1. Introduction
The contamination of groundwater is a widespread problem, which can cause serious environmental and public health drawbacks. Many different pollutants are commonly found in groundwater, both inorganic (e.g., heavy metals) and organic (e.g., hydrocarbons), and multiple contaminations often occur simultaneously. The main direct pathways of exposure for humans are represented by the consumption of drinking water, dermal absorption and the inhalation of volatiles emitted from the contaminated groundwater. However, indirect sources can also be individuated, such as the contamination of soils and surface waters, the consumption of agricultural products irrigated with contaminated groundwater, the ingestion of food prepared in contaminated water and by bio-magnification in the food chain [
1].
Aromatic organic compounds, such as BTEX (benzene, toluene, ethylbenzene and xylene) or PAHs (polycyclic aromatic hydrocarbons) are frequently detected in groundwater with releases occurring during their production, transportation and storage [
2,
3,
4]. The first ones are important industrial solvents, frequently encountered in many industrial operations, such as solvents for organic synthesis, equipment cleansing and other downstream processing purposes [
2,
5]. The others are ubiquitous contaminants resulting from the burning of fossil fuels and, more generally, of organic materials; they are present in the environment as a mixture of many compounds [
5]. All of these compounds can potentially harm human receptors; additionally, these contaminants would render surface and/or groundwater unsuitable for many purposes, due to their toxic and carcinogenic properties [
6].
The physico-chemical properties of organic compounds have a great influence on their environmental fate. They include the possibility of transport across phases by volatilization, sorption and dissolution phenomena. Moreover, under specific conditions, slow biodegradation phenomena might occur, both in aerobic and anaerobic conditions, which can lead to the transformation of these compounds into simpler and less hazardous molecules. However, the occurrence and persistence in groundwater are mainly related to their solubility in water, which, in turn, is a function of different properties, such as molecule dimensions, the number of aromatic rings, substitutions,
etc. [
3,
7]. For instance, PAHs with a low number of aromatic rings (e.g., naphthalene) are the most soluble ones in water and can cause persistent contamination of the environment [
5]. In the water environment, PAHs go through a very slow biodegradation process, because of their general xenobiotic nature. These compounds are generally resistant to hydrolysis, and a combination of different processes must be adopted for a biological transformation, e.g., coupled with advanced oxidation processes (AOPs) [
8,
9].
The high hazard level associated with these aromatic compounds has led to the adoption of very stringent regulation limits by the European Union and the U.S. Environmental Protection Agency. In order to comply with the standards on groundwater quality and wastewater emissions, several researchers have worked on the individuation of efficient and cost-effective remediation technologies over the last few years. Currently, the removal of aromatic compounds from groundwater is carried out by means of several methods and also by combining different technologies, such as air stripping, often coupled with thermal treatments [
10], advanced oxidation processes (Fenton, ozone, hydrogen peroxide,
etc.) [
8,
11], biological processes (bioremediation, biodegradation in reactors and phytoremediation) [
12,
13] or adsorption [
14,
15,
16,
17].
Among the depuration technologies for groundwater remediation, adsorption has been widely used, because it combines good efficiencies with a reliable and robust process configuration. It is a very versatile process that can be used for both organic compounds and heavy metal capture [
15,
18,
19], as well as for single or multiple contaminations [
20,
21,
22]. The wide application of the adsorption process is also due to the possibility of using different kinds of adsorbents, including natural materials [
23,
24], waste materials [
25] or by-products [
26,
27]. In addition, the removal of pollutants from contaminated groundwater by adsorption can be pursued either by an
ex situ technology (
i.e., pump and treat) or by
in situ technology (
i.e., permeable adsorptive barrier (PAB)) [
28,
29,
30]. In the latter case, different PAB configurations can be adopted, according to the nature of the aquifer, its contamination level and the properties of the pollutants present [
28,
31,
32,
33].
For all of the described applications, the identification of the mechanisms that govern adsorption phenomena and of all of the factors affecting the removal performances of an adsorbent are of fundamental importance. A correct approach should take into account the main thermodynamic parameters of the adsorption process and should investigate both single-compound and multicomponent systems, to assess the adsorption capacity of each pollutant and the possible occurrence of competitive effects [
14,
18,
34,
35,
36].
In this work, the performances of a commercial activated carbon (Filtrasorb 400, provided by Calgon) were experimentally tested for the adsorption of different aromatic compounds, i.e., toluene (tol), o-xylene (oxy), ethylbenzene (eth) and naphthalene (nap), from synthetic groundwater. Batch adsorption tests were conducted at constant pH (7) and temperature (20 °C) for a comparative determination of the factors affecting the adsorption of such compounds. Simultaneously, two different binary systems (i.e., toluene + naphthalene and o-xylene + ethylbenzene) were investigated to assess any adsorption interaction between pollutants of similar chemical characteristics. Finally, a critical interpretation of the experimental results was provided, based on a modeling analysis of the experimental data set, so as to evaluate the field of potential application of activated carbon for groundwater remediation from aromatic organic compounds.
3. Results and Discussion
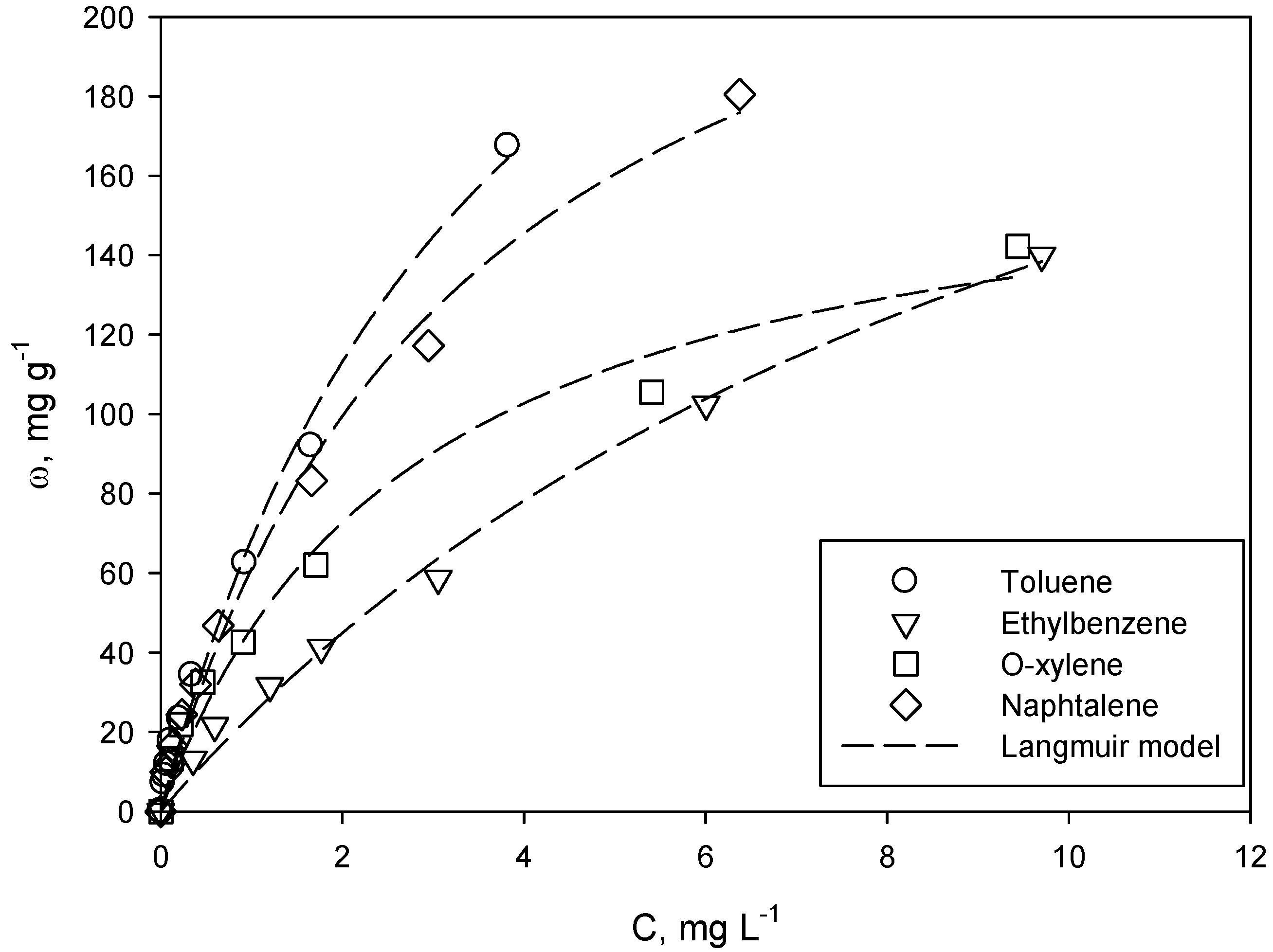
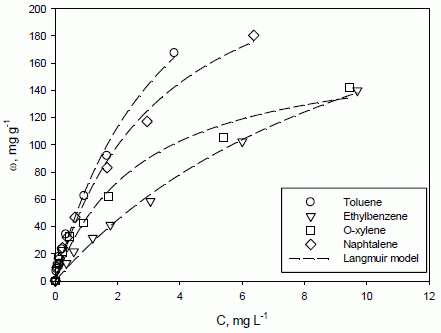
Single-compound adsorption isotherms of all of the investigated analytes, such as the relationships between the adsorbed mass of each compound per mass of the adsorbate (ω, mg g
−1) and the equilibrium liquid concentration (C, mg L
−1), are reported in
Figure 1.
Figure 1.
Adsorption isotherms of toluene, ethylbenzene, o-xylene and naphthalene onto F400 activated carbon. T = 20 °C, equilibrium pH = 7. A comparison between experimental data (symbols) and the Langmuir model (lines).
Figure 1.
Adsorption isotherms of toluene, ethylbenzene, o-xylene and naphthalene onto F400 activated carbon. T = 20 °C, equilibrium pH = 7. A comparison between experimental data (symbols) and the Langmuir model (lines).
For all of the analytes, the adsorption capacity increases with equilibrium concentration, but an asymptotic value is not reached in the investigated range of equilibrium concentration. The results suggest that the F400 activated carbon has a good capability to remove those aromatic compounds from simulated groundwater. In particular, toluene and naphthalene adsorption capacities are the highest and of a similar value. Among all of the investigated compounds, naphthalene is the most hydrophobic, as its octanol-water partition coefficient log-value is equal to 3.37, while for toluene, o-xylene and ethylbenzene, it is 2.74, 3.13 and 3.14, respectively [
38,
39]. In addition, naphthalene is also the least soluble in water, its solubility being 0.03 g L
−1 [
40]. However, the molecule diameter of this compound is 0.91 nm [
40]; hence, it can be inferred that the adsorption of naphthalene is partially limited by its high molecular size. This evidence should be evaluated together with activated carbon properties; in fact, the microporous character of F400 determines a high adsorption surface, but steric hindrance phenomena can likely arise.
The activated carbon structure and the interaction with the different aromatic molecules have a great influence on their adsorption capacity, determining the ranking observed (
Figure 1), as explained in the following. Aromatic compound adsorption is likely to be related to London-van der Waals interactions, and adsorption properties are likely to be independent of sorbent ionization phenomena and ionic interaction with a solid surface [
35,
36,
37,
38]. Actually, the dispersion interactions are exerted between the delocalized π-electrons of the polyaromatic activated carbon sheets and the aromatic rings of the investigated molecules. However, the substitution of hydrogen with aliphatic functional groups can determine a reduction in the interaction with the activated carbon surface, due to the polarization of the molecule. When the molecule size increases, such as for ethylbenzene and o-xylene with respect to toluene, the adsorption capacity can be limited by the occurrence of lateral interactions between adjacent adsorbed molecules, which exert an influence proportional to the molecule dimension and charge [
35,
41]. This effect can be also enhanced by the basic character of the activated carbon, which, in turn, can influence the interactions with adsorbates with aliphatic functional groups, which decrease the electrophilic character of the molecule [
35,
37,
41]. Hence, it can be concluded that the molecular properties of toluene play a major role, determining the highest adsorption capacity. To sum up, the removal of aromatic organic compounds from water on activated carbon mainly occurs by a π–π electron-donor-acceptor mechanism, and it is influenced by various factors, such as the chemical properties of the molecules, molecular size, the hydrophobic nature of the adsorbate and the characteristics of the activated carbon.
The analysis of the entire experimental data set was integrated with a dedicated modeling analysis for the determination of the best fitting model and the correlated adsorption parameters. To this aim, different adsorption models were considered among those commonly adopted in the literature for the description of adsorption phenomena of similar systems [
21,
35]:
- (1)
The Henry model, which hypothesizes a linear trend between adsorption capacity and equilibrium liquid concentration;
- (2)
The Langmuir model, which hypothesizes a homogeneous sorbent surface in terms of the energy of adsorption and no interaction between adsorbed species;
- (3)
The Freundlich model, which hypothesizes an exponential distribution of the energy of active sites typical of site-specific interactions.
In all of the cases, the least residual sum-of-squares was used as a criterion to determine the best fitting parameters for each model. The correlation coefficient (R2) and the standard error in parameter evaluation were determined as indexes of the accuracy of optimal data fitting analysis.
It is worth observing that in the study of adsorption isotherms, linear regression with linearly transformed isotherms is frequently used to determine the best-fitting isotherm. However, such transformations of non-linear isotherms to linear forms implicitly alters their error structure and may violate the error variance and normality assumptions of standard least squares. For this reason, a non-linear regression analysis was performed, where appropriate, avoiding any linearization of the model equations.
The isotherm equations and the model parameters derived from adsorption regression analysis, at T = 20 °C (from
Figure 1) and for all of the investigated aromatic compounds are reported in
Table 2.
Table 2.
Isotherm equations and model parameters for toluene, ethylbenzene, o-xylene and naphthalene adsorption regression analysis at T = 20 °C.
The regression analysis of experimental data at 20 °C shows that, for each model, the coefficient of determination (R
2) is very high. However, in particular for o-xylene and naphthalene, the Henry model shows less accuracy in the description of experimental data, suggesting that adsorption of these compounds does not occur through a simple partitioning mechanism. The accuracy of the experimental data and the Freundlich and Langmuir models’ appropriateness are testified to also by the low value of the standard errors, always an order of magnitude less than the correspondent parameter. The slight differences in the R
2 parameter for these two models is likely ascribed to an “algebraic” effect during calculations, rather than to a significant differences in accuracy. It is worth observing that the experimental data reported in
Figure 1 refer to equilibrium concentration significantly lower than the maximum adsorption capacity (e.g., calculable from
ωmax values reported in
Table 2). This is coherent with the specific application dealt with in the present work,
i.e., polluted groundwater, where contaminations are usually at low concentrations (even if higher than regulatory limits). In these conditions, the adsorption of the investigated aromatic compounds is likely to occur according to a monolayer coverage; hence, the Langmuir model can be chosen for experimental data description. This choice is confirmed by several studies dealing with similar experimental conditions and available in the pertinent literature [
28,
34,
42,
43]. Moreover, as reported in the following, for the interpretation and prediction of binary systems adsorption data, the Langmuir model (in its multicomponent formulation [
21,
35]) can be proficiently adopted. Hence, the model parameters referring to the single-compound systems are essential for the subsequent model calculations.
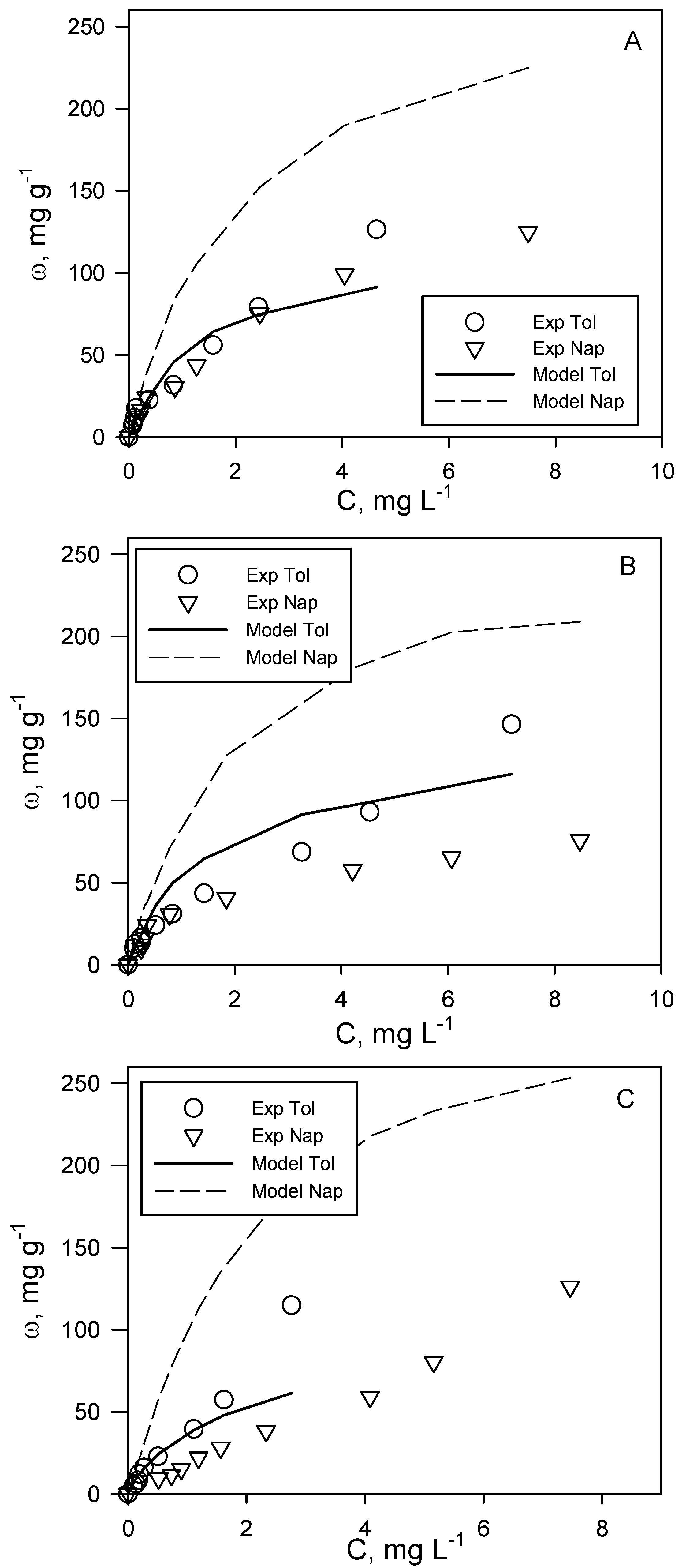
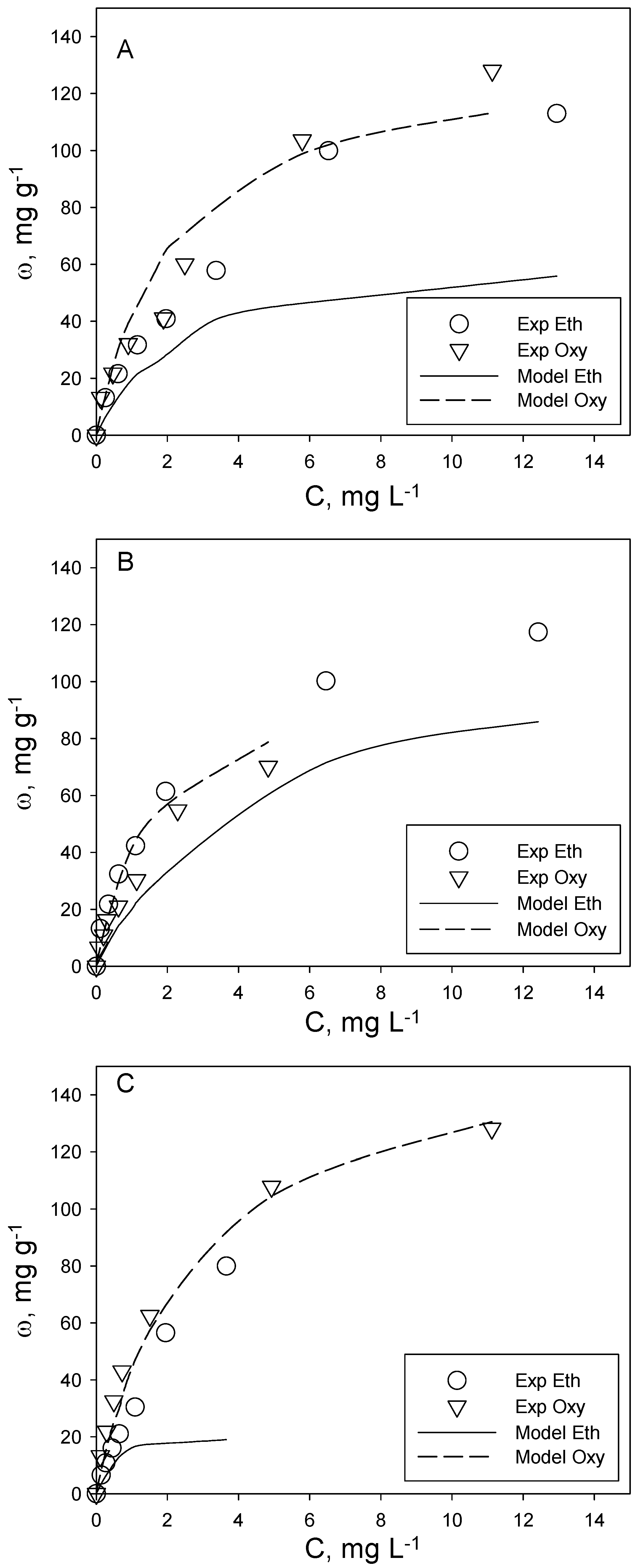
The analysis of the adsorption of the investigated aromatic organic compounds included the realization of adsorption isotherms at T = 20 °C for two binary systems, i.e., toluene + naphthalene and o-xylene + ethylbenzene. The aim was to assess the possible occurrence of competition phenomena during their simultaneous adsorption on the same activated carbon. It is commonly known that adsorption phenomena are strongly influenced by analyte concentration. For this reason, the experimental tests on binary systems were carried out with samples having the same volume, activated carbon dosage and initial concentration ratio of the two analytes (C0tol:C0nap and C0eth:C0oxy), but each experimental point corresponding to different analyte initial concentrations, so as to describe two complete adsorption isotherms in the concentration range of typical polluted groundwater. Following this scheme, three different tests were carried out for each of the two binary systems investigated, corresponding to a different analyte initial concentration ratio. In particular, for the toluene + naphthalene system, the tested ratios are C0tol:C0nap = 1:1, 1:1.4 and 1.4:1, while for o-xylene + ethylbenzene, they are C0eth:C0oxy = 2:1, 1:1 and 1:2.
Figure 2 and
Figure 3 report the experimental results for each adsorption run, inclusive of the three tested ratios and for toluene + naphthalene and o-xylene + ethylbenzene, respectively.
This experimental procedure allowed the determination of binary adsorption isotherms as a function of the initial concentration ratio of the two couples of analytes investigated. In both of the cases, the layout of the curve varies significantly with this parameter, testifying to the intrinsic complexity of the study of binary adsorption systems. For the toluene + naphthalene system (
Figure 2), the experimental data show that, for each binary adsorption run, toluene adsorption capacity is higher than that of naphthalene, and the differences increase when equilibrium concentrations are higher. This result almost reflects the trend observed for single-compound systems. A change in the initial concentration ratio determines a marked effect on naphthalene adsorption capacity and a lower effect on toluene adsorption. However, for both compounds, the adsorption capacity in binary systems is lower than the corresponding one in single-compound systems (
Figure 1), which is likely to be due to competition for the same active sites.
Figure 2.
Toluene (tol) + naphthalene (nap) binary adsorption tests at different initial concentration ratios: (A) C0tol:C0nap = 1:1; (B) C0tol:C0nap = 1:1.4; (C) C0tol:C0nap = 1.4:1. T = 20 °C, pH = 7. A comparison between experimental (exp) data (symbols) and Langmuir model predictions (lines).
Figure 2.
Toluene (tol) + naphthalene (nap) binary adsorption tests at different initial concentration ratios: (A) C0tol:C0nap = 1:1; (B) C0tol:C0nap = 1:1.4; (C) C0tol:C0nap = 1.4:1. T = 20 °C, pH = 7. A comparison between experimental (exp) data (symbols) and Langmuir model predictions (lines).
Figure 3.
O-xylene (oxy) + ethylbenzene (eth) binary adsorption tests at different initial concentration ratios: (A) C0eth:C0oxy = 1:1; (B) C0eth:C0oxy = 2:1; (C) C0eth:C0oxy = 1:2. T = 20 °C, pH = 7. A comparison between experimental data (symbols) and Langmuir model predictions (lines).
Figure 3.
O-xylene (oxy) + ethylbenzene (eth) binary adsorption tests at different initial concentration ratios: (A) C0eth:C0oxy = 1:1; (B) C0eth:C0oxy = 2:1; (C) C0eth:C0oxy = 1:2. T = 20 °C, pH = 7. A comparison between experimental data (symbols) and Langmuir model predictions (lines).
For the o-xylene + ethylbenzene system (
Figure 3), the experimental evidences appear more complex. For this system, the result of binary adsorption does not reflect the trend observed for single-compound systems. Actually, for all of the investigated initial concentration ratios, o-xylene adsorbs to a lesser extent than ethylbenzene, and the difference in adsorption capacity keeps almost constant in the investigated range of equilibrium concentration. Moreover, the adsorption capacity of o-xylene in binary systems is lower than (for C
0eth:C
0oxy = 2:1 and 1:1) or almost equal to (for C
0eth:C
0oxy = 1:2) its adsorption capacity in single-compound systems (
Figure 1). This result shows the occurrence of competition phenomena with ethylbenzene, which affect the adsorption of o-xylene. Moreover, as expected, the o-xylene adsorption capacity in binary systems depends on the analyte concentration ratio,
i.e., when the competitor concentration (
i.e., ethylbenzene) increases, the o-xylene adsorption capacity in binary systems decreases. A similar result was obtained in a previous study on trichloroethylene adsorption in a binary mixture with tetrachloroethylene, confirming that the competition effect depends on the concentration of the competitive compound [
21].
The ethylbenzene adsorption capacity data in binary systems are different. In fact, in two binary tests (i.e., C0eth:C0oxy = 2:1 and 1:2), the ethylbenzene adsorption capacity is slightly higher than its single-compound counterpart over a large part of the equilibrium concentration range, but is lower at higher concentrations (i.e., >5 mg L−1). In the other binary test, the ethylbenzene adsorption capacity is almost similar to its adsorption capacity in a single-compound system at low equilibrium concentrations, and it gets lower at higher concentrations. This is more likely to be due to experimental uncertainties rather than to a synergistic effect with o-xylene on ethylbenzene adsorption.
The experimental data show that the presence of o-xylene has a negligible effect on the ethylbenzene adsorption capacity in a wide range of equilibrium concentrations (i.e., <6 mg L−1). This does not hold true when the surface coverage increases, as competition effects may occur.
It can be concluded that, also for this binary system, the adsorption of the two analytes is likely to occur on the same active sites. The differences in the overall behavior of the two binary systems testifies, once more, to the variety of experimental results when dealing with binary or multicomponent systems, which drives further investigations.
For a deeper analysis of the experimental evidence involving the binary systems, a modeling analysis by a multicomponent Langmuir model, chosen for its simplicity and low number of fitting parameters, easily valuable through single-compound experimental tests, was carried out [
21,
35].
The Langmuir model for multicomponent adsorption systems, hypothesizing a competition between the analytes towards the same adsorption sites, is based on the same assumptions as its single-compound counterpart, which are briefly reported in the following:
- (i)
a homogeneous sorbent surface in terms of energy of adsorption;
- (ii)
no interaction between adsorbed species;
- (iii)
all adsorption sites are equally available to all adsorbed species.
For two-component systems, such as those investigated in this work, the model is expressed by Equation (1):
where
ω1 is Compound 1’s adsorption capacity,
ω1, MAX is the correspondent maximum value and
Ci and
Ki (
I = 1,2) are the equilibrium concentration and adsorption constant, respectively.
As can be observed, the adsorption capacity of Species 1 is reduced in the presence of Species 2 and depends on its concentration (
C2) and their tendency to adsorb (
K1 and
K2). In addition, it is worth noticing that based on the hypotheses stated, the adsorption constants of both compounds (
ωi,MAX and
Ki) should be derived individually from single-component adsorption tests (
Table 2), as they represent intrinsic properties of the adsorbate-adsorbent couple [
21,
35].
In this sense, the application of this model is not a regression analysis, but a prediction of binary data (also extendible to multicomponent data systems) starting from the knowledge of experimental data on single-compound systems.
In
Figure 2, the comparison between the Langmuir multicomponent model (Equation (1)) and the binary experimental data for the toluene + naphthalene system is reported. It is possible to observe that the proposed model provides an acceptable prediction of toluene adsorption data, except for high values of equilibrium concentration, where the adsorption capacity is underestimated. Conversely, a significant deviation between model predictions and experimental values can be observed for naphthalene data for which the adsorption capacity is largely overestimated.
In
Figure 3, the homologous analysis for the o-xylene + ethylbenzene system is reported. The modeling analysis shows that the Langmuir multicomponent model provides very good results in the prediction of o-xylene adsorption, while it is substantially inadequate for ethylbenzene, as it greatly underestimates the experimental data. Indeed, this result was expected based on the general trend of experimental data on o-xylene + ethylbenzene binary systems. In fact, the Langmuir multicomponent model can be applied only when its basic assumptions are verified, in particular the assumption that adsorbates compete for the same active sites, which was not verified for ethylbenzene adsorption. However, given the absence of competition effects on ethylbenzene adsorption and in order to preserve the predictive character of the model, the prediction of ethylbenzene binary adsorption data could be made by the Langmuir single-compound model (with the constants derived from ethylbenzene adsorption single-compound data reported in
Table 2), assuring satisfactory results.
As an alternative, for the model to provide better fitting results, some of its basic hypotheses could be removed; e.g., calculating the adsorption constants (Ki) by the non-linear regression of binary experimental data. However, since this has no theoretical foundation and physical meaning, the model was not applied under these simplified hypotheses.
An overall analysis of the modeling data allows one to conclude that, for a low equilibrium concentration, such as those more commonly found in groundwater, the Langmuir multicomponent model can predict with good approximation the binary adsorption data, provided the occurrence of competition for the same active sites between adsorbates, as dictated by the base hypotheses of the model.
As a final remark, the analysis underlines the crucial importance of consistent thermodynamic data (i.e., adsorption isotherms), for both single-compound and binary systems, as well as of reliable adsorption models for the design of interventions aimed at the restoration of a polluted aquifer. The performance of an adsorption treatment, in fact, mainly depends on the thermodynamic aspects of solute-solvent-sorbent interactions and on the transport phenomena involving the porous media. The equilibrium conditions are the most significant limits for the application of a given sorbent, since the uptake of organic compounds may change within orders of magnitude by varying the process parameters, such as concentration and the chemical composition of the aqueous solution. Consequently, the overall cost of the intervention can significantly vary, because the acquisition of the adsorbent material represents one of the major operating costs.
4. Conclusions
In this work, the adsorption of four aromatic organic compounds (toluene, o-xylene, ethylbenzene and naphthalene) onto a commercial activated carbon (Filtrasorb 400) from simulated polluted groundwater was investigated at constant temperature (20 °C) and pH (7). Experimental tests were carried out both for single compounds and two binary systems (i.e., toluene + naphthalene and o-xylene + ethylbenzene), so as to assess the suitability of this adsorbent in the capture of these compounds and to find out the possible occurrence of competitive phenomena, which could affect the treatment of groundwater with multiple contaminations.
For a single-compound system, all of the analytes showed a significant adsorption capacity on the tested activated carbon, which confirms the suitability of adsorption in the remediation of polluted groundwater. In particular, the toluene and naphthalene adsorption capacities are the highest and almost similar, while for o-xylene and ethylbenzene, the performances are lower. The adsorption of such compounds seems to be influenced by several parameters, exerting a positive (e.g., hydrophobicity, low molecule size) or negative (e.g., substitution of electrophilic groups) effect on the adsorption capacity. Moreover, the activated carbon properties, mainly the porous structure, are expected to play a crucial role in determining both a high adsorption surface and steric hindrance phenomena.
The analysis of the entire experimental data set was integrated with a dedicated modeling analysis for the determination of the best fitting model and the correlated adsorption parameters. The Langmuir model was chosen as the best fitting model; this choice is justified by the low concentration levels, which make likely the occurrence of a mono-layer adsorption mechanism.
Adsorption tests carried out for the two binary systems, at three different values of analyte initial concentration ratios (i.e., three different C0tol:C0nap and C0eth:C0oxy), showed a variegate behavior, which testifies to the unpredictable character of binary or multicomponent adsorption systems. In the case of the toluene + naphthalene system, the experimental results showed the occurrence of competitive effects on the adsorption of both compounds, even if toluene is always adsorbed to a greater extent. Conversely, for the o-xylene + ethylbenzene system, the former undergoes competitive effects, while the latter seems to be almost independent of the presence of the other compound.
Finally, a modeling analysis of binary system data showed that the Langmuir multicomponent model can predict with good approximation the adsorption capacity of those compounds that compete for the same active sites, as dictated by the base hypotheses of the model. In all of the cases, a better prediction can be achieved for low equilibrium concentrations, such as those more commonly found in groundwater.