1. Introduction
Bioelectrochemical systems (BES) have emerged as a niche technology for conversion of waste streams and algae into usable energy [
1]. Microbial fuel cells (MFCs) and microbial electrolysis cells (MECs) are two types of BES capable of producing electricity and hydrogen, respectively, from organic material in aqueous streams [
2,
3]. Conversion of biomass to biofuels results in a number of waste streams, some of which may be amenable to further energy recovery via use of BES systems [
4,
5]. Synergy between biorefineries and BES has been reported previously, demonstrating the ability to produce electricity [
6] and hydrogen [
7,
8] from the streams [
9]. The impact of including BES in biorefinery process can be worthwhile due to the increased energy recovery from biomass, leading to improved energy efficiency [
4], reduction in waste streams produced in the biorefinery [
6], and reduction in lifecycle greenhouse gas emissions. These factors can contribute to the improvement in the sustainability of biorefinery processes. A few studies have reported the assessment of sustainability parameters for bioelectrochemical systems. Pant
et al. provided recommendations on how to carry out life cycle analysis of wastewater systems implementing BESs [
10]. The positive factors, such as reduction in aeration costs, as well as benefits due to production of electric power and reduction in cost, have to be factored in such an analysis. A comparison of MFCs
vs. anaerobic digestion was reported by Pham
et al. [
11]. While sustainability of BES systems themselves has been reported previously, similar analysis of biorefinery scenarios integrating BESs has not been done. In this paper, various process scenarios are outlined which bring together biofuel production processes and BES systems. A discussion of the impact of integrating the BESs into thermochemical, biochemical and algal process scenarios is presented and the influence of such pathways on sustainability parameters is discussed. The novelty of this work is in the integration of the upcoming waste-to-energy conversion pathways into existing biorefinery schemes. Significant advances have been made in the primary biorefinery unit operations such as pretreatment, hydrolysis, bioconversion or thermoconversion and downstream separations and upgrading processes [
12], but utilization of the wastes to improve resource recovery has been a low priority. This report identifies the need to consider these unit operations in the biorefinery to improve resource utilization and energy efficiency.
2. Baseline Biorefinery Process Pathway
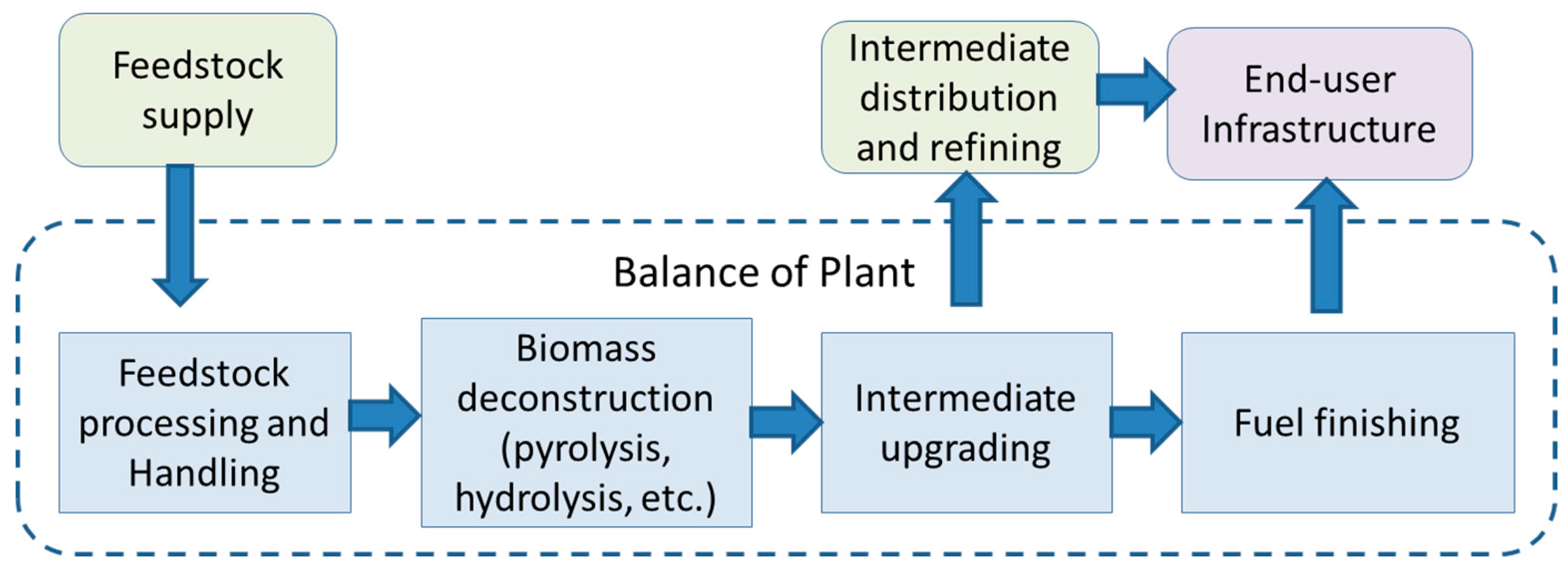
Production of drop-in fuels from biomass is a major goal to reduce utilization of fossil fuels for transportation. Hydrocarbons generated from biomass are the most ideal blendstock for moving towards this goal. However, biomass contains over 40% oxygen by weight [
13]. This requires a significant amount of hydrogen to carry out the deoxygenation to make hydrocarbons. About 22,250 standard cubic feet (scf) of hydrogen is needed to process one metric ton of biomass into fuel [
13]. Thermochemical, biochemical and algal pathways approach this goal using different steps. However, a generalized scheme can be outlined as shown in
Figure 1. In a thermochemical pathway, pyrolysis or gasification is used as the deconstruction step. In the biochemical approach, hydrolysis using chemicals or enzymes is employed, while algal biomass is deconstructed via dilute acid treatment or hydrothermal liquefaction. The product formation steps include fermentation in the biochemical route and hydrotreatment in the thermochemical and algal routes, which may be carried out with or without a catalyst. The high oxygen content of the biomass limits yield of hydrocarbon, and efforts have been made in recent years to increase the yield by supplementing the fuel finishing step with a source of hydrogen. In the thermochemical routes, natural gas reforming has been used to generate the hydrogen, while aqueous phase reforming or similar unit operations have been used in association with biochemical routes. Hydrogen is a key reagent to increase fuel yield in most cases. At present, renewable sources of hydrogen are not commercially available, requiring use of fossil resources, which in turn, impact sustainability. Here, we include the potential to derive hydrogen from the aqueous and waste streams generated during biomass processing via microbial electrolysis, and discuss its impact on sustainability parameters. Other process alternatives to convert biomass-derived streams into hydrogen also exist. These include steam reforming, gasification, autothermal reforming and dark-light fermentation [
4]. Steam reforming of pyrolysis derived bio-oil aqueous phase has been investigated for hydrogen production, however, several issues including low yields, coking, catalyst fouling have limited the success of this approach [
14,
15]. Gasification of biomass followed by gas-phase separation can generate hydrogen, but it has limited application for aqueous waste streams. Autothermal reforming requires use of a catalyst employing rhodium, which makes it a challenge due to the high cost of rhodium and its limited availability. Microbial electrolysis is a nascent technology, but uses self-regenerable catalysts and has high conversion efficiency [
4]. There are opportunities to introduce microbial electrolysis into each of the three biorefinery scenarios. These scenarios are considered below with discussion of the conversion steps and resulting changes related to GHG emissions and water use.
Figure 1.
Block diagram showing biomass conversion to hydrocarbon fuels via a thermochemical pyrolysis pathway [
16].
Figure 1.
Block diagram showing biomass conversion to hydrocarbon fuels via a thermochemical pyrolysis pathway [
16].
3. Thermochemical Biomass Conversion
Pyrolysis produces a bio-oil, which contains about 37.5% oxygen by weight [
13]. The bio-oil intermediate requires upgrading to form a stable product, which can then be hydrotreated to produce gasoline and diesel fuels or blendstock. The process is shown in
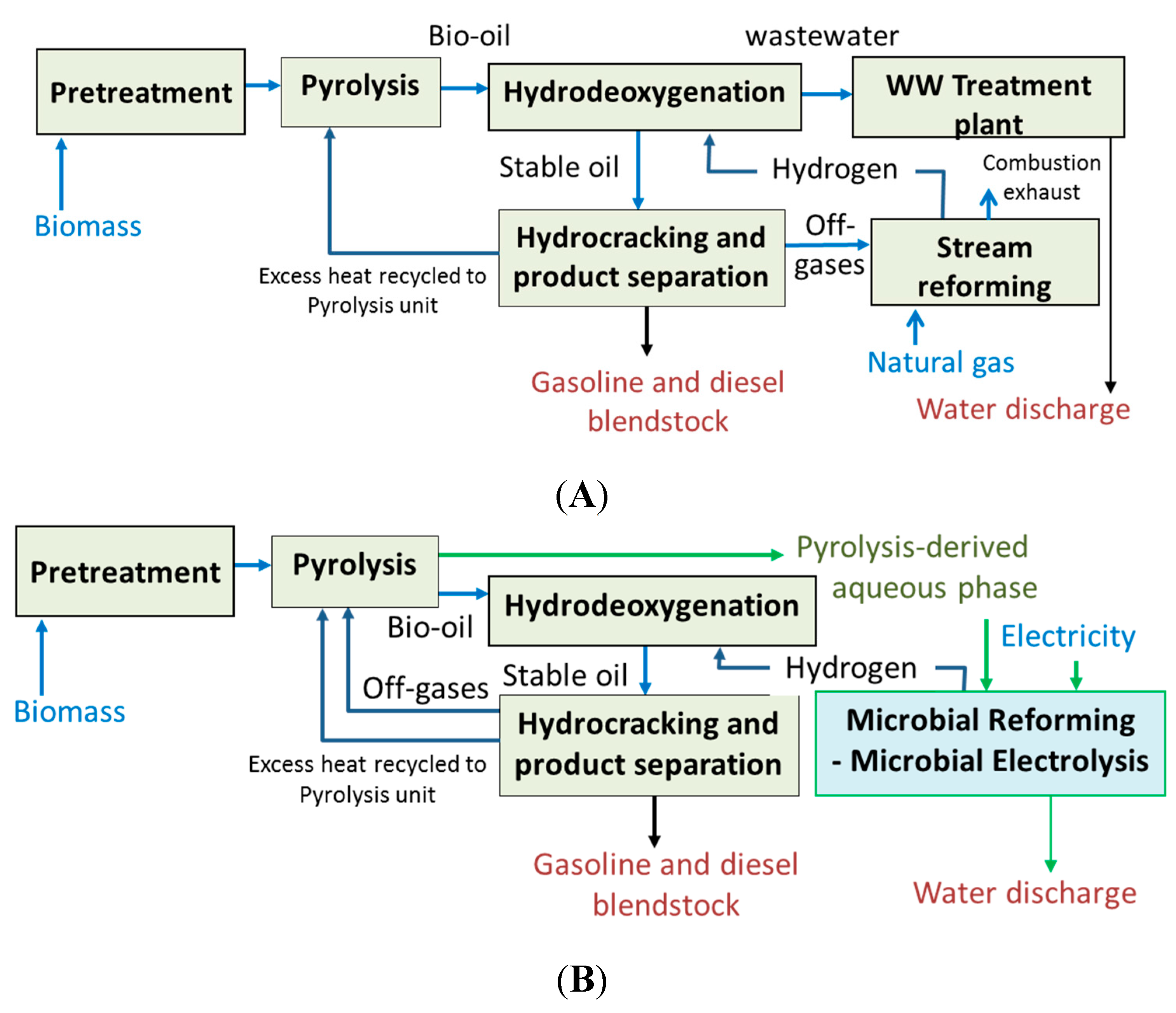
Figure 2.
The hydrogen requirement for conversion of pyrolysis bio-oil to hydrocarbons can be met by steam reforming of natural gas (
Figure 2A). This contributes to 17.9% of the capital costs in the process [
13]. The operating temperature of this process is >350 °C with an operating pressure >300 psi. While significant heat integration is incorporated into the process model, the overall energy balance indicates an energy loss of 51.6%. Thus, the use of low temperature processes can potentially result in a reduction in heat loss from the plant and improve energy efficiency.
Figure 2.
Thermochemical pathways for conversion of biomass to biofuels. (A) Conventional pathway using pyrolysis with natural gas as a source of hydrogen; (B) Alternate pathway integrating microbial electrolysis for hydrogen production.
Figure 2.
Thermochemical pathways for conversion of biomass to biofuels. (A) Conventional pathway using pyrolysis with natural gas as a source of hydrogen; (B) Alternate pathway integrating microbial electrolysis for hydrogen production.
The pyrolysis process produces a mixture of organic and aqueous phases, typically referred to as the bio-oil, however, a separate aqueous phase may also be generated, depending on the moisture content of the biomass and the reaction conditions. Alternately, an aqueous phase can be separated out from the bio-oil via solvent addition and other methods [
17]. The aqueous phase contains a larger portion of dissolved organic compounds derived from biomass. This stream can be separated and used for hydrogen generation via microbial electrolysis, which can be considered as a microbial-electrochemical reforming of the organic compounds present in the aqueous phase to hydrogen. The organic compounds present in the bio-oil aqueous phase include organic acids, furan aldehydes, phenolic compounds and sugar derivatives [
18]. Candidates from each of these class of compounds have been shown to serve as substrates in the bioanode [
6,
19]. In addition, conversion of the whole bio-oil aqueous phase in an MEC has been recently demonstrated in our laboratory [
7,
20].
Energy efficiency is an important consideration for biorefineries. Conversion of model substrates in MECs has shown relatively high coulombic efficiencies. Acetate has been shown to be transformed into hydrogen at a Coulombic efficiency >90% with an overall energy efficiency of 82% [
21]. Transformation of sugars and other non-fermentable compounds have also been shown to be at Coulombic efficiencies >80% [
22,
23]. The yield of hydrogen from sugars has been reported to be 64% [
21], while that from non-fermentable substrates such as acetate has been reported to be 23%–93%, depending on reactor volume and other process conditions [
2]. Thus, effective conversion of the key compounds present in bio-oil aqueous phase to hydrogen is possible with low energy losses. The MEC technology has been demonstrated at the laboratory scale as well as in small scale pilot reactors with a volumes of up to 10 L using a municipal wastewater stream. Laboratory-scale studies of two biorefinery waste streams have been reported using the proposed MEC or bioanode configurations [
7,
19]. Further work is necessary using the streams at pilot scale to demonstrate feasibility. Preliminary economic analyses reported previously have indicated a minimal current density of 20 A/m
2 for MECs to be commercially viable [
24]. This would require operation of the MECs with loading rates greater than 20 g/L-day and applied voltage greater than 0.6V.
4. Approach for Assessment of Environmental Sustainability of Biorefinery Schemes
Several reports describing assessment of sustainability parameters for biorefinery schemes exist in the literature. Tan
et al. reported analysis of bioenergy systems including three main performance indicators: land use, water and carbon footprint, recognizing the demand for agricultural resources for bioenergy production [
25]. They used a fuzzy-multiple-objective approach for optimization of bioenergy system footprint for integrated production of biodiesel, ethanol and electricity. Heyne and Harvey included energy market scenarios in their analysis to better define the economic sustainability for production of synthetic natural gas from biomass [
26]. Liew
et al., as well as Kim and Dale included health effects and safety in their analysis to account for social impacts due to biofuel production [
27,
28]. Kim and Dale evaluated distributed vs centralized processing alternatives using an eco-efficiency indicator, which integrated economic and environmental features. A study focused on EU Renewable Energy Directive reported assessment of sustainability guidelines for the European Directive 2009/28/EC and included a review of the legal definition of waste [
29]. They identified a certain level of uncertainty in understanding the sustainability impacts and called for new methods to complement the sustainability analyses. In certain cases such as algal biorefineries, sustainability issues are predominantly favorable, but economic feasibility for biofuel production is difficult. Inclusion of higher value products was considered and a sustainability analysis of the process was described by Zhu to assess algal biorefineries. Luo
et al. used an 8-point system to investigate sustainability of cellulosic ethanol production. Allocation methodology was reported to be important factor in their LCA results. A broader approach including energy conservation, environmental impact and cost-benefit was suggested to improve sustainability assessment.
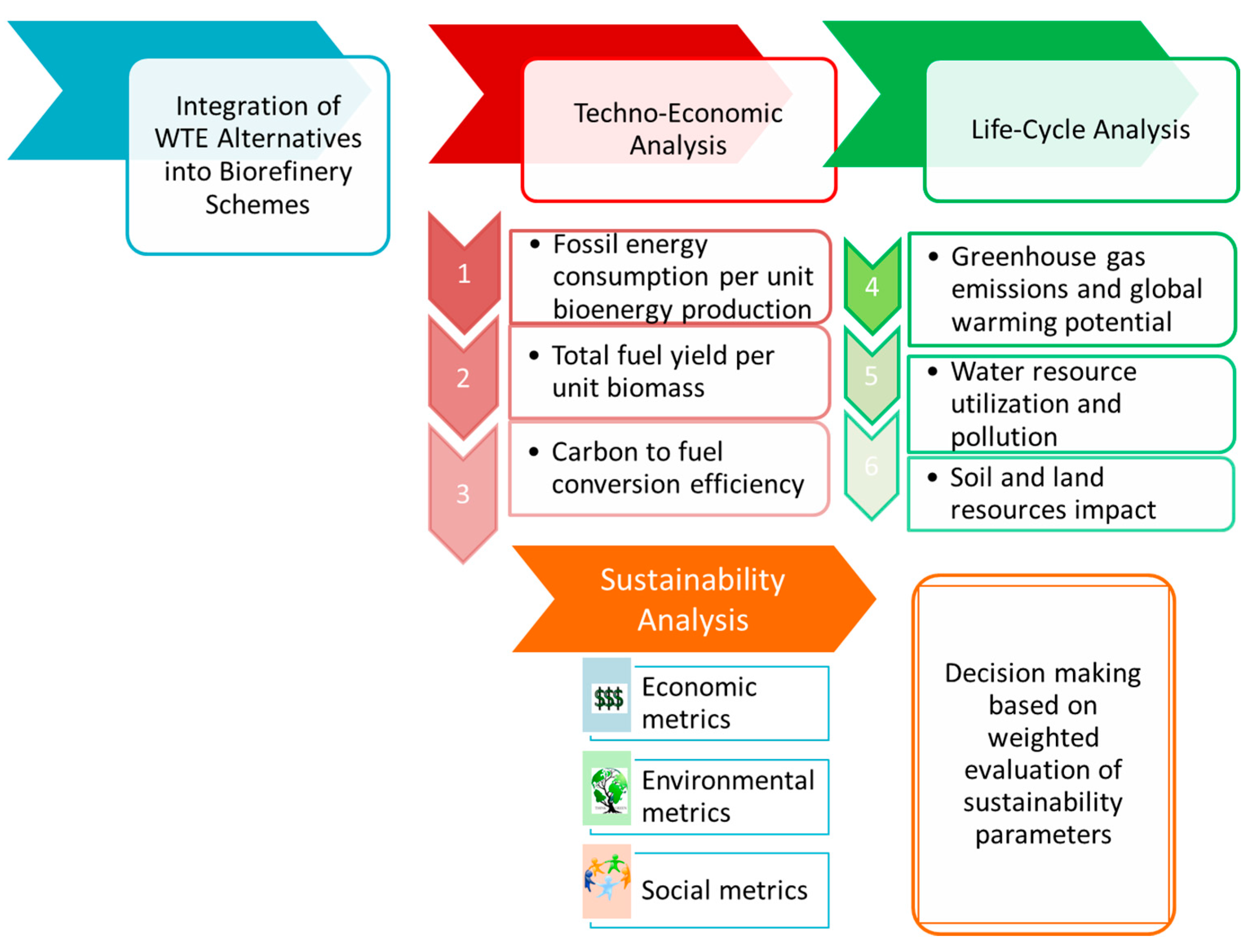
Figure 3 shows our proposed approach to understand the impact of biorefinery processes on the earth’s resources, society, economics and the environment. The following sustainability parameters are considered in the first step for comparison of the baseline process schemes: greenhouse gas emissions (both, fossil-derived as well as biogenic), fossil energy consumption per unit bioenergy production, total fuel yield per unit biomass, carbon to fuel conversion efficiency, water consumption and wastewater generation. The thermochemical, biochemical and algal pathways have been modeled previously and their techno-economic as well as life cycle analysis have been reported. The primary parameters for these processes related to sustainability are compiled here. Additional sustainability metrics need to be developed using criteria reported in the literature [
25,
29,
30,
31]. This will allow complete analysis of the economic, environmental and social impacts resulting in a holistic assessment of the various pathways. In this paper, we primarily focus on the 6 parameters listed above and the potential changes in these parameters resulting from implementation of BES systems.
Figure 3.
Sustainability assessment approach to quantify impact on resources and environment.
Figure 3.
Sustainability assessment approach to quantify impact on resources and environment.
5. Sustainability Considerations during Thermochemical Biomass Processing
The renewable fuel standard (RFS2) mandates set by the US Environmental Protection Agency (EPA) require 50% reduction in lifecycle greenhouse gas (GHG) emissions for advanced biofuels and 60% reduction for cellulosic fuels to qualify as renewable fuels, as legislated by the Energy Independence and Security Act (EISA). As such, the sustainability criteria are as important as economic criteria for production of biofuels. Water is another important component of the sustainability criteria, although at present no fixed guidelines are set for use of water in the biorefinery. A number of unit operations have to be considered when evaluating the GHG emissions and water use in the biorefinery. An important component in assessing the environmental impact of biorefinery operations is the “balance of plant” part in the biorefinery (
Figure 1). This encompasses the process units and site operations that support the main-biomass-to-fuel conversion steps. Primary components of the balance of plant include operations for emissions abatement, wastewater treatment, heat and power generation, waste disposal,
etc. Hydrogen generation may be included in the balance of plant or as a separate unit operation. Either way, it is an important component of the GHG analysis since generating hydrogen at the plant is intimately associated with gas emissions as well as wastewater treatment and heat and power generation.
In the thermochemical conversion process (
Figure 2A), hydrogen is produced partially from the pyrolysis and hydrotreatment effluent gas streams, supplemented by natural gas to balance the energy and carbon flow in the process. The techno-economic analysis for the process indicates a requirement of 3.7 MMscf/day requirement of natural gas to support a 2000 metric ton per day plant converting biomass to hydrocarbon fuels [
13]. This amounts to about 3.95 kg of natural gas per 100 kg of biomass processed. A mass balance on the thermochemical process indicates almost four-fold higher hydrogen requirement for the upgrading and hydrotreatment of bio-oil to gasoline and diesel fuel/blendstock. This extra hydrogen is derived from the off-gases from the pyrolysis and hydrotreating units. In this process, the unit operations are tweaked to produce a syngas which can serve as the feed to the reformer, however, it still produces a bio-oil which contains water-soluble compounds which are difficult substrates for hydrotreatment. Despite this integration, the overall energy efficiency of the process is approximately 48.4%. The alternative process scheme shown in
Figure 2B using MEC converts the acidic, polar compounds in the bio-oil, which are difficult to treat in the hydrotreater, to generate the hydrogen via MEC. This pathway can potentially reduce the energy losses by producing hydrogen from the bio-oil aqueous phase directly and operation under ambient conditions. Potential bioelectrochemical losses, however, do exist via this pathway [
1], which still need to be evaluated and quantified. Nevertheless, this alternative has many advantages that might make it more sustainable and energy efficient. These include direct conversion of the compounds, such as acetic acid and furan aldehydes and other bio-oil compounds, to hydrogen as compared to reforming of the bio-oil or the off-gas steam, reduced heat loss due to operation of MEC at ambient temperature, a combined biocatalytic-electrocatalytic pathway
vs. thermocatalysis route for production of hydrogen, and reduced need for water for the reforming process to produce hydrocarbons.
Table 1 lists the environmental sustainability metrics for the conventional biomass conversion processes [
16]. The GHG emissions from the thermochemical process (
Figure 2A) for conversion of lignocellulosic feedstock to gasoline/diesel blendstock is 19.8 g CO
2e/MJ fuel for the state of technology that existed in 2012 [
16]. A reduction in this amount is projected with a target of 18.9 g CO
2e/MJ fuel by 2017. Use of natural gas for hydrogen production contributes about 47% or 9.2 g CO
2e/MJ fuel. Reducing use of natural gas in the process is one alternative to reduce the GHG emissions. Partitioning of the organic compounds present in the bio-oil into an aqueous phase can remove more than 50% of the carbon [
32]. A typical bio-oil yield from fast pyrolysis of biomass is about 60 wt %. Thus, there is sufficient organic matter in the aqueous phase to produce hydrogen entirely from the biomass-derived bio-oil aqueous phase. Recent work has demonstrated a 54% overall efficiency in converting the bio-oil aqueous phase to hydrogen using MEC with a switchgrass-derived stream [
7,
20]. The second metric for sustainability is the amount of fossil energy used to generate biofuel. Reduction in natural gas use for hydrogen production also reduces this amount. Although the target for 2017 listed in
Table 1 is higher than the 2012 SOT, it may be due to economic considerations being more dominant compared to environmental considerations. The total fuel yield per dry ton of biomass is the third metric, which is projected to increase by 2017. This parameter will decrease if use of natural gas is replaced by biomass in the proposed pathway, since the energy required to upgrade biomass into hydrocarbon fuel will then come from biomass itself
vs. natural gas, reducing the yield of fuel. The trend in this metric may need to be evaluated on the basis of economic considerations. As long as the RFS2 targets are met, it may make sense to derive hydrogen partially from sources other than the MEC process. The fourth metric is carbon to fuel efficiency, which can certainly be increased using the proposed pathway utilizing MEC. The ability to get to 47% via the steam reforming pathway can be very difficult, since reducing input of natural gas will reduce biofuel yield
vs. increasing it.
Table 1.
Baseline sustainability metrics for key biomass to biofuel conversion processes [
16].
Table 1.
Baseline sustainability metrics for key biomass to biofuel conversion processes [16].
| Environmental Sustainability Metric | Thermochemical Process—Figure 2A [13] | Biochemical Process—Figure 3A [33] | Algae-based Process—Figure 4A [34] |
|---|
| | 2012 SOT */2017 projection | 2017 projection | 2014 |
| Greenhouse gases (g CO2e/MJ fuel)-fossil emissions; biogenic emissions) | 19.8; 104/18.9; 85 | 15.9; NA | 10.4 |
| Fossil energy consumption (MJ fossil energy/MJ fuel product) | 0.294/0.301 | 0.27 | 0.17 |
| Total fuel yield (gal /dry biomass; GGE/dry ton biomass) | 74; 78/84; 87 | 43.3; NA | 141.1; NA |
| Carbon-to-fuel efficiency (C in fuel/C in biomass) | 38%/47% | 26.2% | 63% |
| Water consumption (m3/day; gal/GGE) | 998; 1.5/1050; 1.4 | 5209; 13.7 | 1876 #; 2.6 |
| Wastewater generation (m3/day; gal/GGE) | 917; 1.4/932; 1.3 | NA | NA; −5.1 |
Water consumption in the conventional pathway is high due to the loss of water via flue gases, need for make-up water for steam reforming, use of steam for power generation and the use of cooling towers to remove heat from product streams. About 51% of the total water used is needed for steam reformer. Eliminating this unit operation can reduce the water requirement by half. Additionally, water loss via flue gases will reduce since the water is maintained at ambient conditions, post-pyrolysis via its separation from bio-oil and routing via MEC to remove the organic matter in that stream. The MEC effluent can potentially be recycled or cleaned to discharge limits via reducing the total water needs for the biorefinery [
6]. This will result in reduction in the sixth metric, which is wastewater generation in the plant. Typical chemical oxygen demand (COD) of the thermochemical process wastewater was reported to be about 10 g/L at a flow rate of 917 m
3/day for a 2000 metric ton per day plant [
13]. The volume of the wastewater can be reduced when MECs are employed to treat the bio-oil aqueous phase, by reducing the feed to the hydrotreater. Secondly, the COD of the wastewater emanating from the MEC/BES can be well below 0.3 g/L [
6], enabling its recycle, reducing volume and concentration of the wastewater generated.
6. Sustainability Considerations for Biochemical Conversion Process
Focus on production of gasoline and diesel blendstock has warranted changes in the traditional biochemical conversion platform to generate intermediates for hydrocarbon production
vs. ethanol.
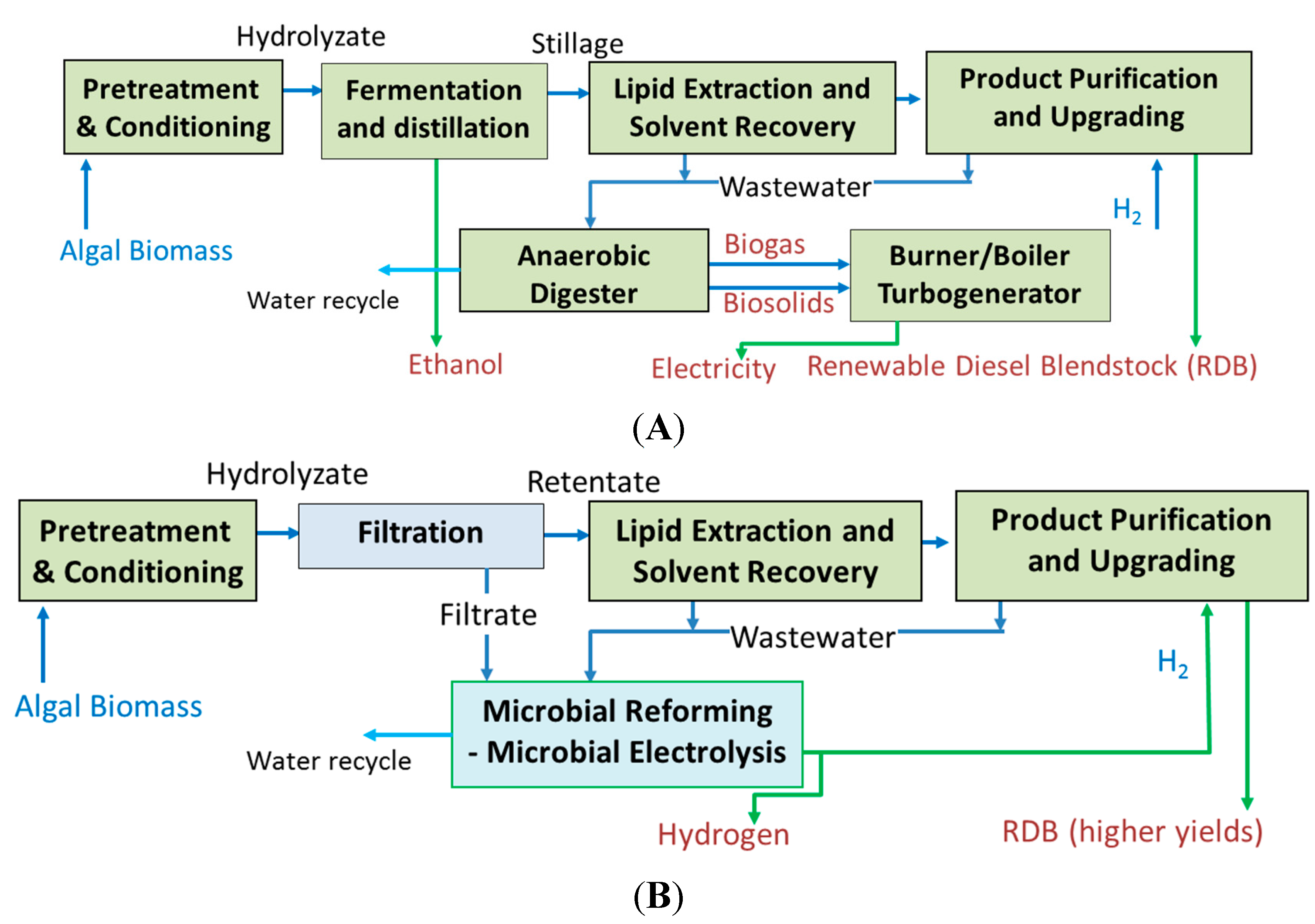
Figure 4 shows the pathways for production of hydrocarbon fuels via the bioconversion platform. The sugar stream resulting from hydrolysis is sent to a bioconversion reactor to produce free fatty acids (FFA), which are then sent to an upgrading process to make renewable diesel blendstock (
Figure 4A). The upgrading process requires hydrogen. Therefore, a separate source of hydrogen (besides the biomass resource) is needed. In the existing pathway, the wastewater from pretreatment containing acetic acid and stillage streams are sent to anaerobic digester, followed by utilization of the resulting biogas and biosolids sludge in a combustor. In an alternate
Figure 4B, use of microbial electrolysis is included. This can eliminate the need for an external source of hydrogen. Secondly, the lignin generated in the process may be better utilized if it is converted to hydrocarbons or a bio-oil [
35,
36]. This can produce a low-oxygen product which can serve as a feedstock for the upgrading reactor, increasing hydrocarbon yield and aromatic content of the blendstock (
Figure 4C).
Figure 4.
Biochemical pathways for conversion of biomass to biofuels. (A) Pathway based on bioconversion of sugars to free fatty acids, followed by upgrading to produce renewable diesel blendstock (RDB); (B) Pathway integrating microbial electrolysis for hydrogen production from stillage and pretreatment waste streams; (C) Further modification including lignin pyrolysis and hydrotreatment to generate upgraded gasoline/diesel blendstock.
Figure 4.
Biochemical pathways for conversion of biomass to biofuels. (A) Pathway based on bioconversion of sugars to free fatty acids, followed by upgrading to produce renewable diesel blendstock (RDB); (B) Pathway integrating microbial electrolysis for hydrogen production from stillage and pretreatment waste streams; (C) Further modification including lignin pyrolysis and hydrotreatment to generate upgraded gasoline/diesel blendstock.
Table 1 shows the sustainability metrics for the process shown in
Figure 4A [
33]. The GHG emissions from the process outlined in
Figure 3C is reported to be 15.9 g CO
2e/MJ. If a co-product credit is included in the analysis for the renewable electricity produced from lignin, the GHG emissions reduce significantly by over 95%. The net fossil energy consumption for the process is 0.27 MJ/MJ, however, utilizing the co-product credits, this usage drops to 0.085 MJ/MJ. The consumptive water use is significantly high at 5209 m
3/day or 13.7 gallons/gge, about 9 times that of the thermochemical process. This is due to the losses resulting from renewable electricity production from lignin, which also results in evaporation and loss of water. Incorporation of MEC into the process can enable reduction in water usage due to recycle of the effluent from MEC to the pretreatment step. This is possible due to the potential of BESs to reach much lower effluent COD compared to an anaerobic digester [
11]. Total fuel production efficiency for the
Figure 4A is 43.3 gal/dry ton of biomass. Production of hydrogen
vs. electricity from the waste streams instead of conversion via an anaerobic digester-combustor/turbogenerator can reduce the fossil energy usage significantly. A true understanding of the effect of these two alternatives on net change in GHG emissions and other sustainability metrics requires a techno-economic analysis, such as the one reported for
Figure 4A [
33].
7. Sustainability Considerations for Algal Conversion Process
Conversion of algal biomass to hydrocarbon blendstock has been modeled via a lipid-extraction step to remove the non-lipid component, followed by hydrotreatment of the lipids to generate renewable diesel blendstock (RDB) [
34]. In addition, ethanol is produced via fermentation of the non-lipid component and electricity is generated from anaerobic digestion of waste components and combined heat and power system (CHP) (
Figure 5A). This process also requires hydrogen to hydrotreat the lipids to make the RDB. The flow diagram shown in
Figure 5A is based on non-storage option where all algal biomass is used in the plant directly. Implementing an MEC in this process (
Figure 5B) can potentially eliminate two major unit operations and potentially replace the fermentation unit operation with a simpler filtration step. Since hydrogen is required in the process, replacing the anaerobic digester and CHP unit with MEC can enable hydrogen generation internally and minimize use of natural gas. This can potentially reduce the capital costs related to the steam reformer for hydrogen production, but will add costs related to MEC installation.
The GHG emissions from the algal process are relatively low compared to the biochemical and thermochemical conversion processes (
Table 1). The net fossil energy consumption is also lower, although the process model in
Figure 5A does not include algal production steps. While the fossil energy consumption is lower, it can be further reduced by including MEC in the process scheme as shown in
Figure 5B. The water use included in
Table 1 does not include water needed for algal biomass production. The use of MEC can benefit on-site growth of algal biomass as well, since the MEC produces effluent which retains most of the nutrients in the influent, while removing only the COD. The total carbon efficiency for the algal process is relatively high at 63%, although this does not include the contribution of the natural gas for hydrogen production used in the process. Inclusion of the carbon consumed due to natural gas usage decreases the carbon efficiency to 58%. The production of hydrogen in the process can be increased further by diverting the non-lipid portion of the extracted biomass to MEC for hydrogen production instead of ethanol production. The excess hydrogen may serve as a co-product and will be at least as valuable as ethanol in contributing to the economic feasibility of the process.
Figure 5.
Algal processing into biofuels and products. (
A) Conversion of algal biomass into renewable diesel blendstock [
34]; (
B) Process incorporating microbial electrolysis cells (MEC) in place of anaerobic digestion and combined heat and power (CHP) to generate hydrogen.
Figure 5.
Algal processing into biofuels and products. (
A) Conversion of algal biomass into renewable diesel blendstock [
34]; (
B) Process incorporating microbial electrolysis cells (MEC) in place of anaerobic digestion and combined heat and power (CHP) to generate hydrogen.