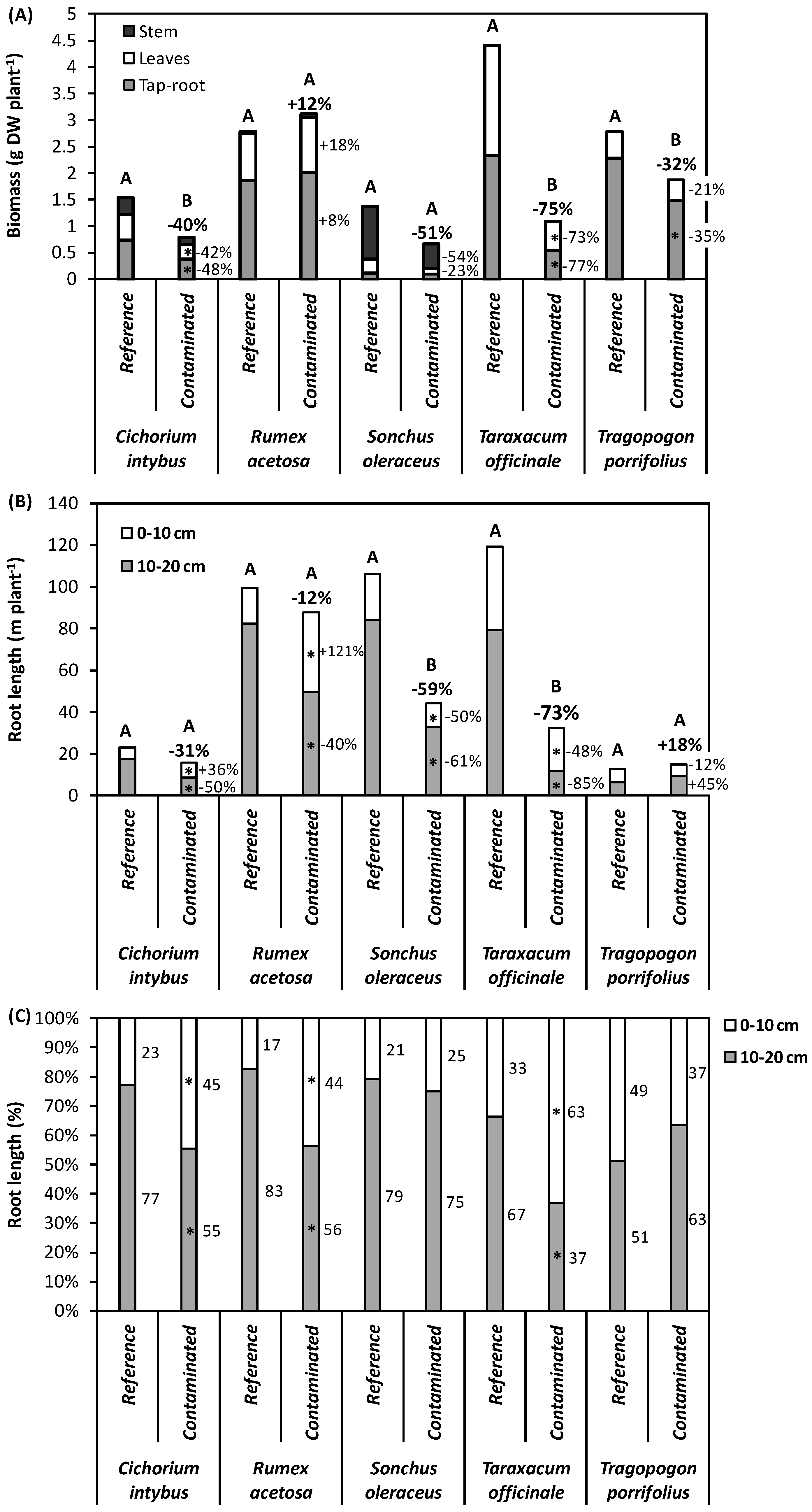
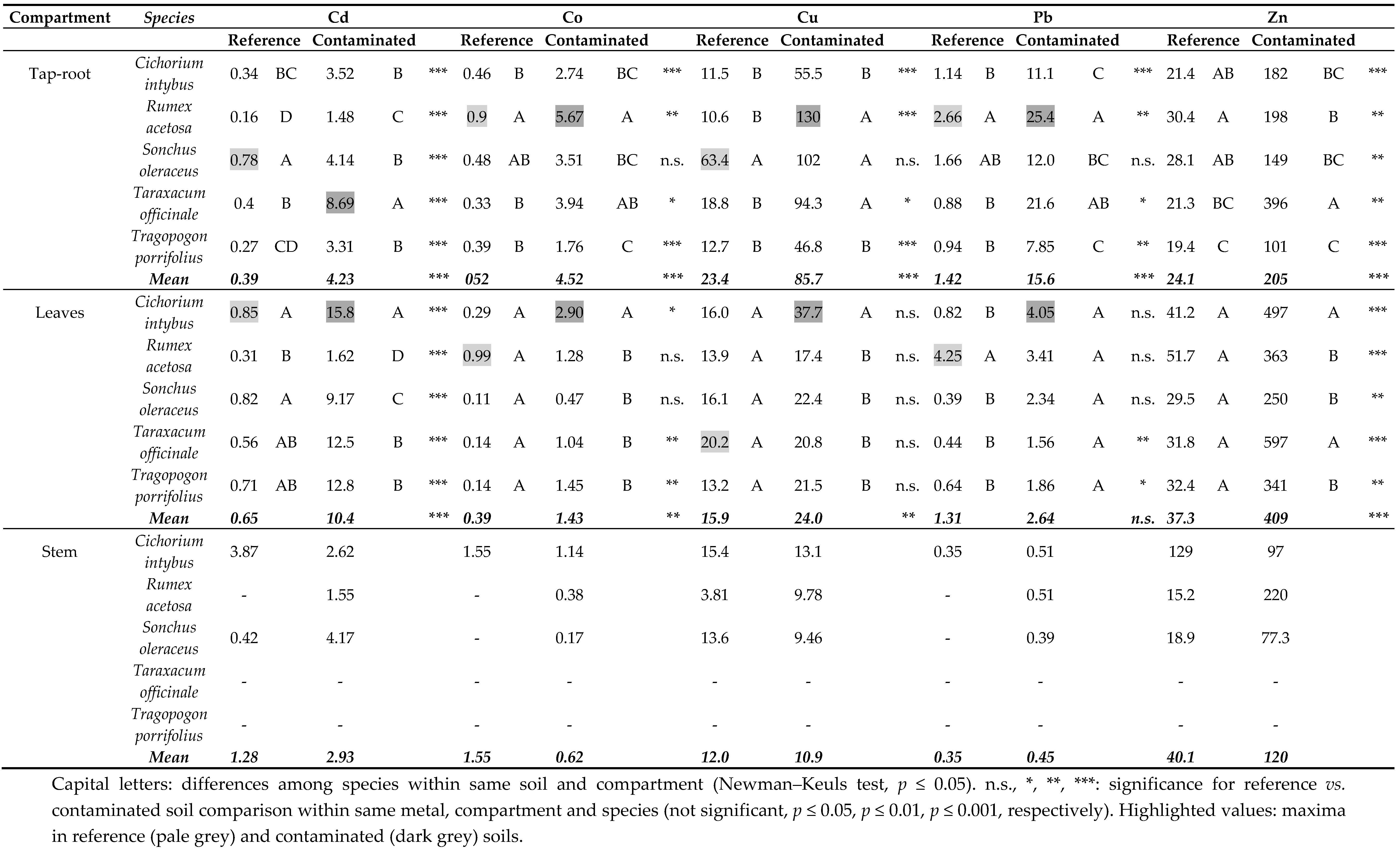
3.2. Plant Growth and Metal Uptake
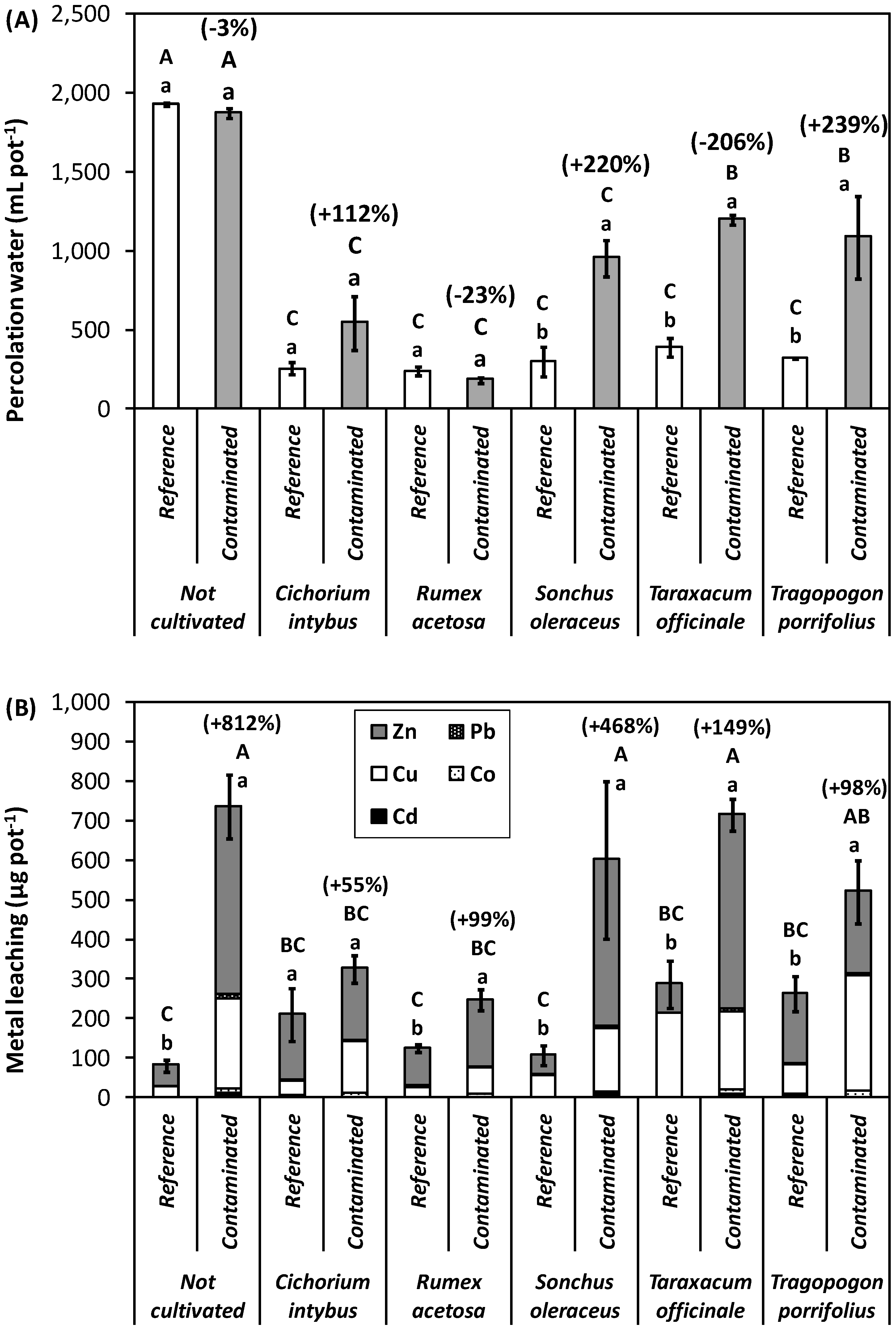
3.3. Metal Leaching
3.4. Vegetation Grown at Potentially Polluted Sites
4. Discussion
4.1. Plant Responses to Metal Contaminants and Perspectives for Phytoremediation
4.2. Food Safety
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Counter, S.A.; Buchanan, L.H.; Ortega, F. Neurocognitive screening of lead-exposed Andean adolescents and young adults. J. Toxicol. Environ. Health A 2009, 72, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Naja, G.M.; Volesky, B. Toxicity and sources of Pb, Cd, Hg, Cr, As, and radionuclides in the environment. In Heavy Metals in the Environment; Wang, L.K., Chen, J.P., Hung, Y., Shammas, N.K., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 13–61. [Google Scholar]
- García-Lestón, J.; Roma-Torres, J.; Mayan, O.; Schroecksnadel, S.; Fuchs, D.; Moreira, A.O.; Pásaro, E.; Méndez, J.; Teixeira, J.P.; Laffon, B. Assessment of immunotoxicity parameters in individuals occupationally exposed to lead. J. Toxicol. Environ. Health A 2012, 75, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Haswell-Elkins, M.R.; Moore, M.R. Safe levels of cadmium intake to prevent renal toxicity in human subjects. Br. J. Nutr. 2000, 84, 791–802. [Google Scholar] [PubMed]
- Joseph, P. Mechanisms of cadmium carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Erdman, J.W., Jr.; MacDonald, I.A.; Zeisel, S.H. Present Knowledge in Nutrition, 10th ed.; Wiley-Blackwell: New York, NY, USA, 2012. [Google Scholar]
- Jomova, Y.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Meng, H.; Xing, G.; Chen, C.; Zhao, Y.; Jia, G.; Wang, T.; Yuan, H.; Ye, C.; Zhao, F.; et al. Acute toxicological effects of copper nanoparticles in vivo. Toxicol. Lett. 2006, 163, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Nriagu, J. Zinc toxicity in humans. In Encyclopedia of Environmental Health; Nriagu, J., Ed.; Elsevier B.V.: Amsterdam, NL, The Netherlands, 2007; pp. 1–7. [Google Scholar]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. Trace elements in human physiology and pathology. Copper. Biomed. Pharmacother. 2003, 57, 386–398. [Google Scholar] [CrossRef]
- Robinson, B.H.; Bañuelos, G.; Conesa, H.M.; Evangelou, M.W.H.; Schulin, R. The phytomanagement of trace elements in soil. Crit. Rev. Plant Sci. 2009, 28, 240–266. [Google Scholar] [CrossRef]
- Vamerali, T.; Marchiol, L.; Bandiera, M.; Fellet, G.; Dickinson, N.M.; Lucchini, P.; Mosca, G.; Zerbi, G. Advances in agronomic management of phytoremediation: Methods and results from a 10-year study of metal-polluted soils. Ital. J. Agron. 2012, 7, 323–330. [Google Scholar] [CrossRef]
- Palmgren, M.G.; Clemens, S.; Williams, L.E.; Kramer, U.; Borg, S.; Schjorring, J.K.; Sanders, D. Zinc biofortification of cereals: Problems and solutions. Trends Plant Sci. 2008, 13, 464–473. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets—Iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.J.M.; Brooks, R.R. Terrestrial higher plants which hyperaccumulate metallic elements—A review of their distribution; ecology and phytochemistry. Biorecovery 1989, 1, 81–126. [Google Scholar]
- Barrutia, O.; Epelde, L.; García-Plazaola, J.I.; Garbisu, C.; Becerril, J.M. Phytoextraction potential of two Rumex acetosa L. accessions collected from metalliferous and non-metalliferous sites: Effect of fertilization. Chemosphere 2009, 74, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Bech, J.; Duran, P.; Roca, N.; Poma, W.; Sánchez, I.; Barceló, J.; Boluda, R.; Roca-Pérez, L.; Poschenrieder, C. Shoot accumulation of several trace elements in native plant species from contaminated soils in the Peruvian Andes. J. Geochem. Explor. 2012, 113, 106–111. [Google Scholar] [CrossRef]
- Martin, H.W.; Young, T.R.; Kaplan, D.I.; Simon, L.; Adriano, D.C. Evaluation of three herbaceous index plant species for bioavailability of soil cadmium; chromium; nickel and vanadium. Plant Soil 1996, 182, 199–207. [Google Scholar]
- Maria do Rosário, G.O.; van Noordwijk, M.; Gaze, S.R.; Brouwer, G.; Bona, S.; Mosca, G.; Hairiah, H.K. Auger sampling, ingrowth cores and pinboard methods. In Root Methods—A Handbook; Smit, A.L., Bengough, A.G., Engels, C., van Noordwijk, M., Pellerin, S., van de Geijn, S.C., Eds.; Springer: Berlin, Germany, 2000; pp. 175–210. [Google Scholar]
- Vamerali, T.; Guarise, M.; Ganis, A.; Bona, S.; Mosca, G. Analysis of root images from auger sampling with a fast procedure: A case of application to sugar beet. Plant Soil 2003, 255, 387–397. [Google Scholar] [CrossRef]
- US Environmental Protection Agency (USEPA). EPA method 3051, microwave assisted acid digestion of sediments; sludges; soils; and oils. In Test Methods for Evaluating Solid Waste, 3rd ed.USEPA: Washington, DC, USA, 1995. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese and copper. Soil. Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- US Environmental Protection Agency (USEPA). EPA Method 3052; microwave assisted acid digestion of siliceous and organically based matrices. In Test Methods for Evaluating Solid Waste, 3rd ed.USEPA: Washington, DC, USA, 1995. [Google Scholar]
- Keane, B.; Collier, M.H.; Shann, J.R.; Rogstad, S.H. Metal content of dandelion (Taraxacum officinale) leaves in relation to soil contamination and airborne particulate matter. Sci. Total Environ. 2001, 281, 63–78. [Google Scholar] [CrossRef]
- Malizia, D.; Giuliano, A.; Ortaggi, G.; Masotti, A. Common plants as alternative analytical tools to monitor heavy metals in soil. Chem. Cen. J. 2012, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, C.; Stihi, C.; Barbes, L.; Chilian, A.; Chelarescu, D.E. Studies concerning heavy metals accumulation of Carduus nutans L. and Taraxacum officinale as potential soil bioindicator species. Rev. Chim.-Buchar. 2013, 64, 754–760. [Google Scholar]
- Simon, L.; Martin, H.W.; Adriano, D.C. Chicory (Cichorium intybus L.) and dandelion (Taraxacum officinale Web.) as phytoindicators of cadmium contamination. Water Air Soil Pollut. 1996, 91, 351–362. [Google Scholar] [CrossRef]
- Liao, M.T.; Hedley, M.J.; Woolley, D.J.; Brooks, R.R.; Nichols, M.A. Copper uptake and translocation in chicory (Cichorium intybus L. cv. Grasslands Puna) and tomato (Lycopersicon esculentum Mill. cv. Rondy) plants grown in NFT system. I. Copper uptake and distribution in plants. Plant Soil 2000, 221, 135–142. [Google Scholar] [CrossRef]
- Aksoy, A. Chicory (Cichorium intybus L.): A possible biomonitor of metal pollution. Pak. J. Bot. 2008, 40, 791–797. [Google Scholar]
- Del Río-Celestino, M.; Font, R.; Moreno-Rojas, R.; De Haro-Bailón, A. Uptake of lead and zinc by wild plants growing on contaminated soils. Ind. Crop. Prod. 2006, 24, 230–237. [Google Scholar] [CrossRef]
- Xiong, Z.T. Bioaccumulation and physiological effects of excess lead in a roadside pioneer species Sonchus oleraceus L. Environ. Pollut. 1997, 97, 275–279. [Google Scholar] [CrossRef]
- Wang, Q.R.; Cui, Y.S.; Liu, X.; Dong, Y.T.; Christie, P. Soil contamination and plant uptake of heavy metals at polluted sites in China. J. Environ. Sci. Health A 2003, 38, 823–838. [Google Scholar] [CrossRef]
- Ernst, W.H.O.; Knolle, F.; Kratz, S.; Schnug, E. Aspects of ecotoxicology of heavy metals in the Harz region—A guided excursion. Lanbauforsch. Völk. 2004, 54, 53–71. [Google Scholar]
- Zhao, L.; Yuan, L.; Wang, Z.; Lei, T.; Yin, X. Phytoremediation of zinc-contaminated soil and zinc-biofortification for human nutrition. In Phytoremediation and Biofortification. Two Sides of One Coin; Yin, X., Yuan, L., Eds.; Springer: New York, NY, USA, 2012; pp. 33–57. [Google Scholar]
- Vamerali, T.; Bandiera, M.; Lucchini, P.; Dickinson, N.M.; Mosca, G. Long-term phytomanagement of metal-contaminated land with field crops: Integrated remediation and biofortification. Eur. J. Agr. 2014, 53, 56–66. [Google Scholar] [CrossRef]
- Bandiera, M.; Mosca, G.; Vamerali, T. Humic acids affect root characteristics of fodder radish (Raphanus sativus L. var. oleiformis Pers.) in metal-polluted wastes. Desalination 2009, 247, 79–92. [Google Scholar]
- Marchiol, L.; Fellet, G.; Perosa, D.; Zerbi, G. Removal of trace metals by Sorghum bicolor and Helianthus annuus in a site polluted by industrial wastes: A field experience. Plant Physiol. Biochem. 2007, 45, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Vamerali, T.; Bandiera, M.; Coletto, L.; Zanetti, F.; Dickinson, N.M.; Mosca, G. Phytoremediation trials on metal- and arsenic-contaminated pyrite wastes (Torviscosa; Italy). Environ. Pollut. 2009, 157, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Vamerali, T.; Bandiera, M.; Mosca, G. In situ phytoremediation of arsenic- and metal-polluted pyrite waste with field crops: Effects of soil management. Chemosphere 2011, 83, 1241–1248. [Google Scholar] [PubMed]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Bandiera, M.; Mosca, G.; Vamerali, T. Phytotoxicity and metal leaching in EDDS-assisted phytoextraction from pyrite wastes with Ethiopian mustard and fodder radish. Plant Biosyst. 2010, 144, 490–498. [Google Scholar] [CrossRef]
- Cotter-Howells, J.D.; Caporn, S.J. Remediation of contaminated land by formation of heavy metal phosphates. Appl. Geochem. 1996, 11, 335–342. [Google Scholar] [CrossRef]
- Dong, J.; Mao, W.H.; Zhang, G.P.; Wu, F.B.; Cai, Y. Root excretion and plant tolerance to cadmium toxicity—A review. Plant Soil Environ. 2007, 53, 193–200. [Google Scholar]
- Tangahu, B.V.; Abdullah, S.R.S.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int. J. Chem. Eng. 2011, 2011, 1–31. [Google Scholar] [CrossRef]
- Huang, B.; Xin, J.; Dai, H.; Liu, A.; Zhou, W.; Yi, Y.; Liao, K. Root morphological responses of three hot pepper cultivars to Cd exposure and their correlations with Cd accumulation. Environ. Sci. Poll. Res. 2015, 22, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Verkleij, J.A.C.; Golan-Goldhirsh, A.; Antosiewisz, D.M.; Schwitzguébel, J.P.; Schröder, P. Dualities in plant tolerance to pollutants and their uptake and translocation to the upper plant parts. Environ. Exp. Bot. 2009, 67, 10–22. [Google Scholar] [CrossRef]
- Robinson, B.H.; Green, S.R.; Chancerel, B.; Mills, T.M.; Clothier, B. Poplar for the phytomanagement of boron contaminated sites. Environ. Pollut. 2007, 150, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Naszradi, T.; Badacsonyi, A.; Németh, N.; Tuba, Z.; Batiĉ, F. Zinc, lead and cadmium content in meadow plants and mosses along the M3 motorway (Hungary). J. Atmos. Chem. 2004, 49, 593–603. [Google Scholar] [CrossRef]
- WHO. WHO Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues. Available online: http://apps.who.int/medicinedocs/documents/s14878e/s14878e.pdf (accessed on 7 April 2016).
- Jankowski, K.; Ciepiela, A.G.; Jankowska, J.; Szulc, W.; Kolczarek, R.; Sosnowski, J.; Wiśniewska-Kadżajan, B.; Malinowska, E.; Radzka, E.; Czeluściński, W.; et al. Content of lead and cadmium in aboveground plant organs of grasses growing on the areas adjacent to a route of big traffic. Environ. Sci. Pollut. Res. 2015, 22, 978–987. [Google Scholar] [CrossRef] [PubMed]



| Metal | IGV (A) | Pseudo-Total (B) | (B/A) | DTPA Extractable | |||
|---|---|---|---|---|---|---|---|
| Reference | Contaminated | Reference | Contaminated | Reference | Contaminated | ||
| Cd | 2 | <0.20 | 3.66 | <0.10 | 1.83 | 0.09 (45%) | 3.05 (83.3%) |
| Co | 20 | 5.54 | 36.8 | 0.28 | 1.84 | 0.05 (0.9%) | 4.82 (13.1%) |
| Cu | 120 | 20.5 | 496 | 0.17 | 4.13 | 4.4 (21.5%) | 226 (45.5%) |
| Pb | 100 | 12.5 | 163 | 0.13 | 1.63 | 2.93 (23.5%) | 40.9 (25.1%) |
| Zn | 150 | 46.7 | 906 | 0.31 | 6.04 | 1.54 (3.3%) | 368 (40.6%) |
| Soil | Cichorium intybus | Rumex acetosa | Sonchus oleraceus | Taraxacum officinale | Tragopogon porrifolius | |
|---|---|---|---|---|---|---|
| Cd | Reference (pot) | 0.19 | 0.05 | 0.18 | 0.11 | 0.18 |
| Contaminated (pot) | ||||||
| Roadside | - | 0.03 | - | 0.05 | - | |
| Landfill | - | - | - | 0.04 | - | |
| Field ditch | - | - | - | 0.04 | - | |
| Pb | Reference (pot) | 0.18 | 0.09 | 0.09 | 0.16 | |
| Contaminated (pot) | 0.26 | |||||
| Roadside | - | - | 0.21 | - | ||
| Landfill | - | - | - | 0.21 | - | |
| Field ditch | - | - | - | 0.21 | - |
| Metal | Compartment | Rumex acetosa | Taraxacum officinale | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Roadside | Landfill | Field Ditch | Roadside | ||||||
| Cd | Soil | 0.60 (27%) | a | 0.16 (45%) | c | 0.17 (34%) | c | 0.33 (25%) | b |
| Tap-root | 0.27 | a | 0.32 | a | 0.28 | a | 0.33 | a | |
| Leaves | 0.17 | a | 0.22 | a | 0.20 | a | 0.25 | a | |
| Cu | Soil | 97.8 (21%) | b | 23.6 (16%) | b | 41.9 (11%) | b | 322 (14%) | a |
| Tap-root | 12.5 | a | 22.5 | a | 24.1 | a | 25.6 | a | |
| Leaves | 10.0 | b | 10.0 | b | 21.3 | a | 15.3 | ab | |
| Pb | Soil | 139 (21%) | a | 18.3 (16%) | c | 29.6 (11%) | bc | 70.6 (7%) | b |
| Tap-root | 5.38 | a | 0.47 | c | 1.18 | bc | 1.78 | b | |
| Leaves | 1.68 | a | 0.48 | b | 1.03 | ab | 1.03 | ab | |
| Zn | Soil | 229 (15%) | b | 81.0 (3%) | c | 114 (2%) | c | 326 (14%) | a |
| Tap-root | 63.1 | b | 42.1 | b | 174 | a | 59.6 | b | |
| Leaves | 49.3 | b | 43.4 | b | 90.9 | a | 56.3 | b | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bandiera, M.; Dal Cortivo, C.; Barion, G.; Mosca, G.; Vamerali, T. Phytoremediation Opportunities with Alimurgic Species in Metal-Contaminated Environments. Sustainability 2016, 8, 357. https://doi.org/10.3390/su8040357
Bandiera M, Dal Cortivo C, Barion G, Mosca G, Vamerali T. Phytoremediation Opportunities with Alimurgic Species in Metal-Contaminated Environments. Sustainability. 2016; 8(4):357. https://doi.org/10.3390/su8040357
Chicago/Turabian StyleBandiera, Marianna, Cristian Dal Cortivo, Giuseppe Barion, Giuliano Mosca, and Teofilo Vamerali. 2016. "Phytoremediation Opportunities with Alimurgic Species in Metal-Contaminated Environments" Sustainability 8, no. 4: 357. https://doi.org/10.3390/su8040357






