AMF Inoculation Enhances Growth and Improves the Nutrient Uptake Rates of Transplanted, Salt-Stressed Tomato Seedlings
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material and Experimental Set-Up
2.2. Plant Sampling and Measurements
2.3. Statistics
3. Results
3.1. Growth
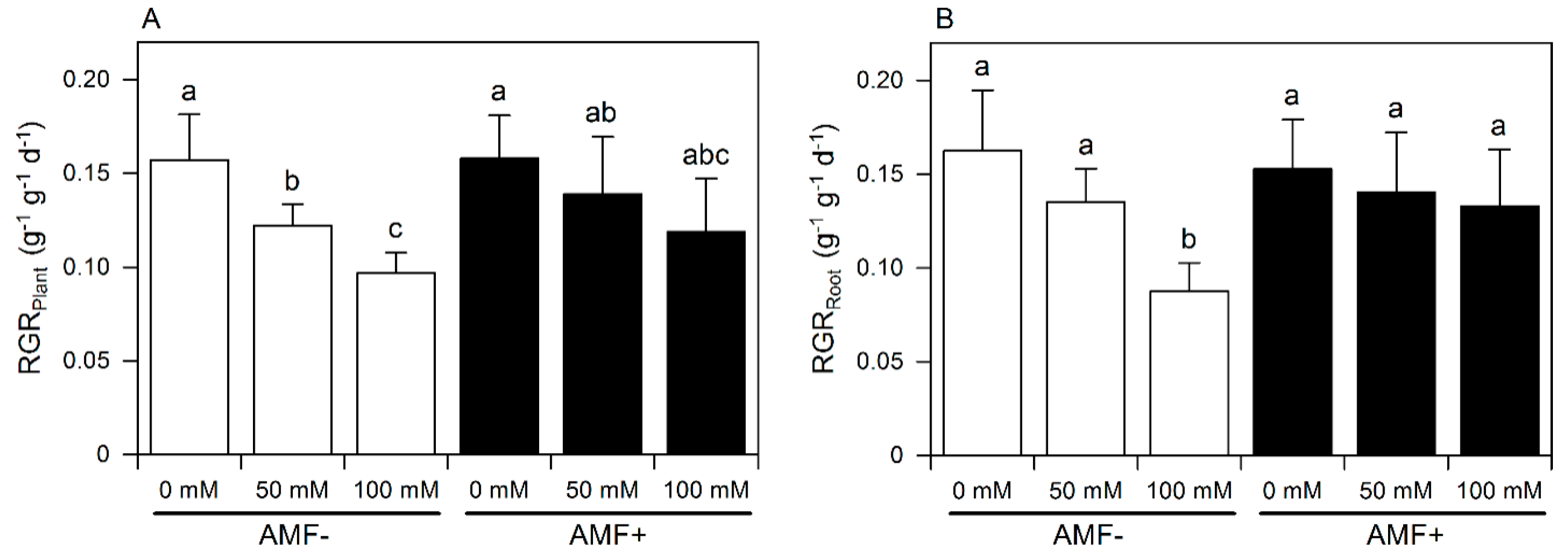
| Sources of Variation | DMPlant | DMRoot | R:S | RGRPlant | RGRRoot |
|---|---|---|---|---|---|
| AMF | 0.855 | 0.205 | 0.234 | 0.053 | 0.079 |
| Salinity | 0.000 | 0.000 | 0.182 | 0.000 | 0.000 |
| AMF × salinity | 0.263 | 0.010 | 0.190 | 0.408 | 0.147 |

3.2. C and N Content and C:N Ratio
| Sources of Variation | N | C | C:N |
|---|---|---|---|
| AMF | 0.053 | 0.031 | 0.004 |
| Salinity | 0.000 | 0.000 | 0.000 |
| AMF × salinity | 0.046 | 0.271 | 0.000 |
| Factors | N (mg g−1) | C (mg g−1) | C:N | |
|---|---|---|---|---|
| AMF− | 0 mM·NaCl | 1.75 ± 0.33 b | 18.7 ± 2.96 b | 10.7 ± 0.64 c |
| 50 mM·NaCl | 1.05 ± 0.25 d | 12.5 ± 2.26 c | 11.2 ± 1.14 b | |
| 100 mM·NaCl | 1.09 ± 0.12 d | 12.2 ± 1.12 c | 12.1 ± 0.54 b | |
| AMF+ | 0 mM·NaCl | 2.06 ± 0.23 a | 22.4 ± 2.84 a | 10.8 ± 0.35 c |
| 50 mM·NaCl | 1.35 ± 0.11 c | 16.1 ± 1.71 b | 11.8 ± 0.40 b | |
| 100 mM·NaCl | 0.95 ± 0.14 d | 12.9 ± 1.83 c | 13.5 ± 0.24 a | |
3.3. Leaf Nutrient Concentration and Relative Uptake Rate (RUR)
| Sources of Variation | N | K | Ca | Mg | P | Na | Mn | Fe | Al | S |
|---|---|---|---|---|---|---|---|---|---|---|
| AMF | 0.000 | 0.375 | 0.000 | 0.000 | 0.000 | 0.000 | 0.007 | 0.865 | 0.000 | 0.446 |
| Salinity | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| AMF × salinity | 0.028 | 0.015 | 0.130 | 0.557 | 0.037 | 0.108 | 0.557 | 0.058 | 0.408 | 0.196 |
| Factors | N (mg·mg−1·day−1) | K (mg·mg−1·day−1) | Ca (mg·mg−1·day−1) | Mg (mg·mg−1·day−1) | P (µg·µg−1·day−1) | Na (µg·µg−1·day−1) | Mn (µg·µg−1·day−1) | Fe (µg·µg−1·day−1) | Al (µg·µg−1·day−1) | S (µg·µg−1·day−1) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AMF− | 0 mM | 0.153 ± 0.02 b,c | 0.179 ± 0.03 b | 0.151 ± 0.03 b | 0.147 ± 0.03 b | 0.064 ± 0.03 b | 0.206 ± 0.00 d | 0.155 ± 0.03 b | 0.150 ± 0.02 c | 0.139 ± 0.00 a | 0.167 ± 0.03 a |
| 50 mM | 0.078 ± 0.03 c | 0.142 ± 0.03 b | 0.089 ± 0.03 c | 0.110 ± 0.03 c | 0.037 ± 0.01 c | 0.348 ± 0.00 b | 0.110 ± 0.03 c | 0.063 ± 0.03 d | 0.060 ± 0.00 b | 0.063 ± 0.03 b | |
| 100 mM | 0.087 ± 0.01 c | 0.135 ± 0.02 c | 0.094 ± 0.00 c | 0.100 ± 0.00 c | 0.021 ± 0.01 c | 0.293 ± 0.00 c | 0.102 ± 0.01 c | 0.076 ± 0.01 d | 0.025 ± 0.00 c | 0.070 ± 0.01 b | |
| AMF+ | 0 mM | 0.220 ± 0.01 a | 0.223 ± 0.02 a | 0.205 ± 0.02 a | 0.201 ± 0.02 a | 0.130 ± 0.02 a | 0.271 ± 0.00 c | 0.201 ± 0.03 a | 0.160 ± 0.02 a | 0.074 ± 0.00 b | 0.173 ± 0.02 a |
| 50 mM | 0.160 ± 0.01 b | 0.156 ± 0.02 b | 0.140 ± 0.02 b | 0.153 ± 0.01 b | 0.055 ± 0.01 b | 0.381 ± 0.00 a | 0.145 ± 0.03 b | 0.082 ± 0.02 b | 0.033 ± 0.00 c | 0.074 ± 0.02 b | |
| 100 mM | 0.109 ± 0.02 c | 0.104 ± 0.02 c | 0.103 ± 0.02 c | 0.130 ± 0.02 c | 0.045 ± 0.02 b | 0.373 ± 0.00 a | 0.118 ± 0.02 c | 0.042 ± 0.02 e | 0.040 ± 0.00 c | 0.038 ± 0.01c | |
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Flowers, T.J. Improving crop salt tolerance. J. Exp. Bot. 2004, 55, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Shelden, M.C.; Roessner, U. Advances in functional genomics for investigating salinity stress tolerance mechanisms in cereals. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Cuartero, J.; Bolarín, M.C.; Asíns, M.J.; Moreno, V. Increasing salt tolerance in the tomato. J. Exp. Bot. 2006, 57, 1045–1058. [Google Scholar] [CrossRef] [PubMed]
- Läuchli, A.; James, R.A.; Huang, C.X.; McCully, M.; Munns, R. Cell-specific localization of Na+ in roots of durum wheat and possible control points for salt exclusion. Plant Cell Environ. 2008, 31, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; Annunziata, M.G.; Pontecorvo, G.; Fuggi, A.; Woodrow, P. Salinity Stress and Salt Tolerance. In Abiotic Stress in Plants-Mechanisms and Adaptations; InTech: Rijeka, Croatia, 2011; pp. 21–38. [Google Scholar]
- Munns, R.; Goyal, S.; Passioura, J. Salinity Stress and Its Mitigation. Available online: http://www.plantstress.com/Articles/index.asp (accessed on 26 November 2015).
- Costa, J.M.; Heuvelink, E. Introduction: The Tomato Crop and Industry. In Tomatoes; Heuvelink, E., Ed.; CABI Publishing, CAB International: Oxfordshire, UK, 2005; pp. 1–21. [Google Scholar]
- Perez-Alfocea, F.; Balibrea, M.E.; Cruz, A.S.; Estae, M.T. Agronomical and physiological characterization of salinity tolerance in a commercial tomato hybrid. Plant Soil 1996, 180, 251–257. [Google Scholar] [CrossRef]
- Abeer, H.; Ef, A.; Alqarawi, A.A.; Mona, A. Arbuscular mycorrhizal fungi mitigates NaCl induced adverse effects on Solanum lycopersicum L. Pak. J. Bot. 2015, 47, 327–340. [Google Scholar]
- Jahromi, F.; Aroca, R.; Porcel, R.; Ruiz-Lozano, J.M. Influence of Salinity on the In Vitro Development of Glomus intraradices and on the In Vivo Physiological and Molecular Responses of Mycorrhizal Lettuce Plants. Microb. Ecol. 2008, 55, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Abdel Latef, A.A.H.; He, C. Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Sci. Hortic. 2011, 127, 228–233. [Google Scholar] [CrossRef]
- Cavagnaro, T.R.; Jackson, L.E.; Six, J.; Ferris, H.; Goyal, S.; Asami, D.; Scow, K.M. Arbuscular mycorrhizas, microbial communities, nutrient availability, and soil aggregates in organic tomato production. Plant Soil 2006, 282, 209–225. [Google Scholar] [CrossRef]
- Feng, G.; Li, X.L.; Zhang, F.S.; Li, S.X. Effect of phosphorus and arbuscular mycorrhizal fungus on response of maize plant to saline environment. J. Plant Res. Environ. 2000, 9, 22–26. [Google Scholar]
- Huang, J.C.; Lai, W.A.; Singh, S.; Hameed, A.; Young, C.C. Response of mycorrhizal hybrid tomato cultivars under saline stress. J. Soil Sci. Plant Nutr. 2013, 13, 469–484. [Google Scholar] [CrossRef]
- Huang, Z.; He, C.X.; He, Z.Q.; Zou, Z.R.; Zhang, Z.B. The effects of arbuscular mycorrhizal fungi on reactive oxyradical scavenging system of tomato under salt tolerance. Agric. Sci. China 2010, 9, 1150–1159. [Google Scholar] [CrossRef]
- Tüzel, Y.; Öztekin, G.B.; Tüzel, I.H. Does mycorrhiza improve salinity tolerance in grafted plants? Acta Hortic. 2012, 960, 57–69. [Google Scholar] [CrossRef]
- Porcel, R.; Aroca, R.; Ruiz-Lozano, J.M. Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron. Sustain. Dev. 2012, 32, 181–299. [Google Scholar] [CrossRef]
- Rewald, B.; Holzer, L.; Göransson, H. Arbuscular mycorrhiza inoculum reduces root respiration and improves biomass accumulation of salt-stressed Ulmus glabra seedlings. Urban For. Urban Green. 2015, 14, 432–437. [Google Scholar] [CrossRef]
- Nouri, E.; Breuillin-Sessoms, F.; Feller, U.; Reinhardt, D. Phosphorus and nitrogen regulate arbuscular mycorrhizal symbiosis in petunia hybrida. PLoS ONE 2014. [Google Scholar] [CrossRef] [PubMed]
- Giananazzi, S.; Schuepp, H.; Barea, J.M.; Haselwandter, K. Mycorrhizal Technology in Agriculture: From Genes to Bioproducts; Birkhauser: Basel, Switzerland, 2001; p. 296. [Google Scholar]
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.N. Ascorbic acid and its effects on alleviation of salt stress in sunflower. Ann. Res. Rev. Biol. 2014, 4, 3656–3665. [Google Scholar] [CrossRef]
- Munns, R.; Termaat, A. Whole-Plant Responses to Salinity. Aust. J. Plant Physiol. 1986, 13, 143–160. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Ann. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [PubMed]
- Azcón-Aguilar, C.; Azcón, R.; Barea, J.M. Endomycorrhizal fungi and Rhizobium as biological fertilizers for Medicago sativa in normal cultivation. Nature 1979, 279, 325–327. [Google Scholar]
- Ruiz-Lozano, J.M.; Azcón, R. Symbiotic efficiency and infectivity of an autochthonous arbuscular mycorrhizal Glomus sp. from saline soils and Glomus deserticola under salinity. Mycorrhiza 2000, 10, 137–143. [Google Scholar] [CrossRef]
- Kadian, N.; Yadav, K.; Badda, N.; Aggarwal, A. AM fungi ameliorates growth, yield and nutrient uptake in Cicer arietinum L. under salt stress. Russ. Agric. Sci. 2013, 39, 321–329. [Google Scholar] [CrossRef]
- Harb, A.; Krishnan, A.; Ambavaram, M.M.R.; Pereira, A. Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiol. 2010, 154, 1254–1271. [Google Scholar] [CrossRef] [PubMed]
- Thwe, A.A.; Vercambre, G.; Gautier, H.; Pagès, L.; Jourdan, C.; Gay, F.; Kasemsap, P. Dynamic shoot and root growth at different developmental stages of tomato (Solanum lycopersicum Mill.) under acute ozone stress. Sci. Hortic. 2013, 150, 317–325. [Google Scholar] [CrossRef]
- Akinci, S.; Yilmaz, K.; Akinci, I.E. Response of tomato (Lycopersicon esculentum Mill.) to salinity in the early growth stages for agricultural cultivation in saline environments. J. Environ. Boil. Acad. Environ. Biol. India 2004, 25, 351–357. [Google Scholar]
- Cruz, V.; Cuartero, J.; Gómez-Guillamón, M.; Fernández-Muñoz, R. Effects of salinity at several developmental stages of six genotypes of tomato (Lycopersicon spp). In Proceedings of the XIth Eucarpia Meeting on Tomato Genetics and Breeding, Málaga, Spain, 6–8 March 1990; pp. 81–86.
- Dumbroff, E.; Cooper, A. Effects of salt stress applied in balanced nutrient solutions at several stages during growth of tomato. Bot. Gaz. 1974, 135, 219–224. [Google Scholar] [CrossRef]
- Rubinigg, M.; Elzenga, J.T.M.; Stulen, I. Effects of NaCl salinity on nitrate uptake and partitioning of N and C in Festuca rubra L. in relation to growth rate. Phyt. Ann. Rei Bot. 2002, 42, 251–267. [Google Scholar]
- Hoffmann, W.A.; Poorter, H. Avoiding bias in calculations of relative growth rate. Ann. Bot. 2002, 90, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Hunt, R.; Causton, D.R.; Shipley, B.; Askew, A.P. A modern tool for classical plant growth analysis. Ann. Bot. 2002, 90, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Aprile, G.G.; Di Salvatore, M.; Carratù, G.; Mingo, A.; Carafa, A.M. Comparison of the suitability of two lichen species and one higher plant for monitoring airborne heavy metals. Environ. Monit. Assess. 2010. [Google Scholar] [CrossRef] [PubMed]
- Leskovar, D.I. Root and Shoot Modification by. Hortic. Technol. 1998, 8, 510–514. [Google Scholar]
- Del Amor, F.M.; Martinez, V.; Cerdá, A. Salt tolerance of tomato plants as affected by stage of plant development. HortScience 2001, 36, 1260–1263. [Google Scholar]
- Babaj, I.; Sallaku, G.; Balliu, A. The effects of endogenous mycorrhiza (Glomus spp.) on plant growth and yield of grafted cucumber (Cucumis sativum L.) under common commercial greenhouse conditions. Alban. J. Agric. Sci. 2014, 13, 24–28. [Google Scholar]
- Balliu, A.; Sallaku, G.; Islami, E. Root Pruning Effects on Seedlings’ Growth and Plant Stand Establishment Rate of Watermelon Grafted Seedlings. Acta Hortic. 2014, 1033, 19–24. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Sonmez, O.; Aydemir, S.; Tuna, A.L.; Cullu, M.A. The influence of arbuscular mycorrhizal colonisation on key growth parameters and fruit yield of pepper plants grown at high salinity. Sci. Hortic. 2009, 121, 1–6. [Google Scholar] [CrossRef]
- Al-Karaki, G.N. Growth of mycorrhizal tomato and mineral acquisition under salt stress. Mycorrhiza 2000, 10, 51–54. [Google Scholar] [CrossRef]
- Al-Karaki, G.N. Nursery inoculation of tomato with arbuscular mycorrhizal fungi and subsequent performance under irrigation with saline water. Sci. Hortic. 2006, 109, 1–7. [Google Scholar] [CrossRef]
- Copeman, R.H.; Martin, C.A.; Stutz, J.C. Tomato growth in response to salinity and mycorrhizal fungi from saline or nonsaline soils. HortScience. 1996, 31, 341–344. [Google Scholar]
- Gomes, M.A.D.C.; Suzuki, M.S.; Cunha, M.D.; Tullii, C.F. Effect of salt stress on nutrient concentration, photosynthetic pigments, proline and foliar morphology of Salvinia auriculata Aubl. Acta Limnol. Bras. 2011, 23, 164–176. [Google Scholar] [CrossRef]
- Grattan, S.R.; Grieve, C.M. Salinity-mineral nutrient relations in horticultural crops. Sci. Hortic. 1998, 78, 127–157. [Google Scholar] [CrossRef]
- Evelin, H.; Giri, B.; Kapoor, R. Contribution of Glomus intraradices inoculation to nutrient acquisition and mitigation of ionic imbalance in NaCl-stressed Trigonella foenum-graecum. Mycorrhiza 2012, 22, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Ågren, G.I. The C:N:P stoichiometry of autotrophs—Theory and observations. Ecol. Lett. 2004, 7, 185–191. [Google Scholar] [CrossRef]
- Peng, Y.; Niklas, K.J.; Sun, S. The relationship between relative growth rate and whole-plant C:N:P stoichiometry in plant seedlings grown under nutrient-enriched conditions. J. Plant Ecol. 2011, 4, 147–156. [Google Scholar] [CrossRef]
- Bach Allen, E.; Cunningham, G.L. Effects of vesicular-arbuscular mycorrhizae on Distichlis spicata under three salinity levels. New Phytol. 1983, 93, 227–236. [Google Scholar] [CrossRef]
- Cantrell, I.C.; Linderman, R.G. Preinoculation of lettuce and onion with VA mycorrhizal fungi reduces deleterious effects of soil salinity. Plant Soil 2001, 233, 269–281. [Google Scholar] [CrossRef]
- Liu, J. A calcium sensor homolog required for plant salt tolerance. Science 1998, 280, 1943–1945. [Google Scholar] [CrossRef] [PubMed]
- Giri, B.; Kapoor, R.; Mukerji, K.G. Improved tolerance of Acacia nilotica to salt stress by arbuscular mycorrhiza, Glomus fasciculatum may be partly related to elevated K/Na ratios in root and shoot tissues. Microb. Ecol. 2007, 54, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.V.; Satti, S.M.E. Calcium and potassium-enhanced growth and yield of tomato under sodium chloride stress. Plant Sci. 1996, 114, 19–27. [Google Scholar] [CrossRef]
- Giri, B.; Mukerji, K.G. Mycorrhizal inoculant alleviates salt stress in Sesbania aegyptiaca and Sesbania grandiflora under field conditions: Evidence for reduced sodium and improved magnesium uptake. Mycorrhiza 2004, 14, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Giri, B.; Kapoor, R.; Mukerji, K.G. Influence of arbuscular mycorrhizal fungi and salinity on growth, biomass, and mineral nutrition of Acacia auriculiformis. Biol. Fertil. Soils 2003, 38, 170–175. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic: London, UK, 1995; p. 889. [Google Scholar]
- Al-Harbi, A.R. Growth and nutrient composition of tomato and cucumber seedlings as affected by sodium chloride salinity and supplemental calcium. J. Plant Nutr. 1995, 18, 1403–1416. [Google Scholar] [CrossRef]
- Mass, E.; Ogata, G.; Garber, M. Influence of salinity on Fe, Mn, and Zn uptake by plants. Agron. J. 1972, 64, 793–795. [Google Scholar] [CrossRef]
- Rengel, Z. Role of calcium in aluminium toxicity. New Phytol. 1992, 121, 499–513. [Google Scholar] [CrossRef]
- Mor, R.; Manchanda, H. Influence of phosphorus on the tolerance of table pea to chloride and sulfate salinity in a sandy soil. Arid Land Res. Manag. 1992, 6, 41–52. [Google Scholar] [CrossRef]
- Schnoor, T.K.; Lekberg, Y.; Rosendahl, S.; Olsson, P.A. Mechanical soil disturbance as a determinant of arbuscular mycorrhizal fungal communities in semi-natural grassland. Mycorrhiza 2011, 21, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, E.; Röling, W.F.; Gamper, H.A.; Kowalchuk, G.A.; Verhoef, H.A.; van der Heijden, M.G. Positive effects of organic farming on below-ground mutualists: Large-scale comparison of mycorrhizal fungal communities in agricultural soils. New Phytol. 2010, 186, 968–979. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balliu, A.; Sallaku, G.; Rewald, B. AMF Inoculation Enhances Growth and Improves the Nutrient Uptake Rates of Transplanted, Salt-Stressed Tomato Seedlings. Sustainability 2015, 7, 15967-15981. https://doi.org/10.3390/su71215799
Balliu A, Sallaku G, Rewald B. AMF Inoculation Enhances Growth and Improves the Nutrient Uptake Rates of Transplanted, Salt-Stressed Tomato Seedlings. Sustainability. 2015; 7(12):15967-15981. https://doi.org/10.3390/su71215799
Chicago/Turabian StyleBalliu, Astrit, Glenda Sallaku, and Boris Rewald. 2015. "AMF Inoculation Enhances Growth and Improves the Nutrient Uptake Rates of Transplanted, Salt-Stressed Tomato Seedlings" Sustainability 7, no. 12: 15967-15981. https://doi.org/10.3390/su71215799







