Abstract
Two genetically improved tilapia strains (GIFT and Akosombo) have been created with Oreochromis niloticus (Nile tilapia), which is native to Africa. In particular, GIFT has been shown to be significantly superior to local African tilapia strains in terms of growth rate. While development economists see the potential for food security and poverty reduction in Africa from culture of these new strains of tilapia, conservationists are wary of potential ecological and genetic impacts on receiving ecosystems and native stocks of tilapia. This study reviews the history of the GIFT technology, and identifies potential environmental and genetic risks of improved and farmed strains and tilapia in general. We also estimate the potential economic gains from the introduction of genetically improved strains in Africa, using Ghana as a case country. Employing a combination of the Economic-Surplus model and Monte Carlo simulation, we found the mean net present value (NPV) of the introduction of the GIFT strain in Ghana to be approximately 1% of the country’s gross domestic product. Sensitivity analysis indicated that the difference in growth or yield between the GIFT and locally-available strains has the largest effect on mean NPV. We conclude that improvements in management practices and infrastructure could increase the yield and profitability of the local strains even if genetically-improved strains are not introduced. These improvements also will ensure the realization of the full potential of introduced strains.
1. The Importance of Aquaculture to Africa’s Development
Capture fisheries production has levelled off and is no longer considered capable of sustaining the supply of fisheries products needed to meet growing global demand [1]. Aquaculture, especially of tilapias, has the potential to play a leading role in the fight against food insecurity, malnutrition, and poverty in Africa [2]. The continent has an immense biological diversity of native fish resources. However, due to poor management and genetic erosion, most aquaculture stocks in current use on the continent are genetically inferior to wild, undomesticated stocks [3,4]. It is widely accepted that successful aquaculture development in Africa requires improvements in feed quality and availability, business and marketing models, and local technical capacity. Another important factor that should be considered is the effective utilization and management of fish genetic resources [4,5]. Specifically, improved strains that are faster growing, resistant to disease, and suited for culture in a variety of fish farming conditions could go a long way to meet the demand for fish protein [6].
2. Tilapia Characteristics and Production
Tilapias (Family: Cichlidae) are suitable for various aquaculture systems due to their ease of propagation, tolerance to handling, fast growth on both natural and manufactured feeds, tolerance of a wide range of environmental conditions, and high palatability, marketability and nutrient content [7]. They are especially well-suited for culture in developing countries due to their fast growth and short generation time, tolerance to a wide range of environmental conditions, resistance to stress and disease, ability to reproduce in captivity, and their acceptance of artificial feeds right after yolk-sac absorption [8].
Global aquaculture production of tilapias increased from 28,000 tonnes to over 3 million tonnes from 1970 to 2010 [9]. Globally, the tilapias were the dominant species group caught in inland fisheries between 2000 and 2005 (the tilapias were surpassed in 2005 by the cyprinids [10]). In terms of aquaculture production, the tilapias comprise approximately 5 percent of total global fish farming, second to the carps, which account for more than 70 percent [11]. However, aquaculture of tilapia in Africa constitutes only approximately 19% of the world’s tilapia production [12].
3. Social Benefits of Tilapia
Historically and from a social standpoint, the most important use of tilapias has been production for home consumption, with millions of small-scale fish farmers in more than 100 countries supplementing their diets with tilapia [13]. There also has been a steady increase in the number of family-owned tilapia marketing microenterprises in many countries. The fish often are retrieved from nearby ponds or tanks, cleaned, fried, and offered for sale. This fried tilapia provides a considerable proportion of dietary protein and calories in these developing countries [13]. More commonly, fresh tilapia also is provided for sale either at the farm gate or in local markets. However, tilapias have grown in importance from being just a low-cost, high-protein food fish (“aquatic chicken” [14]) employed by development agencies to feed the poor in the world’s rural areas, to a highly-domesticated “livestock” with annual sales amounting to over $2 billion globally [10,13]. In terms of economic importance, tilapia surpassed the salmonids in 2004, and they are expected to eventually equal the carps [13]. The tilapias have been referred to as the “most important global whitefish commodity” [15].
4. Distribution of Tilapias
The natural distribution of tilapias is restricted to Africa, Jordan, and Israel, where 112 species and subspecies of the genera Oreochromis, Sarotherodon, and Tilapia have been identified [8,16,17,18,19]. However, only a few of these species are commercially important, and fewer still are of aquacultural importance [11]. Oreochromis niloticus, O. aureus, and various hybrids of these with O. mossambicus are regarded as the most important aquaculture species [11]. For example, in China, of the reported 1.1 million tonnes of O. niloticus produced in 2008, approximately one-quarter was a hybrid between Nile tilapia (O. niloticus) and blue tilapia (O. aureus) [10]. All of the important aquaculture species have been introduced extensively outside of their native range. In the 20th century alone, tilapias were introduced into 90 countries for aquaculture, fisheries, the aquarium trade, or inadvertently [20,21,22]. However, their intolerance to low temperatures (below 20 °C) restricts culture to warmer areas [11].
5. Background to Genetic Improvement of Tilapias
Due to the increasing importance of the tilapias in global fish farming, the intensity and diversity of efforts to improve the genetic baseline of these species have intensified over the last few decades. Ponzoni et al. [23] showed that genetic improvement is one of the most powerful and least expensive means of increasing the efficiency of aquaculture. Both traditional animal breeding and science-based quantitative genetic approaches have been used to improve tilapia phenotypes [24]. Other important genetic improvement methods include innovative chromosomal manipulations, physiological alteration of sex determination, gene transfer, and genetic marker-assisted breeding [24,25]. Traditional animal breeding approaches are still the most practical means of improving tilapia stocks for low-tech producers in most countries, and these approaches basically exploit additive and non-additive gene effects [24].
One common traditional approach to genetic improvement is the practice of individual selection, or selective breeding, which is based on the underlying principle that some significant portion of the variation in observable performance is due to individual genotypes, and that a component of these genotypic influences is directly heritable from parent to offspring [24]. Even in cases of high heritability, a measurable amount of phenotypic variation is needed to enhance growth rate through selection. Random genetic drift and excessive inbreeding result in lower heritabilities due to reduced genetic variation, posing a frequent problem in tilapia culture since a large number of broodstock on any particular farm most likely originated from a few individuals [24]. Hence, selection is usually a viable approach for tilapia genetic improvement where sufficient genetic variation exists [24]. According to Ponzoni et al. [23], selective breeding has a number of advantages over other genetic approaches: continuous genetic gain is possible, genetic gains can be handed down from one generation to the next, and gains in a nucleus can be multiplied and expressed in millions of individuals in the production sector.
The Genetic Improvement of Farmed Tilapia (GIFT) project, one of the most significant recent innovations in tilapia culture, succeeded in part because it was based upon using selective breeding of a highly diverse synthetic base population [8]. The GIFT technology has been applied in a number of countries to improve local strains of tilapia. One example of applying the GIFT methodology is the Akosombo strain, which was developed in Ghana by the Aquaculture Research and Development Center (ARDEC) in collaboration with the World Fish Center in 2003 [26,27,28].
6. The Genetic Improvement of Farmed Tilapia (GIFT) Project
The GIFT project was a collaborative research effort involving five separate research institutions that was implemented in the Philippines from 1988 to 1997 [29,30]. This project had the overarching goal of “increasing the quantity and quality of protein consumed in low income rural and urban populations in tropical developing countries, and in all regions of the world, leading to an increase in the income of low-income producers” [31]. The collaborating institutions were the International Center for Living Aquatic Resources Management (ICLARM, now the World Fish Center), the Philippines Bureau of Fisheries and Aquatic Resources (BFAR), the Freshwater Aquaculture Center of the Central Luzon State University (FAC-CLSU), the Marine Science Institute of the University of the Philippines (UPMSI), and the Institute of Aquaculture Research, Ltd., in Norway [30]. Funding for this project was provided by the Asian Development Bank, the United Nations Development Program, and ICLARM [30].
The GIFT project had three specific objectives: (1) to develop improved breeds of Nile tilapia (Oreochromis niloticus) and provide those fish breeds to national testing programs and then to the fish farmers; (2) to strengthen national institutions in aquaculture genetics research; and (3) to establish a mechanism for international exchange and evaluation of improved breeds and research methods [31]. Nile tilapia was chosen as the focal species for a number of reasons. It has a short generation time (approximately 8 months), which made it the perfect species for a breeding program [32]. This species also was growing rapidly in importance in aquaculture [14,33]. Additionally, the omnivorous diet of the Nile tilapia makes it an excellent fit for low-cost aquaculture, in contrast to carnivorous species that rely heavily on fishmeal or other expensive animal protein [31]. Also, numerous potentially useful Nile tilapia resource stocks existed in several countries in Africa and the Middle East.
Before the commencement of field trials, a number of consultations were held with fish farmers and experts from various disciplines, and the relative importance of the improved stocks was considered for the various tilapia farming systems. Some of the farming systems considered were cage culture, backyard fish pond, rice-fish integrated culture, and more intensive systems [31]. Fingerling size was set at 3–7 g, and the grow-out period was 90 days, with a harvest weight of about 120 g [31].
Even though the natural tilapia resources are restricted mostly to Africa, the world’s main tilapia aquaculture industries are located in Asia [10,32]. Due to the generality that established farmed tilapia stocks in Asia were derived from few founder individuals, there was the suspicion that loss of variation through random genetic drift, inbreeding and introgression of genes from other less-desirable feral tilapia stocks had occurred. Hence, the project collected wild Nile tilapia germplasm from Egypt (May 1988; August 1989; Nile Delta system), Ghana (October 1988; Upper Volta system), Senegal (October 1988; extreme west of distribution) and Kenya (August 1989; Lake Turkana) [34,35]. These collections represented the first-ever direct transfers of O. niloticus from Africa to Southeast Asia, with all samples belonging to the sub-species Oreochromis niloticus niloticus, with the exception of the Kenyan samples, which belonged to the sub-species Oreochromis niloticus vulcani. Four commercial Nile tilapia strains were collected from the Philippines (three strains originated from Ghana, and one stock originated from Egypt [34]).
The most important criterion determining the number of strains to be developed by the GIFT project was relative performance (growth, maturation and fecundity, and hardiness) in different target environments, or the genotype x environment interaction (GxE) [31]. An insignificant GxE effect (in terms of farming relevance) implies that the best strain in one environment will be the best in most or all environments. A high GxE effect, on the other hand, implies that special strains would have to be developed for specific environments. The GxE interaction effect was found to be low, i.e., overall growth performance was found not to differ significantly with environment, in terms of farming relevance. Hence, it would not be necessary to develop different strains for the different farming systems. The principal breeding objective of the GIFT program was growth rate, while monitoring other traits, such as survival, occurrence of disease and maturation rate [31].
Results indicated that, with the exception of the Ghana strain, the strains from Africa performed as well as or better than the then existing commercial or “domesticated” strains in Asia. The Egyptian strain was the best performer in the first generation, while the Kenyan strain was the best in the second. The Ghana strain performed the worst in both generations [34]. One of the widely cultured strains (the Israel strain, originally derived from Ghana) also performed poorly [34]. Crossbreeding (hybridization) of the different strains did not result in significant improvements. Therefore, individuals from the best-performing purebred and crossbred groups were selected, based on the growth performance of a number of different strain combinations, in order to build a stock with a broad genetic base. This constituted the original GIFT strain [31].
It was no surprise that the GIFT project, after just one generation of selection, had generated considerable interest from the tilapia production industry in Asia. Therefore, a consultation meeting was held in 1992 to discuss strategies and safeguards for fish germplasm transfer and distribution [31]. The consultation meeting involved senior scientists from National Agricultural Research Systems (NARS) of developing countries, international experts on fish genetics and biodiversity, representatives of NGOs, and donor institutions. Following the recommendations from this meeting, the improved strain developed in the Philippines was disseminated (from May 1994 to August 1997) to member countries that formed the Dissemination and Evaluation of Genetically Improved Tilapia in Asia (DEGITA).These countries were Bangladesh, People’s Republic of China, Philippines, Thailand, and Vietnam. This dissemination allowed detailed evaluation of the genetic and socioeconomic performance and environmental impacts of this strain prior to more widespread commercial production and dissemination [31]. The dissemination was carried out according to standard quarantine procedures developed as part of the GIFT project [36].
Dey [37] studied the potential economic impacts of culturing the GIFT tilapia strain in five Asian countries: Bangladesh, China, the Philippines, Thailand and Vietnam. He concluded that adoption of the improved tilapia strain would benefit both producers and consumers of fish in each of the countries studied. Further investigations concluded that the GIFT strain resulted in body weight 18%–58% higher than “non-GIFT” strains on “average” farms in Asia. The break-even price above variable cost was found to be 7%–36% lower for the GIFT strain than for other O. niloticus strains then being farmed [35].
7. The GIFT/GenoMar Supreme (GST) Strain
In 1999, the GIFT Foundation International, which was founded as part of the GIFT project, signed an exclusive agreement with GenoMar, a Norwegian company based in Oslo, for the long-term continuation of the GIFT breeding program [15]. According to GenoMar, and beginning with the 10th GIFT generation DNA marker-assisted selection has been applied in order to increase the selection differential. According to Gjoen [15], the selection differential from this approach, compared to that of the traditional selection approach, was expected to be 40% higher than for the 9th-generation GIFT tilapia in 2000. Genetic maps were generated for tilapia, and experiments were conducted in order to reveal genes that influence traits that are economically important [15,25]. According to Gjoen [15], color, growth, body shape, salt tolerance, and sex determination are some of the traits for which GenoMar detected influential chromosomal regions. GenoMar planned to use this information to facilitate the acceleration of genetic gains, especially for traits such as disease resistance and feed conversion, which are difficult to measure within traditional selective breeding programs [15]. As of 2001, the GIFT/GenoMar strain was officially being distributed in countries in Southeast Asia and Latin America [15]. The GIFT/GenoMar strain is known officially as the GenoMar Supreme Tilapia (GSTTM) [8].
Ponzoni et al. [5] detail the current state of the GIFT strain and also summarize research that the World Fish Center has conducted on the strain since 2000. The World Fish Center took delivery of 63 full-sib groups of 35 fish each (the progeny of single-pair mated parents) at Jitra, Kedah State, Malaysia, towards the end of 2000 and beginning of 2001, from the GIFT Foundation International, Philippines. The aims of this project included, among others, the maintenance and continuous improvement of the GIFT strain, and the distribution to partner countries likely to benefit from its use. According to the authors, the strain has achieved sustained gains of 10%–15% per generation over more than six generations. Importantly, these gains have not been accompanied by any undesirable correlated response to date [5].
8. Dissemination of GIFT/GST Strains in Africa
The outcome of the GIFT project generated interest from developing countries in Asia, the Pacific, and Africa, both in terms of developing their own aquaculture strains, and also in gaining access to the GIFT germplasm [38]. Africa, the origin of the tilapias, benefits the least from the GIFT strain, even though much of the continent has a high potential for tilapia farming [39]. This situation arose due to the policy of the WorldFish Center not to introduce the GIFT strain into countries where O. niloticus is indigenous, concerned that interbreeding of the GIFT strain with locally-adapted native populations might compromise wild aquatic genetic diversity [38]. This decision by the WorldFish Center was given weight by an expert consultation in 2002 in Nairobi, which was sponsored by the WorldFish Center, the Food and Agriculture Organization (FAO), the World Conservation Union (IUCN), the United Nations Environment Program (UNEP), and the Technical Center for Agriculture and Rural Cooperation (CTA) [40]. The expert consultation resulted in the Nairobi Declaration, a set of ten recommendations aimed at realizing the potential of African aquaculture without compromising native ecological and genetic resources [41]. The WorldFish Center, instead, decided to help these countries apply the GIFT methodology to the genetic improvement of indigenous tilapias.
The WorldFish Center established the International Network on Genetics in Aquaculture (INGA) in 1993 to train member-country scientists in quantitative genetics applied to aquaculture, and to coordinate national breeding programs in the 13 member countries (Bangladesh, China, Cote d’Ivoire, Egypt, Fiji, Ghana, India, Indonesia, Malaysia, Malawi, Philippines, Thailand, and Vietnam) using the GIFT methodology to genetically improve their indigenous cultured species [35,38].
The pressure for the dissemination of the actual GIFT germplasm to Africa seemed to reach a head in 2007, when the WorldFish Center approved the Policy on the Transfer of GIFT from Asia to Africa, making the GIFT strain available to any African government that can demonstrate procedures to manage environmental and biodiversity risks, among other conditions [42]. Such a country also must display compliance with the Convention on Biological Diversity (CBD), an international treaty, while at the same time addressing the development objective it intends to achieve with the GIFT introduction [36,42]. The guiding principle for this new policy was that the genetic risks of introducing the actual GIFT strain to Africa was comparable to those associated with the genetic improvement of indigenous O. niloticus strains in Africa [42]. Basically, the new policy acknowledged that there was no point in keeping the GIFT strain from Africa if the genetic improvement of tilapia was going to occur on the continent anyway.
The Nogoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization to the CBD, a multi-lateral agreement, was adopted in 2010. This protocol aims at:
Sharing the benefits arising from the utilization of genetic resources in a fair and equitable way, including by appropriate access to the genetic resources and by appropriate transfer of relevant technologies, taking into account all rights over those resources and to technologies, and by appropriate funding, thereby contributing to the conservation of biological diversity and the sustainable use of its components.[43]
It can be argued that the World Fish Center has equitably shared with Africa the benefits arising from the utilization of the genetic resources (O. niloticus germplasm) collected from Africa to develop the GIFT strain ([6]; p. 139). The center has been training African scientists in application of the GIFT technology to their own national improvement programs, and then eventually allowing African governments’ access (at no cost) to the GIFT strain.
Although the decision to allow the GIFT strain into Africa seems to have been reached through a logical scientific process, the policy evoked mixed reactions in Africa. The Sustainable Aquaculture Research Networks in Sub-Saharan Africa (SARNISSA) is a network of scientists, fish farmers, development partners, and other players in the African aquaculture industry. SARNISSA, hosted by University of Stirling, UK, maintains an online forum on which issues that are pertinent to African aquaculture are discussed, and one of the most hotly-debated topics recently discussed was use of the GIFT strain in Africa. For example, on 29 November 2011, SARNISSA member Hiskia Asino posted an enquiry onto the forum asking from which African countries he could obtain GIFT tilapia fingerlings for farming in Namibia. This request set off a heated debate that lasted several months. While most fish farmers and development agents generally seemed to favor the new policy and its potential benefits towards increasing farm profits and reducing poverty, a number of scientists and conservationists spoke quite passionately against it, citing the potential negative genetic and ecological impacts of the introduction. Instead of the GIFT strain, those against it proposed improvements in the existing aquaculture practices and infrastructure to achieve the same economic goals as with the GIFT. A forum contributor summed up the fears of SARNISSA members opposed to the use of the GIFT strain in Africa, “Once local species or stains are lost to competing non-endemic species or strains, that biodiversity is lost forever”.
The GIFT technology has been disseminated to Kenya, Cote d’Ivoire, and Egypt, along with other developing countries in Asia and the Pacific (Dr. Raul Ponzoni, formerly of WorldFish, personal communication). GenoMar also has supplied the GST strain to partner hatcheries in Zambia and Angola, and by 2008 it was carrying out a feasibility study on setting up another partner hatchery in Uganda [36]. In Ghana, the Aquaculture Research and Development Center (ARDEC) took delivery of the GIFT strain officially in 2012, with the expressed objective of comparing its growth performance with the locally improved Akosombo strain (Dr. Joseph Padi, ARDEC, Water Research Institute, Ghana, personal communication).
Considering the porous nature of African borders and inadequate capacity to monitor the transfer of genetic material, we expect that the GIFT/GST strain is currently in several other countries in Africa. An example of the easy movement of non-native species on the African continent is the current production in Omilende, Nigeria, of the Asian catfish Pangasius sp. fingerlings for sale, as was reported on the SARNISSA forum on 2 March 2013—another non-native species introduction revelation that was followed by a lengthy and contentious discussion on the forum. It is clear, therefore, that once the GIFT/GST strain is legitimately introduced into a country in Africa, it eventually will be found in unapproved parts of that country or in other unauthorized neighboring countries. It is also clear that both benefits and negative impacts are possible with the introduction of the GIFT strains into Africa (Figure 1).
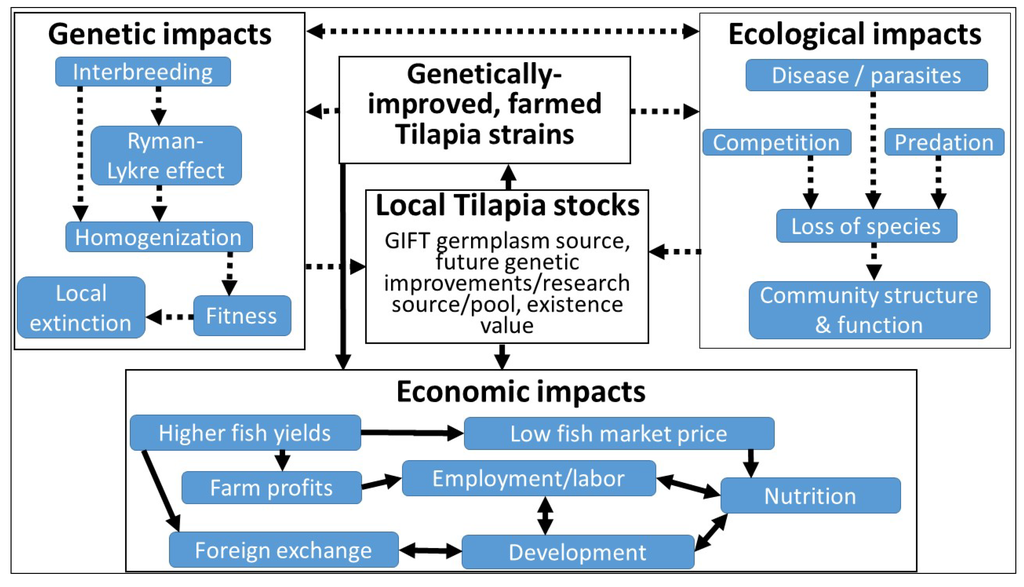
Figure 1.
Interdependence of the potential ecological, genetic, and economic impacts with the introduction of genetically-improved tilapia into Africa. Broken arrows indicate negative impacts and solid arrows indicate positive impacts.
9. Potential Ecological Impacts of Tilapia Introductions
Tilapia production is well on its way to taking center stage in the global aquaculture industry [13]. However, in the context of development, the success of a species is determined by its social, cultural, economic and environmental impacts, in addition to its contribution to production per se [22]. The traditional small-scale, semi-intensive culture of tilapias has proven sustainable in many countries where they are native, with no observable negative ecological impacts on surrounding environments attributable directly to the species (e.g., [8,44]). The recent trend towards intensification, driven by market forces, has been predicted to pose serious environmental and socioeconomic problems [8]. These impacts will arise as a result of the expected increases in use of processed feeds, drugs, hormones [8], and non-indigenous genetic resources in intensive aquaculture. The global transfer of both genetically-altered and unaltered tilapia species outside their native ranges for aquaculture purposes also is expected to result in negative impacts upon natural aquatic ecosystems.
The popularity of the GIFT strain will drive its introduction into African countries outside the natural range of Oreochromis niloticus. Characteristics that make tilapias the perfect aquaculture species also make them invasive species, keeping in mind that even though Nile tilapia is native to Africa, it is not native to all parts of the continent. Escape of both altered and unaltered genetic resources from aquaculture installations into natural ecosystems occurs relatively frequently [45,46]. For example, Attipoe et al. [27] report the loss of the entire control line of the 2003 spawning season from the ARDEC research facility during the development of the Akosombo strain in Ghana as a result of a violent storm.
Pullin et al. [21] studied the establishment success of various tilapine species and found that tilapia species, in general, are moderately to highly invasive, with a 60%–90% probability of becoming established in new open waters. The impacts of any invasive species on an aquatic ecosystem differ significantly, depending on the species, the extent of the introduction (i.e., propagule pressure), and the vulnerability of the invaded ecosystem [18]. Tilapine species invest in significant parental care that facilitates their establishment in novel habitats. The family Cichlidae is grouped into nest-builders (Tilapia spp.), and mouth-brooders (Oreochromis and Sarotherodon spp.) [11]. Mouth-brooders, for example, have no specific substrate requirements for reproduction; they simply carry fertilized eggs and yolk-sac fry in their mouths until yolk sac absorption and dispersal [47,48].
Examples abound in the literature demonstrating the negative impacts of tilapia on natural aquatic ecosystems after their introduction, keeping in mind that Nile tilapia, and tilapia in general, are not native to all parts of Africa. These include predation on eggs and small fish [49], rapid total elimination of submerged and floating aquatic macrophytes that served as essential habitat for native fish species in natural and man-made reservoirs [50,51], and eutrophication (bioturbation and nutrient cycling through ingestion and excretion) stemming from the foraging activity of tilapia on benthic algae [52]. Tilapia introductions into Madagascar, Lake Victoria, and Zimbabwe led to habitat alteration, such as declines in aquatic plants and decreases in the availability of breeding areas for native species [53]. Also, the establishment of Tilapia graham in Kenya’s Lake Nakuru led to the emergence of a fish-eating bird population [54]. Canonico et al. [18] provide an excellent review of impacts of tilapia on receiving aquatic systems using specific case studies from around the world.
Another potential impact of introducing the genetically improved tilapia strain from Asia to Africa is the risk of “hitch-hiker” disease-causing vectors and pathogens from Asia. Two possibilities exist. Firstly, the selectively bred strain might not be able to withstand diseases to which local African stocks have evolved resistance, which could lead to high mortalities on farms producing the selected strains. Secondly, the selectively bred strain, if resistant to certain Asian disease vectors, might carry these vectors and pathogens to Africa and infect local fish stocks. Noga [55] provides detailed descriptions of diseases of tilapia and numerous other fish species. Tilapia diseases include those caused by bacteria of the family Eimeriidae, Mycobacterium spp., and Edwardsiella tarda.
A number of general procedures exist for conducting risk analysis of non-native fishes (e.g., [56]). There are established procedures for analyzing risk regarding pathogen transfer and for quarantine monitoring [57]. Unfortunately, there is no universally-accepted a priori procedure for assessing the potential environmental impacts of non-native tilapias. In fact, scientific and policy decisions on new tilapia introductions frequently are polarized and are largely based on guesswork [21]. As such, a majority of the impacts mentioned above were recognized after introduction and/or establishment.
10. Potential Genetic Impacts of Tilapia Genetic Improvement on Native Populations
There is a paucity of studies on the impacts of aquaculture on locally-adapted gene pools of tilapia in receiving ecosystems. However, potential harms and associated risks stemming from aquaculture escapes have been considered in a more general context, Carvalho and Hauser [58] grouped the genetic impacts of “escapee” genetic resources into direct and indirect effects. Interbreeding of natural fish populations with escaped cultured stocks is arguably the biggest direct effect. When cultured fish escape or are released, the receiving population may experience a reduction in genetically effective population size, Ne, a phenomenon referred to as the Ryman-Laikre effect [59]. In addition, the risk of subsequent inbreeding may be increased if the ratio of “escapees” to the natural population is sufficiently high due to the relatively low Ne of many cultured stocks. Natural selection acts upon alleles at fitness-related loci to ensure adaptation of wild fish populations to their environments. That is, local differences in natural selection over a wide area result in adaptive genetic divergence of populations over time; further, selective forces that operate across adaptively important loci may result in combinations of alleles, or co-adapted gene complexes, which confer fitness upon their carriers [60].
In contrast, selective breeding acts upon alleles at performance-related loci to ensure expression of valued traits within aquaculture systems. Escape of selectively bred individuals into the wild and interbreeding with wild populations may result in offspring that exhibit low fitness, posing the risk of outbreeding depression at a local scale; further, interbreeding of escaped, cultured stocks at multiple sites across a landscape will tend to homogenize among-population variation of wild populations and also could lead to loss of fitness and outbreeding depression. The best-demonstrated case studies involve salmonids, and are reviewed by Ferguson et al. [61]. Among case studies, McGinnity et al. [62] demonstrated that the interaction of farmed with wild Atlantic salmon resulted in lowered fitness, and that repeated fish escapes caused cumulative fitness depression. Araki et al. showed dramatic losses of fitness after just two generations of captive breeding in steelhead salmon [63], as well as reduced fitness of their wild-born descendants [64]. Hindar et al. [65] reviewed studies of the effects of escape of cultured fish and introgression with local populations upon genetic differentiation, noting that in a subset of cases genetic swamping of local populations led to loss of indigenous stock structure in several salmonids. Conceptual models have been developed to predict the effects of introductions of maladapted individuals into locally-adapted gene pools [66,67] or to predict the relative likelihoods of beneficial or negative impacts of gene flow on receiving gene pools [68]. However, empirical studies of the effects of interbreeding of escaped cultured fish upon wild populations are lacking for most fish taxa, including tilapias. Hence, we lack the empirical data to parameterize models in order to predict the effects of introgression of selectively bred tilapias into native gene pools. Knowledge of gene flow rates and ecological differences among source and recipient populations [69], carefully controlled, multigenerational experiments, and thoughtful use of data from “experiments” created unintentionally [70] will be needed to advance our understanding.
A number of indirect effects also may be posed by the release or escape of cultured tilapia stocks into the wild. Indirect effects generally highlight the strong relationship between the ecology of tilapia species and their genetics (Figure 1). Released cultured stock may reduce abundance, and hence the effective population sizes (Ne) of critical species in the receiving ecosystem through competition, predation, habitat alteration, or changes in community trophic structure or food webs [63]. This decrease in Ne could cause a loss of genetic variability and adaptive ability to changing selective pressure, possibly leading to an increase in the likelihood of inbreeding and extinction.
The level of impact stemming from establishment of these “escapee” genetic resources in an ecosystem is influenced by three factors: the species’ invasiveness, the fitness of the selectively-bred stock, and characteristics (specifically, the invasibility or vulnerability to invasion) of the receiving community [71]. Invasiveness refers to the ability of a cultured stock to escape, disperse, and become feral in aquatic communities, and tilapias, along with other aquaculture species, exhibit great abilities to disperse and become established in non-native ecosystems.
Selective breeding that increases fitness may increase the likelihood of a cultured species becoming established in a receiving ecosystem (e.g., significant impacts on native soil, vegetation and animals by feral hogs in southeastern USA [72], and feral horses in Australia [73]). However, experience from selective breeding in domestic farm animals for production purposes suggests that this is not usually the case, and is strictly case-dependent. Physiological imbalances or growth demands in natural environments with limited food availability tend to decrease the fitness of selectively-bred stock in the wild. Nevertheless, it is possible for selectively-bred stocks to overcome one fitness component if other components, such as juvenile viability, adult viability, age at sexual maturity, female fecundity, male fertility, and mating success are enhanced through the selective breeding process [74]. Whether selectively bred tilapia show increased or decreased fitness in the wild has yet to be assessed experimentally.
A stable community is one whose structure and function return to their initial conditions after a perturbation [75]. A stable ecosystem will quickly recover from a fish escape event. It has been shown that decreases in native species are more common in low-diversity aquatic ecosystems following introductions of tilapias (e.g., high elevation lakes of Madagascar with few native species), than in high-diversity ecosystems (e.g., coastal lakes with many native species) [76]. Despite widespread introductions into Asian waters, explicit evidence on the ecological and genetic impacts of these non-native tilapias as a whole is scanty [22]. The scanty evidence of the impacts of tilapias in Asia could be due to the absence of conspecifics and other related fish species in communities of that region. This lack of information also could be attributed in part to poor assessment of “before” and “after” states of the receiving ecosystems as part of deliberate species introductions, especially for aquaculture.
Release or escape of the GIFT/GST strain into African waters may have the potential to be highly damaging. These selectively-bred strains could mate with wild stocks to set off the genetic impact scenarios described above. Apart from there being related, wild tilapia stocks with which to interbreed, African countries currently lack the capacity to prevent the escape of selectively-bred fish from aquaculture facilities or to prevent the intentional release of these fish into the wild. Also, there is little data on the baseline condition of the receiving ecosystems before the introduction of selectively-bred tilapia.
While Brummett and Ponzoni [77] and Ponzoni et al. [23] do not associate use of genetically improved tilapia strains in Africa with a high level of concern, Hallerman and Hilsdorf [78] noted that considerable molecular and adaptive variation exists within the species O. niloticus. The species has an exceptional ability to colonize and adapt to a wide range of habitats, ranging from small forest rivers to large drainages and lakes, as well as alkaline pools with hot springs [79,80]. At a general level, the description of seven subspecies based on eco-morphology [79] reflects adaptive divergence. Multiple putative evolutionarily significant units (ESUs) correspond more strongly to bioregions, however, than to subspecies. Bezault et al. [81] discuss the hypothesis that O. n. filoa and O. n. cancellatus are differentially adapted ecotypes rather than valid subspecies; they may constitute ESUs. Additional ESUs may be detected upon detailed survey; for example, Nyingi et al. [82] found a unique genetic resource in a recently discovered population from a warm water spring, a tributary of the Loboi Swamp in Kenya. Sex determination systems of natural populations adapted to three extreme thermal regimes showed thermosensitivity of sex differentiation [81], indicating either genotype-environment interaction or epigenetic effects [83] upon sex determination. Observation of genetic differentiation among O. niloticus populations within regions supports the existence of multiple management units (MUs) within certain ESUs, for example, in the Ethiopian and Nilotic regions. In the latter, analysis of microsatellite variation among five Egyptian populations [84] indicated distinct groups respectively inhabiting the deeper lotic Nile River, the shallow less lotic Delta lakes, and the upstream Nile River. The economic importance of O. niloticus worldwide makes detailed knowledge of its genetic resources pivotal for sustainable use of the species in aquaculture operations [85]. Hence, detailed consideration of adaptive differentiation is needed to defensibly assess genetic risk from culture of selectively bred O. niloticus in Africa.
Several international and national agreements address management of genetic resources. The major one is the Cartagena Protocol on Biosafety developed to implement the Convention on Biological Diversity, which came into effect in 2003, that which governs the movement of living modified organisms (LMOs) resulting from modern biotechnology from one country to another [86]; Convention articles 15, 16, and 22 outline guidelines on risk assessment, risk management and capacity building with regards to LMOs. Kapuscinski et al. [87] outline the steps to be taken by developing countries to strengthen scientific and technical capacity to address environmental biosafety issues associated with LMOs; while the risks associated with LMOs and selectively bred stocks may differ, the risk assessment framework developed for LMOs could serve as a useful resource for countries considering the prospect of importing existing selectively bred strains or developing their own strains.
11. Potential Economic Benefits of the GIFT Strain in Africa; Case Study of Ghana
11.1. Model
Against this background, we estimated the potential economic benefits of using selectively bred tilapia strains in Africa. We focused upon the GIFT strain for which production traits and prices are known and approached the benefit assessment by applying the economic surplus model, the most common method for analyzing the economic benefits of agricultural research in a partial equilibrium framework [88,89]. This analysis has been used to study, ex ante, the benefits of agricultural research for various innovations and for a number of countries (e.g., marker-assisted rice breeding in Southeastern Asia [90], and cassava breeding in sub-Saharan Africa [91]). Total economic benefits associated with a new technology for a non-traded commodity (Ghana currently exports very little freshwater fish; [92]) can be represented by the formula:
where P and Q are the initial equilibrium price and quantity, respectively; Z = Ke/(e + n) is the relative reduction in price due to the supply shift resulting from the new technology; e is supply elasticity, and n is demand elasticity (absolute value), which reflect how responsive the quantity supplied and quantity demanded are to changes in prices, respectively; and K is the shift in the supply curve as a proportion of the initial price. K is calculated as:
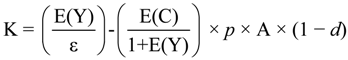 where E(Y) is the expected proportionate yield increase per hectare after the adoption of the new technology; E(C) is the expected proportionate change in variable input cost per hectare; p is the probability of success associated with the research; A is the adoption rate for the technology; and d is the depreciation rate of the new technology [89].
where E(Y) is the expected proportionate yield increase per hectare after the adoption of the new technology; E(C) is the expected proportionate change in variable input cost per hectare; p is the probability of success associated with the research; A is the adoption rate for the technology; and d is the depreciation rate of the new technology [89].
ΔTS = PQK (1 + 0.5Zn)
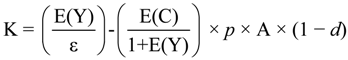
“Economic benefits” is the change in total economic surplus for each year, and the costs are the expenditures on the research projects plus estimated after-project costs related to developing and disseminating the new varieties. The annual costs and benefits are netted and totaled using the discount rate to calculate a net present value (NPV) using the standard NPV formula:
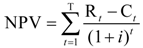 where: Rt is the benefits in year t; Ct is research, development, and dissemination cost in year t; and i is the discount rate. In other words, the NPV is calculated as the sum of future benefits, minus the costs associated with the project discounted over time.
where: Rt is the benefits in year t; Ct is research, development, and dissemination cost in year t; and i is the discount rate. In other words, the NPV is calculated as the sum of future benefits, minus the costs associated with the project discounted over time.
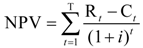
11.2. Data and Key Assumptions
Several factors must be considered to estimate the economic benefits of an agricultural innovation using the economic surplus model: (1) the proportion of farmers who adopt the innovation over time; (2) the price of the commodity; (3) the change in yield of the commodity with the new technology; (4) the nature of the market, as products that are traded may not experience price declines if production increases [37,89]; (5) the time it takes to develop the innovation, and the number of years for maximum adoption to be reached; and (6) the discount rate for future benefits compared to current benefits, since for example, a 5% discount rate implies that after 20 years the calculated benefits of the new technology are not sensitive to the usual depreciation of a new technology that occurs with time.
Dey [37] found that the adoption of the GIFT strain in Bangladesh, China, the Philippines, Thailand, and Vietnam led to yield increases of between 24% and 61%. Since this information is not available for Ghana, we used the mean yield change across these five countries (41.4%), bounded by 24% and 61% for our calculations. The Food and Agricultural Organization estimated Ghana’s Nile tilapia aquaculture production at 18,200 tons in 2011 [93]. Increases in Nile tilapia aquaculture production in the four preceding years were 1600 t, 1576 t, 2748 t, and 8776 t, respectively. Therefore, we extrapolated the aquaculture production of tilapia in 2012 to be 28,200 t, an increase of 10,000 t over 2011’s production value. We calculated the price per ton of production as $2,646 by dividing FAO’s 2011 estimate of the value of production ($48,159,000) by the annual production that year (18,200 t).
Experience from tilapia culture indicates that the only production cost variable expected to be directly impacted by the adoption of the GIFT strain is fingerling price. Fingerling cost constitutes approximately 14% of total costs in a typical enterprise budget for a tilapia farm [94]. Also, according to the Asian Development Bank [95], one of the consequences of the introduction of the GIFT strain in the Philippines was the increase in the price of GIFT fingerlings by 150% compared to non-GIFT fingerlings. Assuming this same increase in fingerling price with the adoption of the GIFT strain in Ghana, total production costs will be increased by 20%. This increase in total costs is expected to incorporate increases and decreases in all input costs resulting from the adoption of the new strain. We also assumed that the technology will be available to Ghanaian farmers from 2013, since the country took delivery of the strain officially in 2012 [96], although primarily for research purposes.
Dey [37] defined “early” GIFT adoption rates to be 30%–40% during the initial dissemination of the technology in Asia, peaking at 70% at the latter stages. We are not aware of any data on supply and demand elasticities of tilapia in Ghana. In the absence of such studies, a supply elasticity of 0.5 usually is applied to perennial crops and other livestock, and demand elasticity of 1 can be used for most livestock [89]. Since the GIFT technology exists already, the probability of success of GIFT research can be assumed to be 100%. Research and development costs also can be assumed to be zero.
Following Ponzoni et al. [23], we anticipate that a government department, such as the Aquaculture Research and Development Center in Ghana, will have to invest in the establishment and running of a nucleus to continuously maintain and improve the strain. Hatchery operators in Ghana will replace their brood stock annually with the latest version of the strain from the nucleus. This arrangement will ensure that production will benefit from the greatest amount of genetic gain. However, this arrangement will have cost implications. Ponzoni et al. ([23], Table 4) provide values for annual or recurrent costs of such a program, with the most likely value being $60,000. We assume that the initial investment cost component is not necessary in our case, since the strain already has been developed. Recurrent costs include dissemination costs, and the costs of continuous improvement and maintenance. We acknowledge that continuous improvement could result in the continuous increase in growth rate and other traits, such as survival and disease resistance, and this expectation should be captured in the sensitivity analysis (see next section).
11.3. Analysis
We included the data above in a modified version of a spreadsheet that incorporates all the model formulas obtained from George W. Norton (Department of Agricultural and Applied Economics, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA). To the typical or most likely value of each key variable, we added a possible minimum and a possible maximum to create a triangular distribution, based on the assumptions or extrapolations from the preceding sub-section, in order to incorporate uncertainty (Table 1), and then conducted economic risk analysis by running Monte Carlo simulations using the @Risk 6 software [97]. We ran the model to calculate the net present values of the annual economic benefits of the GIFT technology for 20 years, at a discount rate of 5%, in Microsoft Excel. The total net present value (NPV) then was calculated as the sum of these annual benefits. We also conducted an analysis to determine how sensitive the average NPV was to the key variables in Table 1.

Table 1.
Values of key variables used in the Monte Carlo simulation to estimate net present value of adopting the GIFT strain in Ghana.
| Variable | Minimum | Most likely | Maximum |
|---|---|---|---|
| Recurrent costs ($) | 30,000 | 60,000 | 90,000 |
| Increase in production costs (%) | 10 | 20 | 30 |
| 2012 Nile tilapia aquaculture production (metric tons) | 26,200 | 28,200 | 30,200 |
| Peak adoption rate (%) | 60 | 70 | 80 |
| Yield change (%) | 24 | 41.4 | 61 |
11.4. Results
The estimated cumulative net benefits of adoption of the Genetically Improved Farmed Tilapia strain in Ghana (discounted at 5%) over twenty years ranged from over $130 million to about $650 million, with a mean close to $400 million (Figure 2). There is a probability of about 40% that the NPV is greater than $400 million (Figure 3). Sensitivity analysis indicated that the variable with the biggest impact on the mean NPV (across the variable’s range of values from Table 1) was the level of change in the aquaculture yield of Nile tilapia resulting from the adoption of the GIFT strain (Figure 4). Not as important were the peak rate of adoption of the strain, level of change in production costs with the adoption of GIFT, the production of Nile tilapia in the year preceding the introduction of the GIFT strain, and the recurrent costs associated with maintaining, improving, and disseminating the new strain, in that order.
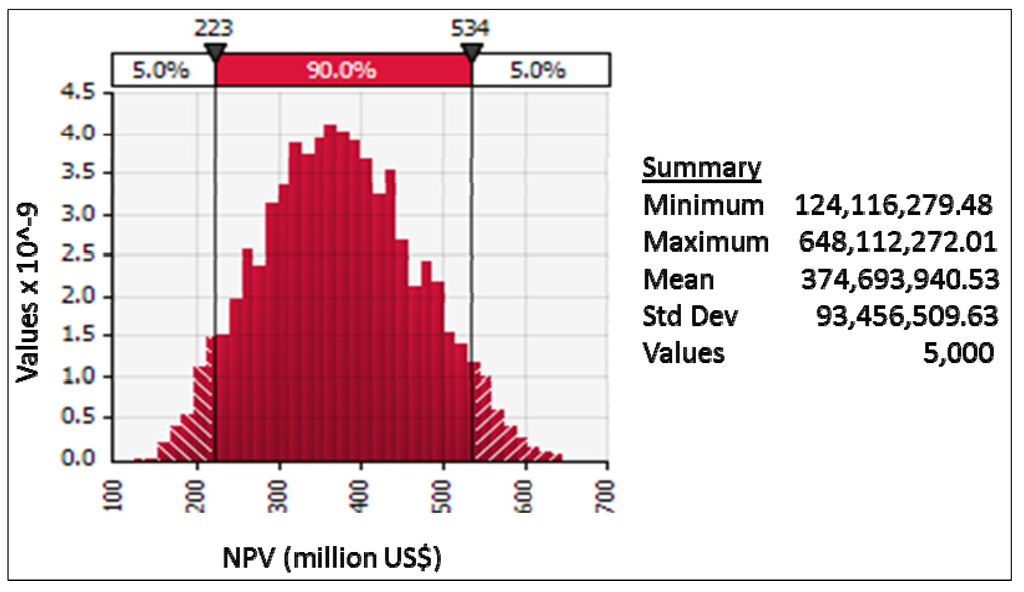
Figure 2.
Summary of results of Monte Carlo simulation to determine net present value (NPV) in US$ for the adoption of GIFT-strain tilapia in Ghana, showing a 90% confidence interval.
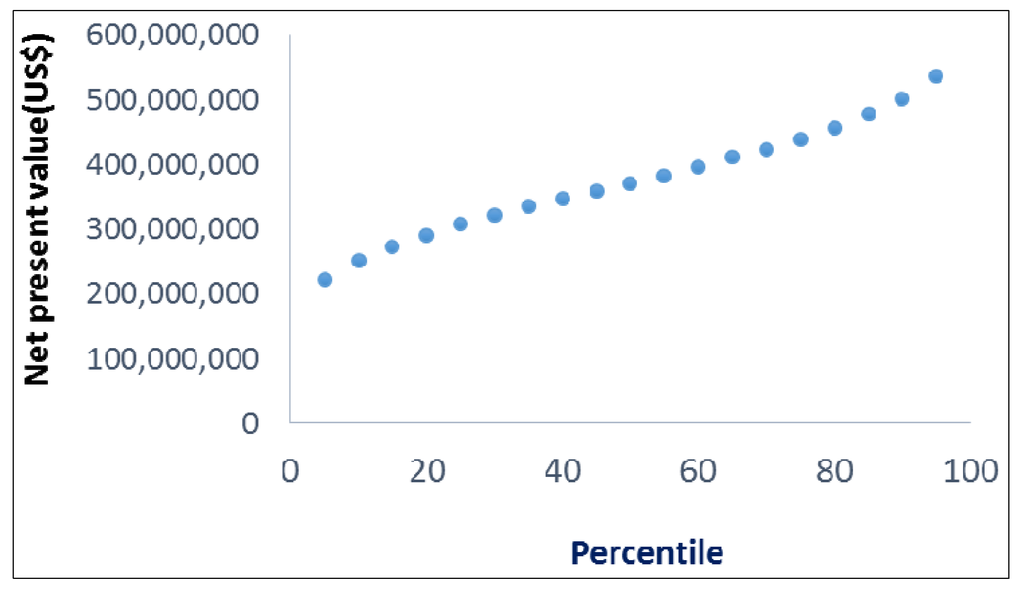
Figure 3.
Net present values (NPV) at different percentiles for the adoption of GIFT-strain tilapia in Ghana.
Figure 4.
Relative impacts of key variables on the mean net present value of adoption of GIFT-strain tilapia in Ghana across the range of key variables.
11.5. Discussion
According to the World Bank [98], the gross domestic product (GDP) of Ghana was $40.71 billion in 2012. This implies that Ghana stands to benefit by approximately 1% of the country’s GDP from the introduction of the GIFT strain. The calculated NPV is also approximately 2.5% of the component of the GDP contributed by the country’s agriculture sector [99]. This percentage is significant, given that the total farmed fish share of the Ghana fish market is currently 3% [100]. The Ghana National Aquaculture Development Plan [100] quotes the value of commercially-farmed fish as $28.44 million in 2010. This value should be close to an optimistic $40 million at the time of developing this manuscript (May 2014) if any progress in growth has been made. Our calculated NPV ($400 million over 20 years) indicates that Ghana could achieve or add on about 50% of the annual farmed fish value from the introduction of the GIFT strain alone. Ponzoni et al. [23] conducted an investment appraisal of a genetic improvement program in Nile tilapia and found the most conservative estimates of economic benefits ranging between 4 and 32 million US$. Their simulated timeframe was 10 years and included the cost of developing the strain from scratch. However, our analysis was done for a period of 20 years with the assumption that the strain was already developed, which is the case with both the GIFT and Akosombo strains.
The version of the economic surplus model that we used assumed that Ghana exports little to no tilapia, as is the case currently. Therefore, the computed NPV values could be higher if this assumption no longer holds true, as might be the case when there is an abundant supply of tilapia. Clearly Ghana, and hence Africa, stands to gain substantial socio-economic benefits from the adoption of the GIFT strain. Some of the possible key benefits include the increased availability of relatively low-cost, high-quality animal protein from the increased yield; increased employment within the expanded aquaculture sector; and possible foreign exchange earnings in the long term.
The Monte Carlo simulation technique allowed us to estimate ranges for variables with uncertain values and to analyze economic risk stemming from changes in these values on net present value. This approach incorporated the element of uncertainty into our computations. Of the five assumed or extrapolated key variables, the cost of maintenance, continuous improvement, and dissemination of the new strain in Ghana (recurring costs) had the least impact on changes in mean NPV. It is intuitive that not much investment will be needed in the dissemination of the GIFT strain in Ghana to cage fish farmers on Volta Lake and to the most progressive pond farmers in other parts of the country. We have observed that this group of tilapia farmers has widely adopted the locally improved Akosombo strain of O. niloticus. However, more work needs to be done to disseminate these relatively new strains of tilapia to the majority of pond farmers, who are still farming inferior, mixed, and unknown stocks of tilapia more than a decade after the development and dissemination of the Akosombo strain by the ARDEC started [28,85]. Our analysis shows how small the investment cost of maintaining, continuous improvement, and dissemination of improved strains of tilapia could be relative to the economic benefits of these strains reaching all farmers. It is a sound policy for the government of Ghana to invest in the dissemination of the improved Akosombo and/or the GIFT strain of tilapia.
On the other hand, the variable with the largest impact on mean NPV was the difference in aquaculture yield between the GIFT and local strains of Nile tilapia. A number of production bottlenecks exist currently in the Ghanaian and African aquaculture industry. The AquaFish Innovation Lab, formerly the Aquaculture and Fisheries Collaborative Research Support Program (AquaFish CRSP) has observed, through on-farm demonstrations in Ghana, that for pond farmers growing the Akosombo strain of O. niloticus, improving pond construction and maintenance, supplementary use of commercial feeds, water quality management, and control of excessive reproduction in ponds (by hormonal sex-reversal and polyculture with a predator) could result in an increase of 2–4 times the current average yields from unimproved local strains. Other significant bottlenecks observed are institutional, including, inadequate access to urban markets due to bad roads, and unreliable electricity supply leading to absence of cold-storage in the rural tilapia value-chain and therefore inefficient and risky post-harvest handling [101]. Addressing these problems would increase the yield and income from improved local strains of tilapia to the extent that the differences in yield between local and GIFT strains could diminish the calculated NPV for the GIFT strain. Notably, some of these bottlenecks have the potential to limit the production and profitability of the GIFT strain, irrespective of its superior growth rate. For example, a farmer who invests in the necessary better management practices and the extra cost of fingerlings of the GIFT strain but who cannot market his or her fish effectively may become skeptical about the value of investing in increased production at an increased cost. Therefore, the problems currently impeding the production of local strains could also affect the realization of the attractive economic benefit computed for GIFT in this study.
Other socio-economic impacts are possible with the adoption of improved strains (see Figure 1). These include higher incomes, better nutrition (more protein), reduced poverty, improved health and welfare. Also, since women are traditionally involved more in processing and marketing of fish, they too will benefit from the adoption of improved strains. This analysis focused generally on the GIFT strain as a representation of an improved tilapia strain. This analysis will still hold true with the Akosombo or any other improved strain. At this time, we are not aware of any completed biological studies comparing the performance of these two strains. These extensions are recommended for future studies.
12. Conclusions
Clearly, the introduction of the GIFT strain in Africa has the potential for substantial economic benefits. However, the potential ecological impacts of the GIFT strain on African aquatic ecosystems cannot be overlooked. After a country makes the decision to introduce the GIFT tilapia strain, each facility that would like to culture this strain must be able to show the structures it has put in place to prevent escapes and both intentional and inadvertent release into the wild. Of course, facilities prone to such events as flooding should not be allowed to culture the GIFT strain. Possible methods to achieve this goal of non-release of the GIFT strain from farms include physical confinement, reproductive confinement, and operations management [63]. Effective physical confinement involves a combination of measures, such as mechanical barriers (e.g., standpipe screens, gravel traps, etc.). Also, effluents could be filtered to exclude any life-stage of the strain. Reproductive confinement is particularly important where physical confinement alone cannot be relied on. Production of GIFT tilapia could be limited to either monosex or sterile individuals. Operations management includes those measures put in place on a tilapia farm to ensure that the activities of all workers are in conformance with the goal of effective confinement, to prevent any unauthorized human access to the facility, and to routinely inspect and maintain all physical barriers to ensure they are constantly operational [63]. Operations management also could ensure that only dead fish on ice are marketed.
African governments, such as Ghana, which have obtained, or are in the process of obtaining, the GIFT germplasm should commission rigorous baseline and ecological risk analyses. Since each country has unique conditions, exhaustive and baseline ecological, genetic, and economic benefit and risk analyses should be conducted separately for each country. Efforts also must be made to improve the culture of locally available strains of tilapia. Improvements in management practices and infrastructure could go a long way to increase the yield of local strains even if genetically-improved strains are not introduced. These improvements also will ensure that the full potential of introduced, improved strains are realized.
Governments also must develop the political will to refuse, or to discontinue the adoption of, the GIFT strain if results of environmental, genetic and economic risk analyses point them in that direction. Each country should develop the capacity to prevent the needless exposure of native aquatic ecosystems to preventable risk, while reaping the substantial economic benefits of adopting the GIFT or similar improved strains of tilapia.
Acknowledgments
This research was partly funded by the Aquaculture and Fisheries (AquaFish) Innovation Lab, supported by the U.S. Agency for International Development (USAID) award number CA/LWA No. EPP-A-00-06-0012-00, and by contributions from participating institutions. The AquaFish Innovation Lab accession number is 1421. We would like to thank Raul Ponzoni (formerly of the WorldFish Center) for making important factual corrections to the review section 8 on dissemination of GIFT/GST Strains in Africa. The opinions expressed herein are those of the authors and do not necessarily reflect the views of the U.S. Agency for International Development.
Author Contributions
The study was conceived by Emmanuel A. Frimpong, and designed with Eric M. Hallerman. Yaw B. Ansah did the desk study, data collection and economic analysis, and a first draft of results. Emmanuel A. Frimpong extended interpretation and discussion of economic analysis results. Each author contributed to writing and editing of specific portions of the manuscript as follows: Yaw B. Ansah (Economics and Ecology); Emmanuel A. Frimpong (Ecology and Economics); and Eric M. Hallerman (Genetics and Ecology).
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- Subasinghe, R.; Soto, D.; Jia, J. Global aquaculture and its role in sustainable development. Rev. Aquac. 2009, 1, 2–9. [Google Scholar] [CrossRef]
- Béné, C.; Heck, S. Fish and food security in Africa. NAGA WorldFish Center Q. 2005, 28, 8–13. [Google Scholar]
- Brummett, R.E.; Angoni, D.E.; Pouomogne, V. On-farm and on-station comparison of wild and domesticated Cameroonian populations of Oreochromis niloticus. Aquaculture 2004, 242, 157–164. [Google Scholar] [CrossRef]
- Lind, C.E.; Brummett, R.E.; Ponzoni, R.W. Exploitation and conservation of fish genetic resources in Africa: Issues and priorities for aquaculture development and research. Rev. Aquac. 2012, 4, 125–141. [Google Scholar] [CrossRef]
- Ponzoni, R.W.; Nguyen, N.H.; Khaw, H.L.; Hamzah, A.; Bakar, K.R.A.; Yee, H.Y. Genetic improvement of Nile tilapia (Oreochromis niloticus) with special reference to the work conducted by the Worldfish Center with the GIFT strain. Rev. Aquac. 2011, 3, 27–41. [Google Scholar] [CrossRef]
- Greer, D.S.; Harvey, B.J. Blue Genes: Sharing and Conserving the World’s Aquatic Biodiversity; Earthscan: London, UK, 2004. [Google Scholar]
- Teichert-Coddington, D.; Popma, T.; Lovshin, L. Attributes of tropical pond-cultured fish. In Dynamics of Pond Aquaculture; Egna, H.S., Boyd, C.E., Eds.; CRC Press: Boca Raton, FL, USA, 1997; pp. 183–198. [Google Scholar]
- El-Sayed, A.F.M. Tilapia Culture; CABI: Cambridge, MA, USA, 2006. [Google Scholar]
- Fitzsimmons, K. Potential to increase global tilapia production. In Global Outlook for Aquaculture Leadership; GOAL Conference: Kuala Lumpur, Malaysia, 2010. [Google Scholar]
- Food and Agriculture Organization. The State of World Fisheries and Aquaculture; Food and Agriculture Organization (United Nations): Rome, Italy, 2010; p. 218. [Google Scholar]
- Shelton, W.L.; Popma, T.J. Biology. In Tilapia: Biology, Culture, and Nutrition; Lim, C.E., Webster, C.D., Eds.; Haworth Press: Binghamton, NY, USA, 2006; pp. 1–49. [Google Scholar]
- Food and Agriculture Organization. State of World Fisheries and Aquaculture; Food and Agriculture Organisation: Rome, Italy, 2012; p. 209. [Google Scholar]
- Fitzsimmons, K. Prospect and potential for global production. In Tilapia: Biology, Culture, and Nutrition; Lim, C., Webster, C., Eds.; Harworth Press: Binghamton, NY, USA, 2006; pp. 51–72. [Google Scholar]
- Pullin, R. Tilapia: Everyman’s fish. Biologist 1985, 32, 84–88. [Google Scholar]
- Gjoen, H. GIFT Program Continues: Distribution of Fast-Growing Tilapia to Expand. Available online: http://pdf.gaalliance.org/pdf/GAA-Gj%F8en-Dec01.pdf (accessed on 30 May 2014).
- McAndrew, B.J. Evolution, phylogenetic relationships and biogeography. In Tilapias: Biology and Exploitation; Beveridge, M.C.M., McAndrew, B.J., Eds.; Kluwer Academic: Dordrecht, The Netherlands, 2000; pp. 1–32. [Google Scholar]
- Pillay, T.V.R.; Kutty, M. Aquaculture: Principles and Practices; Blackwell Publishing: Oxford, UK, 2005. [Google Scholar]
- Canonico, G.C.; Arthington, A.; McCrary, J.K.; Thieme, M.L. The effects of introduced tilapias on native biodiversity. Aquat. Conserv.: Mar. Freshw. Ecosyst. 2005, 15, 463–483. [Google Scholar] [CrossRef]
- Fish Base Tilapia spp. Available online: http://www.fishbase.org/Nomenclature/ScientificNameSearchList.php? (accessed on 14 December 2013).
- Courtenay, W. Tilapias as non-indigenous species in the Americas: Environmental, regulatory and legal issues. Tilapia Aquac. Am. 1997, 1, 18–33. [Google Scholar]
- Pullin, R.S.V.; Palomares, M.; Casal, C.; Dey, M.; Pauly, D. Environmental impacts of tilapia. In Fourth International Symposium on Tilapia in Aquaculture; Fitzsimmons, K., Ed.; Northeast Regional Aquacultural Engineering Services: Ithaca, NY, USA, 1997; pp. 554–570. [Google Scholar]
- De Silva, S.S.; Subasinghe, R.P.; Bartley, D.M.; Lowther, A. Tilapias as Alien Aquatics in Asia and the Pacific: A Review; Food and Agriculture Organization: Rome, Italy, 2004; Volume 453. [Google Scholar]
- Ponzoni, R.W.; Nguyen, N.H.; Khaw, H.L. Investment appraisal of genetic improvement programs in Nile tilapia (Oreochromis niloticus). Aquaculture 2007, 269, 187–199. [Google Scholar] [CrossRef]
- Lutz, C.G. Recent directions in genetics. In Tilapia: Biology, Culture, and Nutrition; Lim, C.E., Webster, C.D., Eds.; Haworth Press: Binghamton, NY, USA, 2006; pp. 139–180. [Google Scholar]
- Poompuang, S.; Hallerman, E.M. Toward detection of quantitative trait loci and marker-assisted selection in fish. Rev. Fish. Sci. 1997, 5, 253–277. [Google Scholar] [CrossRef]
- Attipoe, F.Y.K.; Agyakwah, S.K. Status of Catfish Farming and Research in Ghana. In Proceedings of the a Workshop on the Development of a Genetic Improvement Program for African Catfish Clarias gariepinus, Accra, Ghana, 5–9 November 2007; Ponzoni, R.W., Nguyen, N.H., Eds.; p. 23.
- Attipoe, F.Y.; Blay Jnr, J.; Agyakwah, S.; Ponzoni, R.W.; Khaw, H.L.; Abban, E.K. Genetic parameters and response to selection in the development of Akosombo strain of the Nile tilapia (Oreochromis niloticus) in the Volta Basin, Ghana. In Proceedings of the International Symposium on Tilapia in Aquaculture, Jerusalem, Palestine, 6–10 October 2013.
- Ofori, J.; Abban, E.; Karikari, A.; Brummett, R. Production parameters and economics of small-scale tilapia cage aquaculture in the Volta Lake, ghana. J. Appl. Aquac. 2010, 22, 337–351. [Google Scholar] [CrossRef]
- Bentsen, H.B.; Gjerde, B.; Nguyen, N.H.; Rye, M.; Ponzoni, R.W.; Palada de Vera, M.S.; Bolivar, H.L.; Velasco, R.R.; Danting, J.C.; Dionisio, E.E. Genetic improvement of farmed tilapias: Genetic parameters for body weight at harvest in Nile tilapia (Oreochromis niloticus) during five generations of testing in multiple environments. Aquaculture 2012, 338, 56–65. [Google Scholar]
- Dey, M.M.; Gupta, M.V. Socioeconomics of disseminating genetically improved Nile tilapia in Asia: An introduction. Aquac. Econ. Manag. 2000, 4, 5–11. [Google Scholar] [CrossRef]
- Eknath, A.; Acosta, B. Genetic Improvement of Farmed Tilapias (GIFT) Project: Final Report, March 1988 to December 1997; World Fish Center: Penang, Malaysia, 1998. [Google Scholar]
- Asian Development Bank. An Impact Evaluation of the Development of Genetically Improved Farmed Tilapia and Their Dissemination in Selected Countries; Asian Development Bank: Manila, Phillippines, 2005. [Google Scholar]
- Asian Development Bank. Overview of Freshwater Aquaculture of Tilapia in the Philippines; Asian Development Bank: Manila, Phillippines, 2004. [Google Scholar]
- Eknath, A.E.; Tayamen, M.M.; Palada-de Vera, M.S.; Danting, J.C.; Reyes, R.A.; Dionisio, E.E.; Capili, J.B.; Bolivar, H.L.; Abella, T.A.; Circa, A.V. Genetic improvement of farmed tilapias: The growth performance of eight strains of Oreochromis niloticus tested in different farm environments. Aquaculture 1993, 111, 171–188. [Google Scholar] [CrossRef]
- Gupta, M.V.; Acosta, B.O.; Eknath, A.E.; Dey, M.M. Breakthrough in genetic improvement of tropical finfish through partnership between ICLARM, ASI and developing country NARS. Available online: http://www.fao.org/docs/eims/upload/206603/3_8_cases.PDF (accessed on 30 May 2014).
- Acosta, B.O.; Gupta, M.V. The genetic improvement of farmed tilapias project: Impact and lessons learned. In Success Stories in Asian Aquaculture; De Silva, S.S., Davy, F.B., Eds.; Springer: London, UK, 2010; pp. 149–171. [Google Scholar]
- Dey, M.M. The impact of genetically improved farmed Nile tilapia in Asia. Aquac. Econ. Manag. 2000, 4, 107–124. [Google Scholar] [CrossRef]
- Gupta, M.; Acosta, B. From drawing board to dining table: The success story of the GIFT project. NAGA WorldFish Center Q. 2004, 27, 4–14. [Google Scholar]
- Dey, M.M.; Eknath, A.E. Current Trends in the Asian Tilapia Industry and the Significance of Genetically Improved Tilapia Breeds; INFOFISH: Kuala Lumpur, Malaysia, 1997. [Google Scholar]
- Gupta, M.V.; Bartley, D.M.; Acosta, B.O. Use of Genetically Improved and Alien Species for Aquaculture and Conservation of Aquatic Biodiversity in Africa; The WorldFish Center: Penang, Malaysia, 2004; Volume 68. [Google Scholar]
- Gupta, M.V. Genetic enhancement and conservation of aquatic biodiversity in Africa. NAGA WorldFish Center Q. 2002, 25, 48–53. [Google Scholar]
- WorldFish Center. Policy on the transfer of genetically improved farm tilapia (GIFT) from Asia to Africa by the Worldfish Center. In The WorldFish Center Working Papers; WorldFish Center: Penang, Malaysia, 2007. [Google Scholar]
- Convention on Biological Diversity. The Nagoya Protocol on Access and Benefit-Sharing. Available online: http://www.cbd.int/abs/ (accessed on 21 March 2014).
- Ansah, Y.B.; Frimpong, E.A.; Amisah, S. Biological assessment of aquaculture effects on effluent-receiving streams in Ghana using structural and functional composition of fish and macroinvertebrate assemblages. Environ. Manag. 2012, 50, 166–180. [Google Scholar] [CrossRef]
- Volpe, J.P.; Taylor, E.B.; Rimmer, D.W.; Glickman, B.W. Evidence of natural reproduction of aquaculture-escaped Atlantic salmon in a coastal British Columbia river. Conserv. Biol. 2000, 14, 899–903. [Google Scholar] [CrossRef]
- Naylor, R.L.; Williams, S.L.; Strong, D.R. Aquaculture—A gateway for exotic species. Science 2001, 294, 1655–1656. [Google Scholar] [CrossRef]
- Breder, C.M.; Rosen, D.E. Modes of Reproduction in Fishes; The American Museum of Natural History: New York, NY, USA, 1966. [Google Scholar]
- Balon, E.K. Reproductive guilds of fishes: A proposal and definition. J. Fish. Res. Board Can. 1975, 32, 821–864. [Google Scholar] [CrossRef]
- Arthington, A.; Blüdhorn, D.; Kennard, M. Food resource partitioning by the introduced cichlid, Oreochromis mossambicus, and two native fishes in a subtropical Australian impoundment. In Proceedings of the Third Asian Fisheries Forum, Singapore, 26–30 October 1994; pp. 425–428.
- Crutchfield, J.U., Jr. Establishment and expansion of redbelly tilapia and blue tilapia in a power plant cooling reservoir. Am. Fish. Soc. Symp. 1995, 15, 452–461. [Google Scholar]
- McCrary, J.; van den Berghe, E.; McKaye, K.; Lopez Perez, L. Tilapia cultivation: A threat to native fish species in Nicaragua. Encuentro 2001, 58, 9–19. [Google Scholar]
- Starling, F.; Lazzaro, X.; Cavalcanti, C.; Moreira, R. Contribution of omnivorous tilapia to eutrophication of a shallow tropical reservoir: Evidence from a fish kill. Freshw. Biol. 2002, 47, 2443–2452. [Google Scholar] [CrossRef]
- Moreau, J. A review of introductions of tilapia in open waters of Africa, their influence on ecology and fisheries. In Proceedings of the International Symposium on Tilapia in Aquaculture; Fishelson, L., Yaron, Z., Eds.; Tel Aviv University Press: Nazareth, Israel, 1983; pp. 77–85. [Google Scholar]
- Vareschi, E.; Jacobs, J. The ecology of Lake Nakuru. Oecologia 1985, 65, 412–424. [Google Scholar] [CrossRef]
- Noga, E.J. Fish Disease: Diagnosis and Treatment, 2nd ed.; John Wiley & Sons: Ames, IA, USA, 2010; p. 519. [Google Scholar]
- Zambrano, L.; Martínez-Meyer, E.; Menezes, N.; Peterson, A.T. Invasive potential of common carp (Cyprinus carpio) and Nile tilapia (oreochromis niloticus) in American freshwater systems. Can. J. Fish. Aquat. Sci. 2006, 63, 1903–1910. [Google Scholar] [CrossRef]
- OIE Aquatic animal health. Available online: http://www.oie.int/en/international-standard-setting/aquatic-code/access-online/ (accessed on 20 March 2014).
- Carvalho, G.R.; Hauser, L. Genetic impacts of fish introductions: A perspective on African lakes. In The Impact of Species Changes in African Lakes; Chapman and Hall: London, UK, 1995; pp. 457–493. [Google Scholar]
- Ryman, N.; Laikre, L. Effects of supportive breeding on the genetically effective population size. Conserv. Biol. 1991, 5, 325–329. [Google Scholar] [CrossRef]
- Hallerman, E.M. Population Genetics: Principles and Applications for Fisheries Scientists; Hallerman, E.M., Ed.; American Fisheries Society: Bethesda, MD, USA, 2003; Hallerman, E.M., Ed.; pp. 239–259. [Google Scholar]
- Ferguson, A.; Fleming, I.A.; Hindar, K.; Skaala, O.; McGinnity, P.; Cross, T.; Prodohl, P. The Atlantic Salmon: Genetic Conservation and Management. Blackwell Publishing: Oxford, UK, 2007; Verspoor, E., Stradmeyer, L., Nielsen, J.L., Eds.; pp. 357–398. [Google Scholar]
- McGinnity, P.; Prodöhl, P.; Ferguson, A.; Hynes, R.; ó Maoiléidigh, N.; Baker, N.; Cotter, D.; O’Hea, B.; Cooke, D.; Rogan, G. Fitness reduction and potential extinction of wild populations of Atlantic salmon, Salmo salar, as a result of interactions with escaped farm salmon. Proc. R. Soc. Lond. Ser. B: Biol. Sci. 2003, 270, 2443–2450. [Google Scholar] [CrossRef]
- Araki, H.; Cooper, B.; Blouin, M.S. Genetic effects of captive breeding cause a rapid, cumulative fitness decline in the wild. Science 2007, 318, 100–103. [Google Scholar] [CrossRef]
- Araki, H.; Cooper, B.; Blouin, M.S. Carry-over effect of captive breeding reduces reproductive fitness of wild-born descendants in the wild. Biol. Lett. 2009, 5, 621–624. [Google Scholar] [CrossRef]
- Hindar, K.; Ryman, N.; Utter, F. Genetic effects of cultured fish on natural fish populations. Can. J. Fish. Aquat. Sci. 1991, 48, 945–957. [Google Scholar] [CrossRef]
- Tufto, J. Effects of releasing maladapted individuals: A demographic-evolutionary model. Am. Nat. 2001, 158, 331–340. [Google Scholar] [CrossRef]
- Tufto, J. Gene flow from domesticated species to wild relatives: Migration load in a model of multivariate selection. Evolution 2010, 64, 180–192. [Google Scholar] [CrossRef]
- Garant, D.; Forde, S.E.; Hendry, A.P. The multifarious effects of dispersal and gene flow on contemporary adaptation. Funct. Ecol. 2007, 21, 434–443. [Google Scholar]
- Storfer, A. Gene flow and endangered species translocation. Biol. Conserv. 1999, 87, 173–180. [Google Scholar] [CrossRef]
- Tallmon, D.A.; Luikart, G.; Waples, R.S. The alluring simplicity and complex reality of genetic rescue. Trends Ecol. Evol. 2004, 19, 489–495. [Google Scholar] [CrossRef]
- Hallerman, E. Application of risk analysis to genetic issues in aquaculture. In Understanding and Applying Risk Analysis in Aquaculture; FAO Fisheries and Aquaculture Technical Paper; Bondad-Reantaso, M.G., Arthur, J.R., Subasinghe, R.P., Eds.; Food and Agriculture Organization: Rome, Italy, 2008; Volume 519, pp. 47–66. [Google Scholar]
- Seward, N.W.; VerCauteren, K.C.; Witmer, G.W.; Engeman, R.M. Feral swine impacts on agriculture and the environment. Sheep Goat Res. J. 2004, 19, 34–40. [Google Scholar]
- McLeod, R.; Norris, A. Counting the Cost: Impact of Invasive Animals in Australia, 2004; Cooperative Research Centre for Pest Animal Control Canberra: Canberra, Australia, 2004. [Google Scholar]
- Muir, W.; Howard, R. Fitness components and ecological risk of transgenic release: A model using Japanese medaka (Oryzias latipes). Am. Nat. 2001, 158, 1–16. [Google Scholar]
- Pimm, S.L. The complexity and stability of ecosystems. Nature 1984, 307, 321–326. [Google Scholar] [CrossRef]
- Moyle, P.B.; Light, T. Biological invasions of fresh water: Empirical rules and assembly theory. Biol. Conserv. 1996, 78, 149–161. [Google Scholar] [CrossRef]
- Brummett, R.E.; Ponzoni, R.W. Concepts, alternatives, and environmental considerations in the development and use of improved strains of tilapia in African aquaculture. Rev. Fish. Sci. 2009, 17, 70–77. [Google Scholar] [CrossRef]
- Hallerman, E.; Hilsdorf, A.W.S. Conservation genetics of tilapias: Seeking to define appropriate units for management. Isr. J. Aquac.—Bamidgeh 2014, in press. [Google Scholar]
- Trewavas, E. Tilapiine Fishes of the Genera Sarotherodon, Oreochromis and Danakilia; British Museum (Natural History): London, UK, 1983. [Google Scholar]
- Philippart, J.-C.; Ruwet, J.-C. Ecology and distribution of tilapias. Biol. Cult. Tilapias 1982, 7, 15–60. [Google Scholar]
- Bezault, E.; Balaresque, P.; Toguyeni, A.; Fermon, Y.; Araki, H.; Baroiller, J.-F.; Rognon, X. Spatial and temporal variation in population genetic structure of wild Nile tilapia (Oreochromis niloticus) across Africa. BMC Genet. 2011, 12. [Google Scholar] [CrossRef]
- Nyingi, D.; de Vos, L.; Aman, R.; Agnèse, J.-F. Genetic characterization of an unknown and endangered native population of the Nile tilapia (Oreochromis niloticus (Linnaeus, 1758) (Cichlidae; Teleostei) in the Loboi Swamp (Kenya). Aquaculture 2009, 297, 57–63. [Google Scholar] [CrossRef]
- Navarro-Martín, L.; Vinas, J.; Ribas, L.; Diaz, N.; Gutierrez, A.; di Croce, L.; Piferrer, F. DNA methylation of the gonadal aromatase (cyp19a) promoter is involved in temperature-dependent sex ratio shifts in the European sea bass. PLoS Genet. 2011, 7, e1002447. [Google Scholar] [CrossRef]
- Hassanien, H.A.; Gilbey, J. Genetic diversity and differentiation of Nile tilapia (Oreochromis niloticus) revealed by DNA microsatellites. Aquac. Res. 2005, 36, 1450–1457. [Google Scholar] [CrossRef]
- Eknath, A.E.; Hulata, G. Use and exchange of genetic resources of Nile tilapia (Oreochromis niloticus). Rev. Aquac. 2009, 1, 197–213. [Google Scholar] [CrossRef]
- Convention on Biological Diversity—The Cartegena Protocol on Biosafety. Available online: http://bch.cbd.int/protocol/default.shtml (accessed on 20 March 2014).
- Kapuscinski, A.R.; Hayes, K.R.; Li, S.; Dana, G. Environmental Risk Assessment of Genetically Modified Organisms Volume 3: Methodologies for Transgenic Fish; CABI: Cambridge, MA, USA, 2007. [Google Scholar]
- Masters, A.; Coulibaly, B.; Sanogo, D.; Sidibé, M.; Williams, A. The Economic Impact of Agricultural Research: A Practical Guide; Department of Agricultural Economics, Purdue University: West Lafayette, IN, USA, 1996. [Google Scholar]
- Alston, J.M.; Norton, G.W.; Pardey, P.G. International Service for National Agricultural Research. In Science under Scarcity: Principles and Practice for Agricultural Research Evaluation and Priority Setting; Cornell University Press: Ithaca, NY, USA, 1998. [Google Scholar]
- Alpuerto, V.L.E.B.; Norton, G.W.; Alwang, J.; Ismail, A.M. Economic impact analysis of marker-assisted breeding for tolerance to salinity and phosphorous deficiency in rice. Appl. Econ. Perspect. Policy 2009, 31, 779–792. [Google Scholar]
- Rudi, N.; Norton, G.W.; Alwang, J.; Asumugha, G. Economic impact analysis of marker-assisted breeding for resistance to pests and post-harvest deterioration in cassava. Afr. J. Agric. Resour. Econ. 2010, 4, 110–122. [Google Scholar]
- Food and Agriculture Organization (FAO). FAOSTAT; FAO: Rome, Italy, 2012. [Google Scholar]
- Food and Agriculture Organization (FAO). Global Aquaculture Production 2013; FAO: Rome, Italy, 2013. [Google Scholar]
- Engle, C.R.; Neira, I. Tilapia Farm Business Management and Economics; Aquaculture CRSP, Oregon State University: Corvallis, OR, USA, 2005. [Google Scholar]
- Asian Development Bank. Special Evaluation Study on Small-Scale Freshwater Rural Aquaculture Development for Poverty Reduction; Asian Development Bank: Manila, Phillippines, 2004; p. 67. [Google Scholar]
- Padi, J.; ARDEC, Water Research Institute, Akosombo, Ghana. Personal communication, 2012.
- Palisade Corporation. @Risk, Edition 6 ed; Palisade Corporation: Ithaca, NY, USA, 2013. [Google Scholar]
- World Bank Data by Country; Ghana. The World Bank Group, 2013. Available online: http://data.worldbank.org/country/ghana (accessed on 6 July 2013).
- Overseas Development Institute. Ghana’s Sustained Agricultural Growth: Putting Underused Resources to Work; ODI Publications: London, UK, 2011; p. 27. [Google Scholar]
- Ghana Fisheries Commission. Ghana National Aquaculture Development Plan; Ghana Ministry of Food and Agriculture: Accra, Ghana, 2012; p. 85. [Google Scholar]
- Frimpong, E.A.; Anane-Taabeah, G. Social and Economic Performance of Tilapia Farming in Ghana; Junning, C., Quagrainie, K.K., Hishamunda, N., Eds.; FAO: Rome, Italy, 2014; in press. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).