Adsorption Studies of Coconut Shell Carbons Prepared by KOH Activation for Removal of Lead(II) From Aqueous Solutions
Abstract
:1. Introduction
2. Experimental
2.1. Preparation and Characterizations of Coconut Shell Carbons
2.2. Adsorption Experiments
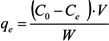
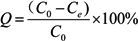
2.3. Adsorption Isotherm
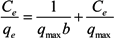
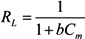
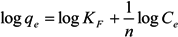
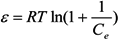
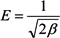
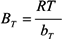
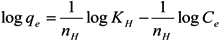
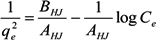
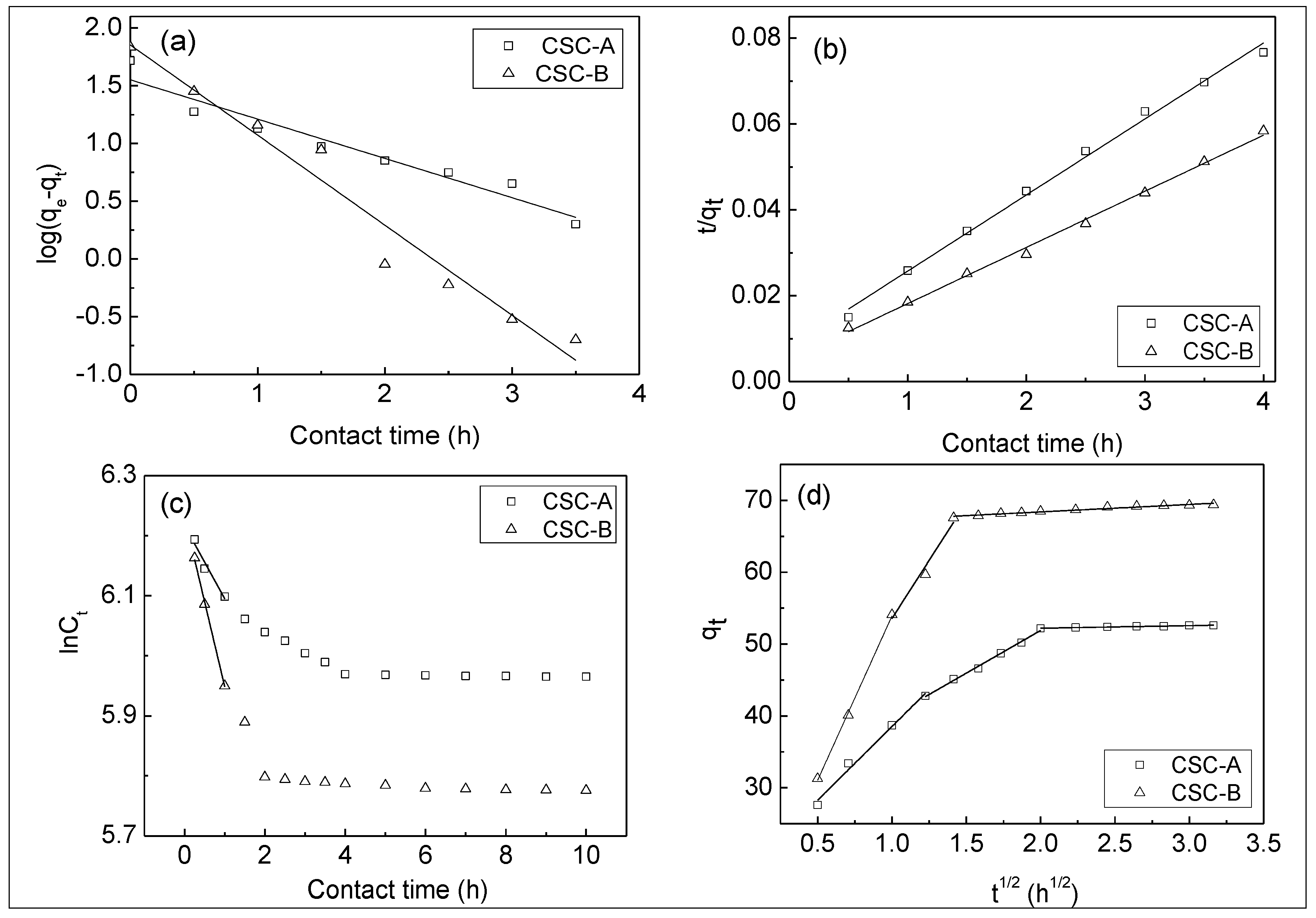
2.4. Adsorption Kinetics
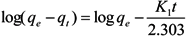
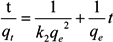
3. Results and Discussion
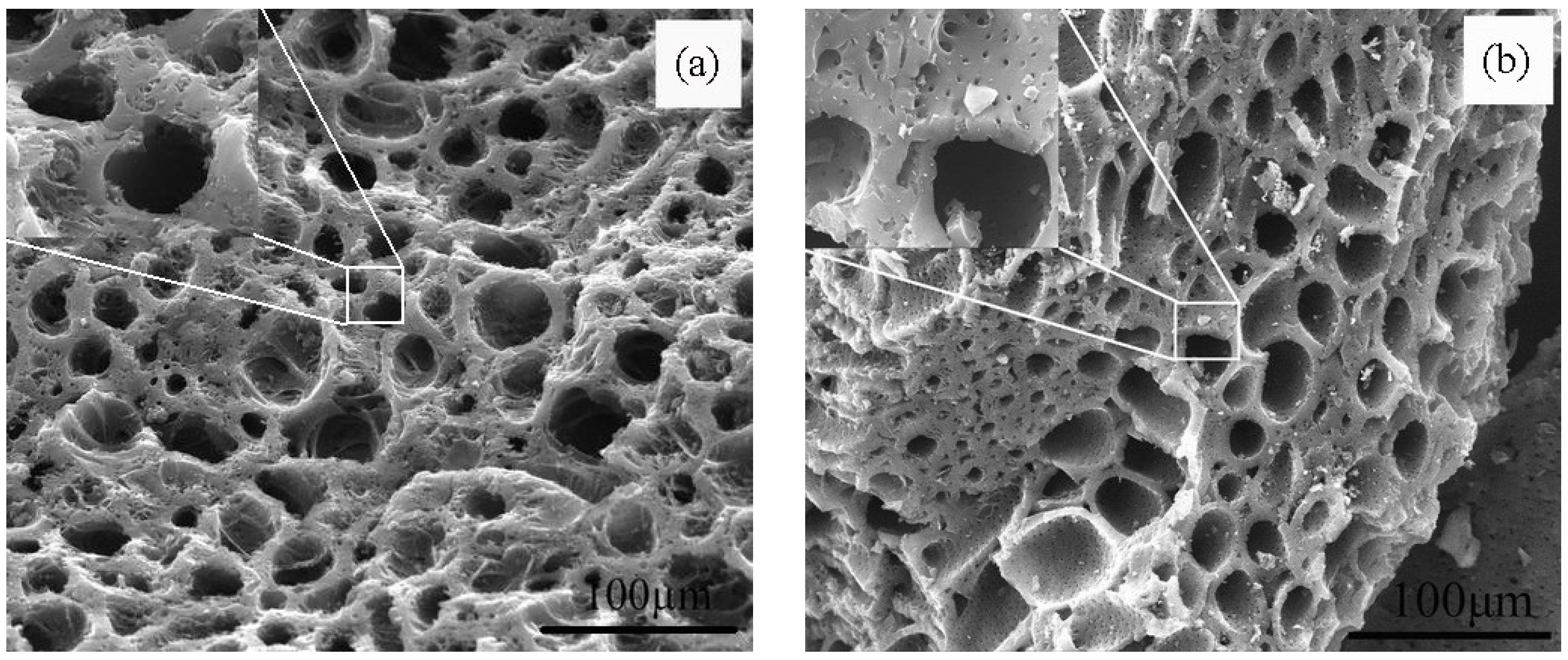
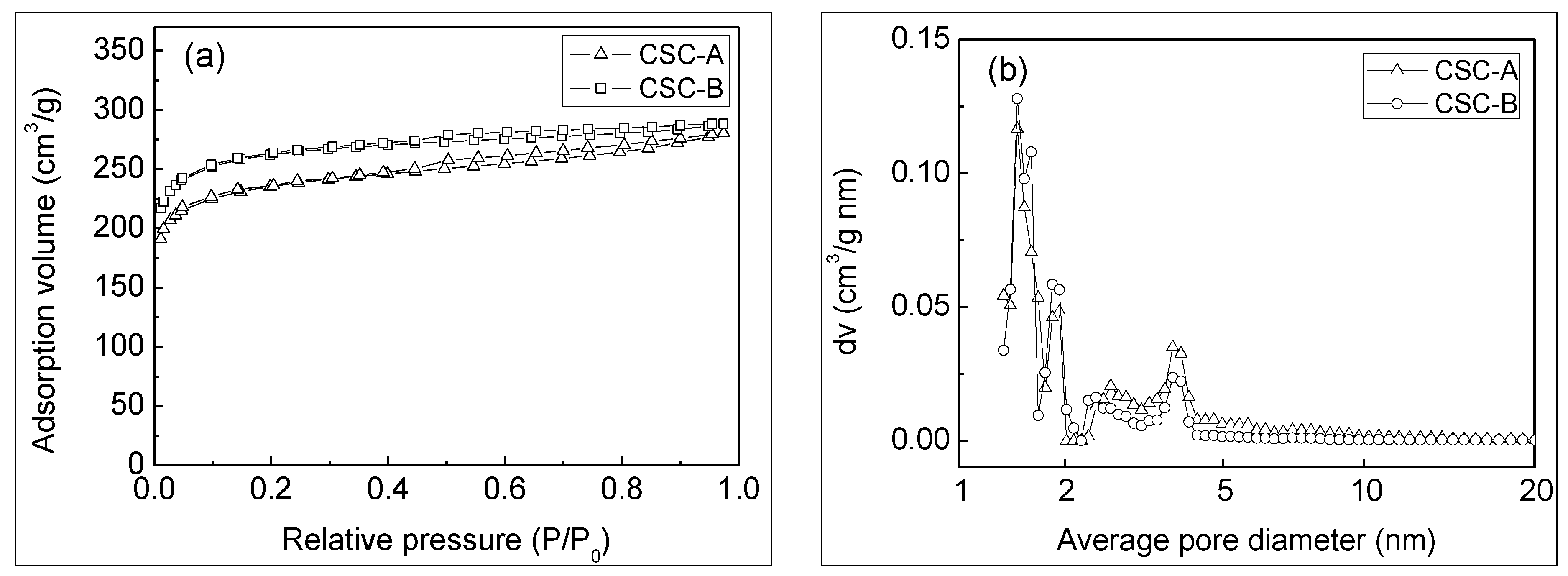
3.1. Morphology and Pore Structure Properties of Coconut Shell Carbons
3.2. Effect of Adsorbent Concentration on Pb2+ Removal

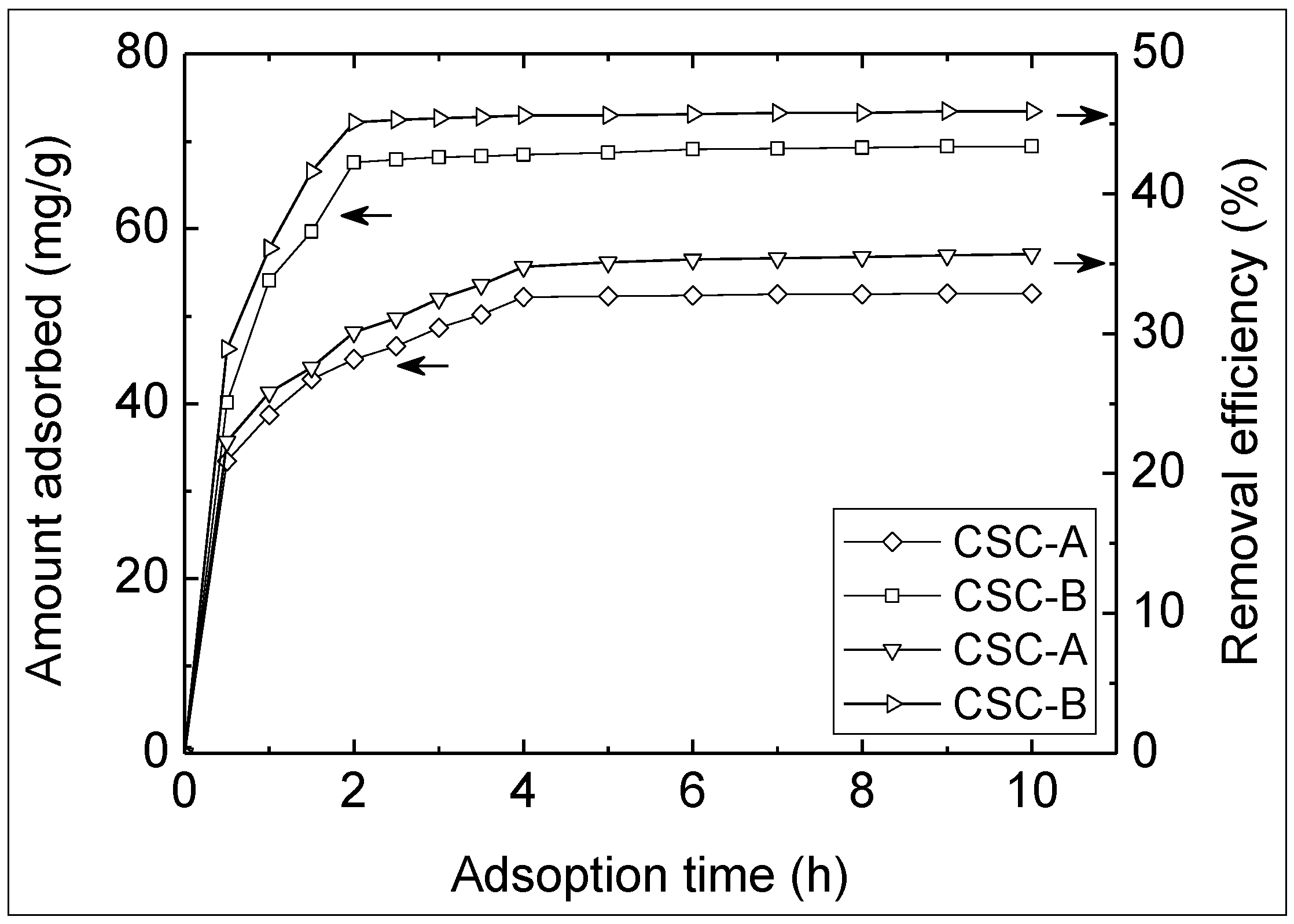
3.3. Effect of Agitation Time on Pb2+ Removal
3.4. Effect of Initial Ions Concentration on Pb2+ Removal

3.5. Adsorption Isotherm
| Parameter | CSC-A | CSC-B |
|---|---|---|
| Langmuir isotherm | ||
| qmax (mg/g) | 112.36 | 151.52 |
| b (L/mg) | 0.0021 | 0.0026 |
| RL | 0.2815 | 0.2401 |
| R2 | 0.9288 | 0.9461 |
| Freundlich isotherm | ||
| n | 1.6784 | 1.6753 |
| KF | 1.3421 | 2.0188 |
| R2 | 0.9906 | 0.9972 |
| Dubinin-Radushkevich (D-R) isotherm | ||
| qm | 53.11 | 73.35 |
| β | 9 × 10−8 | 7 × 10−8 |
| E (kJ/mol) | 2.36 | 2.67 |
| R2 | 0.7820 | 0.8162 |
| Tempkin isotherm | ||
| bT (kJ/mol) | 23.82 | 17.92 |
| KT | 5.77 | 7.38 |
| R2 | 0.9225 | 0.9270 |
| Halsey isotherm | ||
| nH | −1.6784 | −1.6753 |
| KH | 0.6102 | 0.3082 |
| R2 | 0.9906 | 0.9972 |
| Harkin-Jura isotherm | ||
| AHJ | 263.15 | 416.67 |
| BHJ | 2.79 | 2.63 |
| R2 | 0.8261 | 0.7891 |
| Adsorbents | pH | Dosage (g/L) | SBET (m2/g) | Adsorption capacity (mg/g) | Reference |
|---|---|---|---|---|---|
| Enteromorpha prolifera | 5 | 0.5 | 1688 | 146.85 | [8] |
| Pine cone activated carbon | 5 | 2 | 1094.1 | 27.53 | [29] |
| Palm shell activated carbon | 5 | 5 | 957.04 | 95.2 | [30] |
| Apricot stone | 5 | 2 | 566 | 22.84 | [31] |
| CSC-A | 5 | 4 | 728 | 112.36 | In this study |
| CSC-B | 5 | 4 | 1135 | 151.52 | In this study |
3.6. Adsorption Kinetics
| Parameter | CSC-A | CSC-B |
|---|---|---|
| Pseudo-first-order kinetic model | ||
| K1 | 0.7835 | 1.7961 |
| R2 | 0.9352 | 0.9578 |
| Pseudo-second-order kinetic model | ||
| K2 | 0.0392 | 0.0336 |
| R2 | 0.9949 | 0.9962 |
| Spahn and Schlünder model | ||
| Kext | 0.1219 | 0.2827 |
| R2 | 0.9192 | 0.9981 |
| Intra-particle diffusion model | ||
| Stage 1 | ||
| Ki1 | 20.5391 | 45.7459 |
| R2 | 0.9842 | 0.9979 |
| Stage 2 | ||
| Ki2 | 11.8941 | 32.3560 |
| R2 | 0.9946 | 0.9570 |
| Stage 3 | ||
| Ki3 | 0.3556 | 1.0351 |
| R2 | 0.9545 | 0.9488 |
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bahadir, T.; Bakan, G.; Altas, L.; Buyukgungor, H. The investigation of lead removal by biosorption: An application at storage battery industry wastewaters. Enzyme Microb. Technol. 2007, 41, 98–102. [Google Scholar] [CrossRef]
- Wang, S.; Gong, W.; Liu, X.; Yao, Y.; Gao, B.; Yue, Q. Removal of lead(II) from aqueous solution by adsorption onto manganese oxide-coated carbon nanotubes. Sep. Purif. Technol. 2007, 58, 17–23. [Google Scholar] [CrossRef]
- Gupta, V.K.; Agarwal, S.; Saleh, T.A. Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. J. Hazard. Mater. 2011, 185, 17–23. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Wei, J.; Zhang, X.; Xu, C.; Luan, Z.; Wu, D.; Wei, B. Lead adsorption on carbon nanotubes. Chem. Phys. Lett. 2002, 357, 263–266. [Google Scholar] [CrossRef]
- Sekar, M.; Sakthi, V.; Rengaraj, S. Kinetics and equilibrium adsorption study of lead(II) onto activated carbon prepared from coconut shell. J. Colloid Interf. Sci. 2004, 279, 307–313. [Google Scholar] [CrossRef]
- O’Connell, D.W.; Birkinshaw, C.; O’Dwyer, T.F. Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresour. Technol. 2008, 99, 6709–6724. [Google Scholar] [CrossRef]
- Acharya, J.; Sahu, J.N.; Mohanty, C.R.; Meikap, B.C. Removal of lead(II) from wastewater by activated carbon developed from Tamarind wood by zinc chloride activation. Chem. Eng. J. 2009, 149, 249–262. [Google Scholar] [CrossRef]
- Ricordel, S.; Taha, S.; Cisse, I.; Dorange, G. Heavy metals removal by adsorption onto peanut husks carbon: Characterization, kinetic study and modeling. Sep. Purif. Technol. 2001, 24, 389–401. [Google Scholar] [CrossRef]
- Saeed, A.; Iqbal, M.; Akhtar, M.W. Removal and recovery of lead(II) from single and multimetal (Cd, Cu, Ni, Zn) solutions by crop milling waste (black gram husk). J. Hazard. Mater. 2005, 117, 65–73. [Google Scholar] [CrossRef]
- Doyurum, S.; Celik, A. Pb(II) and Cd(II) removal fromaqueous solutions by olive cake. J. Hazard. Mater. 2006, 138, 22–28. [Google Scholar] [CrossRef]
- Goel, J.; Kadirvelu, K.; Rajagopal, C.; Garg, V.K. Removal of lead(II) by adsorption using treated granular activated carbon: Batch and column studies. J. Hazard. Mater. 2005, 125, 211–220. [Google Scholar] [CrossRef]
- Cazetta, A.L.; Vargas, A.M.M.; Nogami, E.M.; Kunita, M.H.; Guilherme, M.R.; Martins, A.C.; Silva, T.L.; Moraes, J.C.G.; Almeida, V.C. NaOH-activated carbon of high surface area produced from coconut shell: Kinetics and equilibrium studies from the methylene blue adsorption. Chem. Eng. J. 2011, 174, 117–125. [Google Scholar] [CrossRef]
- Rao, M.M.; Rao, G.P.C.; Seshaiah, K.; Choudary, N.V.; Wang, M.C. Activated carbon from Ceiba pentandra hulls, an agricultural waste, as an adsorbent in the removal of lead and zinc from aqueous solutions. Waste Manag. 2008, 28, 849–858. [Google Scholar] [CrossRef]
- Li, W.; Yang, K.; Peng, J.; Zhang, L.; Guo, S.; Xia, H. Effects of carbonization temperatures on characteristics of porosity in coconut shell chars and activated carbons derived from carbonized coconut shell chars. Ind. Crop. Prod. 2008, 28, 190–198. [Google Scholar] [CrossRef]
- Bansode, R.R.; Losso, J.N.; Marshall, W.E.; Rao, R.M.; Portier, R.J. Adsorption of metal ions by pecan shell-based granular activated carbons. Bioresour. Technol. 2003, 89, 115–119. [Google Scholar] [CrossRef]
- Jankowska, H.; Swaiatkowski, A.; Choma, J. Activated Carbon; Ellis Horwood: New York, NY, USA, 1991. [Google Scholar]
- Tofighy, M.A.; Mohammadi, T. Adsorption of divalent heavy metal ions from water using carbon nanotube sheets. J. Hazard. Mater. 2011, 185, 140–147. [Google Scholar] [CrossRef]
- González, J.F.; Román, S.; Encinar, J.M.; Martínez, G. Pyrolysis of various biomass residues and char utilization for the production of activated carbons. J. Anal. Appl. Pyrol. 2009, 85, 134–141. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Cr(VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/or chitosan. Chemosphere 2004, 54, 951–967. [Google Scholar] [CrossRef]
- Nomanbhay, S.M.; Palanisamy, K. Removal of heavy metal from industrial wastewater using chitosan coated oil palm shell charcoal. Electron. J. Biotechnol. 2005, 8, 43–53. [Google Scholar]
- Li, Y.; Du, Q.; Wang, X.; Zhang, P.; Wang, D.; Wang, Z.; Xia, Y. Removal of lead from aqueous solution by activated carbon prepared from Enteromorpha prolifera by zinc chloride activation. J. Hazard. Mater. 2010, 183, 583–589. [Google Scholar] [CrossRef]
- Amuda, O.S.; Giwa, A.A.; Bello, I.A. Removal of heavy metal from industrial wastewater using modified activated coconut shell carbon. Biochem. Eng. J. 2007, 36, 174–181. [Google Scholar] [CrossRef]
- Aroua, M.K.; Leong, S.P.P.; Teo, L.Y.; Yin, C.Y.; Daud, W.M.A.W. Real-time determination of kinetics of adsorption of lead(II) onto palm shell-based activated carbon using ion selective electrode. Bioresour. Technol. 2008, 99, 5786–5792. [Google Scholar] [CrossRef]
- Mohamed, A.S.; Ghalia, A.Z.; Samia, A.K. Simultaneous removal of copper(II), lead(II), zinc(II) and cadmium(II) from aqueous solutions by multi-walled carbon nanotubes. C. R. Chim. 2012, 15, 398–408. [Google Scholar] [CrossRef]
- Imamoglu, M.; Tekir, O. Removal of copper(II) and lead(II) ions from aqueous solutions by adsorption on activated carbon from a new precursor hazelnut husks. Desalination 2008, 228, 108–113. [Google Scholar] [CrossRef]
- Gong, J.; Liu, T.; Wang, X.; Hu, X.; Zhang, L. Efficient removal of heavy metal ions from aqueous systems with the assembly of anisotropic layered double hydroxide nanocrystals@carbon nanosphere. Environ. Sci. Technol. 2011, 45, 6181–6187. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X. Novel silica-based hybrid adsorbents: Lead(II) adsorption isotherms. Sci. World J. 2013, 2013. Article 897159. [Google Scholar]
- Shahmohammadi-Kalalagh, S.; Babazadeh, H.; Nazemi, A.H.; Manshouri, M. Isotherm and kinetic studies on adsorption of Pb, Zn and Cu by kaolinite. Caspian J. Environ. Sci. 2011, 9, 243–255. [Google Scholar]
- Momčilović, M.; Purenović, M.; Bojić, A.; Zarubica, A.; Ranđelović, M. Removal of lead(II) ions from aqueous solutions by adsorption onto pine cone activated carbon. Desalination 2011, 276, 53–59. [Google Scholar] [CrossRef]
- Issabayeva, G.; Aroua, M.K.; Sulaiman, N.M.N.S. Removal of lead from aqueous solutions on palm shell activated carbon. Bioresour. Technol. 2006, 97, 2350–2355. [Google Scholar] [CrossRef]
- Kobya, M.; Demirbas, E.; Senturk, E.; Ince, M. Adsorption of heavy metal ions from aqueous solutions by activated carbon prepared from apricot stone. Bioresour. Technol. 2005, 96, 1518–1521. [Google Scholar] [CrossRef]
- Figaro, S.; Avril, J.P.; Brouers, F.; Ouensanga, A.; Gaspard, S. Adsorption studies of molasse’s wastewaters on activated carbon: Modelling with a new fractal kinetic equation and evaluation of kinetic models. J. Hazard. Mater. 2009, 161, 649–656. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Song, C.; Wu, S.; Cheng, M.; Tao, P.; Shao, M.; Gao, G. Adsorption Studies of Coconut Shell Carbons Prepared by KOH Activation for Removal of Lead(II) From Aqueous Solutions. Sustainability 2014, 6, 86-98. https://doi.org/10.3390/su6010086
Song C, Wu S, Cheng M, Tao P, Shao M, Gao G. Adsorption Studies of Coconut Shell Carbons Prepared by KOH Activation for Removal of Lead(II) From Aqueous Solutions. Sustainability. 2014; 6(1):86-98. https://doi.org/10.3390/su6010086
Chicago/Turabian StyleSong, Chengwen, Shuaihua Wu, Murong Cheng, Ping Tao, Mihua Shao, and Guangrui Gao. 2014. "Adsorption Studies of Coconut Shell Carbons Prepared by KOH Activation for Removal of Lead(II) From Aqueous Solutions" Sustainability 6, no. 1: 86-98. https://doi.org/10.3390/su6010086




