Exploring the Impact of Humic Biostimulants on Cassava Yield and Nutrition in Northeast Brazil
Abstract
:1. Introduction
2. Materials and Methods
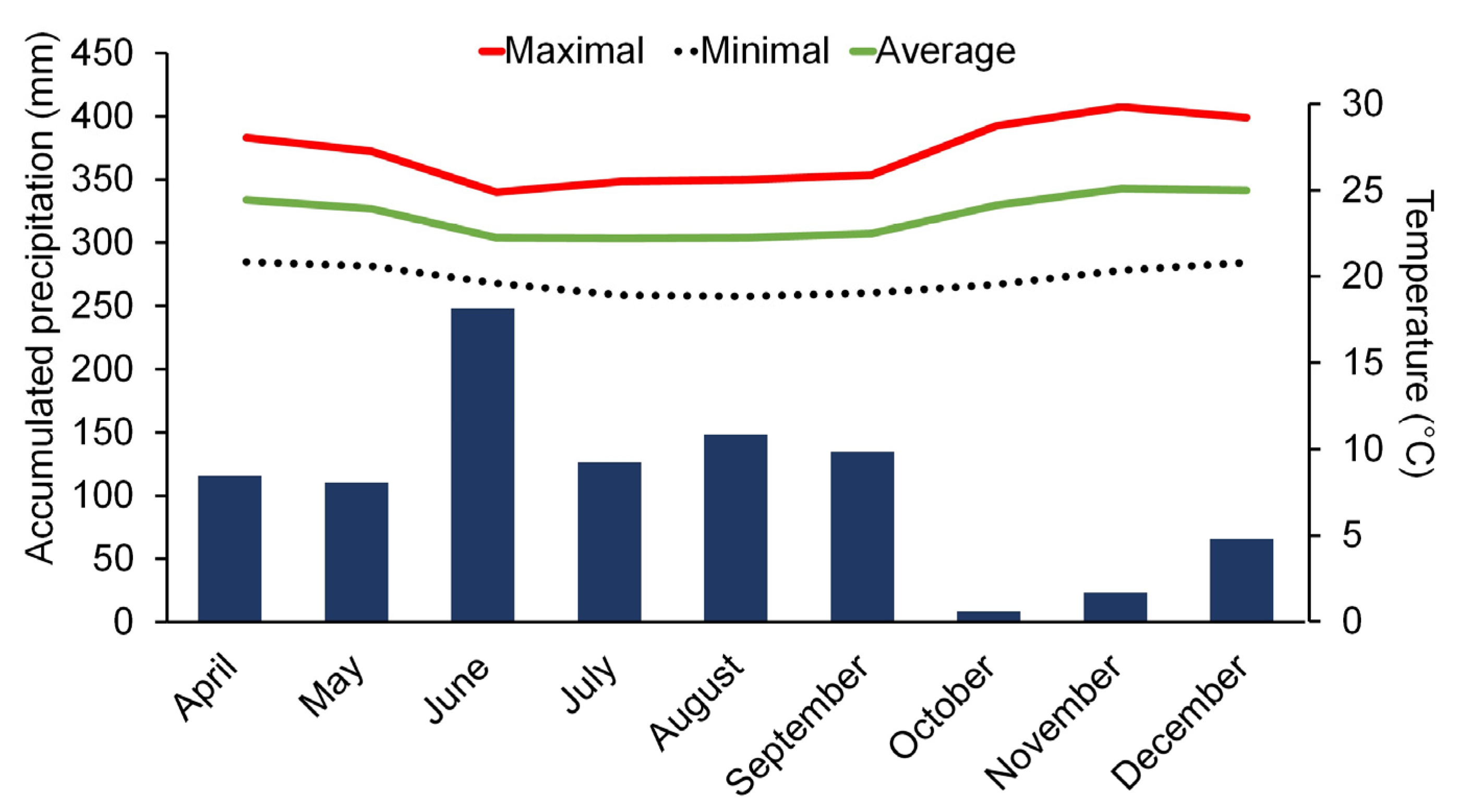
2.1. Experimental Site
2.2. Plant Material and Treatments
2.3. Gas Exchanges and Chlorophyll Index
2.4. Growth and Productivity
2.5. Soil Analysis and Mineral Nutrient Contents in Soil, Roots, and Leaves
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Carvalho, R.R.B.; Bandeira e Sousa, M.; de Oliveira, L.A.; de Oliveira, E.J. Phenotypic diversity and selection in biofortified cassava germplasm for yield and quality root traits. Euphytica 2022, 218, 173. [Google Scholar] [CrossRef] [PubMed]
- Mohidin, S.R.N.S.P.; Moshawih, S.; Hermansyah, A.; Asmuni, M.I.; Shafqat, N.; Ming, L.C. Cassava (Manihot esculenta Crantz): A systematic review for the pharmacological activities, traditional uses, nutritional values, and phytochemistry. J. Evid. Based Integr. Med. 2023, 28, 2515690. [Google Scholar]
- Parmar, A.; Sturm, B.; Hensel, O. Crops that feed the world: Production and improvement of cassava for food, feed, and industrial uses. Food Secur. 2017, 9, 907–927. [Google Scholar] [CrossRef]
- FAOSTAT—Food and Agriculture Organization of the United Nations (2024) Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/ (accessed on 19 March 2024).
- Barbosa, F.R.G.M.; Duarte, V.N.; Staduto, J.A.R.; Kreter, A.C. Land-Use Dynamics for Agricultural and Livestock in Central-West Brazil and its Reflects on the Agricultural Frontier Expansion. Clean. Circ. Bioecon. 2023, 4, 100033. [Google Scholar] [CrossRef]
- Canellas, L.P.; Canellas, N.O.A.; da Silva, R.M.; Spaccini, R.; Mota, G.P.; Olivares, F.L. Biostimulants using humic substances and plant-growth-promoting bacteria: Effects on cassava (Manihot esculentus) and okra (Abelmoschus esculentus) yield. Agronomy 2023, 13, 80. [Google Scholar] [CrossRef]
- Santos, M.N.; Zárate-Salazar, J.R.; de Carvalho, R.; Albuquerque, U.P. Intraspecific variation, knowledge and local management of cassava (Manihot esculenta Crantz) in the semiarid region of Pernambuco, Northeast Brazil. Environ. Dev. Sustain. 2020, 22, 2881–2903. [Google Scholar] [CrossRef]
- Ngongo, Y.; Basuki, T.; Derosari, B.; Mau, Y.S.; Noerwijati, K.; Dasilva, H.; Sitorus, A.; Kotta, N.R.E.; Utomo, W.H.; Wisnubroto, E.I. The roles of cassava in marginal semi-arid farming in East Nusa Tenggara—Indonesia. Sustainability 2022, 14, 5439. [Google Scholar] [CrossRef]
- Ceballos, H.; Hershey, C.; Iglesias, C.; Zhang, X. Fifty years of a public cassava breeding program: Evolution of breeding objectives, methods, and decision-making processes. Theor. Appl. Genet. 2021, 134, 2335–2353. [Google Scholar] [CrossRef] [PubMed]
- Odoemelam, C.S.; Percival, B.; Ahmad, Z.; Chang, M.W.; Scholey, D.; Burton, E.; Okafor, P.N.; Wilson, P.B. Characterization of yellow root cassava and food products: Investigation of cyanide and β-carotene concentrations. BMC Res. Notes 2020, 13, 333. [Google Scholar] [CrossRef]
- Montagnac, J.A.; Davis, C.R.; Tanumihardjo, S.A. Nutritional value of cassava for use as a staple food and recent advances for improvement. Compr. Rev. Food Sci. Food Saf. 2009, 8, 181–194. [Google Scholar] [CrossRef]
- Soares, I.S.; Perrechil, F.; Grandis, A.; Pagliuso, D.; Purgatto, E.; de Oliveira, L.A.; Cavalari, A.A. Cassava waste (stem and leaf) analysis for reuse. Food Chem. Adv. 2024, 4, 100675. [Google Scholar] [CrossRef]
- Pushpalatha, R.; Gangadharan, B. Is cassava (Manihot esculenta Crantz) a climate “smart” crop? A review in the context of bridging future food demand gap. Trop. Plant Biol. 2020, 13, 201–211. [Google Scholar]
- de Araújo Visses, F.A.; Sentelhas, P.C.; Pereira, A.B. Yield gap of cassava crop as a measure of food security—An example for the main Brazilian producing regions. Food Secur. 2018, 10, 1191–1202. [Google Scholar] [CrossRef]
- Howeler, R.H. Long term effect of cassava cultivation on soil productivity. Field Crops Res. 1991, 26, 1–18. [Google Scholar] [CrossRef]
- Adiele, J.G.; Schut, A.G.; Van Den Beuken, R.P.; Ezui, K.S.; Pypers, P.; Ano, A.O.; Egesi, C.N.; Giller, K.E. Towards closing cassava yield gap in West Africa: Agronomic efficiency and storage root yield responses to NPK fertilizers. Field Crops Res. 2020, 253, 107820. [Google Scholar] [CrossRef]
- Nguyen, H.; Schoenau, J.J.; Nguyen, D.; Van Rees, K.; Boehm, M. Effects of long-term nitrogen, phosphorus, and potassium fertilization on cassava yield and plant nutrient composition in North Vietnam. J. Plant Nutr. 2002, 25, 425–442. [Google Scholar] [CrossRef]
- Nanganoa, L.T.; Ngome, F.A.; Suh, C.; Basga, S.D. Assessing soil nutrients variability and adequacy for the cultivation of maize, cassava, and sorghum in selected agroecological zones of Cameroon. Int. J. Agron. 2020, 2020, 8887318. [Google Scholar] [CrossRef]
- Wezel, A.; Gemmill Herren, B.; Kerr, R.B.; Barrios, E.; Luiz, A.; Gonçalves, R.; Sinclair, F. Agroecological principles and elements and their implications for transitioning to sustainable food systems. A review. Agron. Sustain. Dev. 2020, 40, 40. [Google Scholar]
- Wang, Y.; Lu, Y.; Wang, L.; Song, G.; Ni, L.; Xu, M.; Nie, C.; Li, B.; Bai, Y. Analysis of the molecular composition of humic substances and their effects on physiological metabolism in maize based on untargeted metabolomics. Front. Plant Sci. 2023, 14, 1122621. [Google Scholar] [CrossRef]
- De Hita, D.; Fuentes, M.; Fernández, V.; Zamarreño, A.M.; Olaetxea, M.; García-Mina, J.M. Discriminating the short-term action of root and foliar application of humic acids on plant growth: Emerging role of jasmonic acid. Front. Plant Sci. 2020, 11, 510477. [Google Scholar] [CrossRef]
- de Moura, O.V.; Berbara, R.L.; de Oliveira Torchia, D.F.; da Silva, H.F.; de Castro, T.A.; Tavares, O.C.; Rodrigues, N.F.; Zonta, E.; Santos, L.A.; García, A.C. Humic foliar application as sustainable technology for improving the growth, yield, and abiotic stress protection of agricultural crops. A review. J. Saudi Soc. Agric. Sci. 2023, 22, 493–513. [Google Scholar] [CrossRef]
- Nardi, S.; Schiavon, M.; Francioso, O. Chemical structure and biological activity of humic substances define their role as plant growth promoters. Molecules 2021, 26, 2256. [Google Scholar] [CrossRef]
- Jindo, K.; Olivares, F.L.; Malcher, D.J.P.; Sánchez-Monedero, M.A.; Kempenaar, C.; Canellas, L.P. From lab to field: Role of humic substances under open-field and greenhouse conditions as biostimulant and biocontrol agent. Front. Plant Sci. 2020, 11, 426. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalvez, J.D.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Aesa—Agência Executiva de Gestão das Águas. Dados Meteorológicos por Região, Governo da Paraíba. 2024. Available online: http://www.aesa.pb.gov.br/aesa-website/ (accessed on 10 March 2024).
- dos Santos, H.G.; Jacomine, P.K.; dos Anjos, L.H.; De Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; de Almeida, J.A.; de Araujo Filho, J.C.; de Oliveira, J.D.; Cunha, T.J. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa: Brasília, DF, Brazil, 2018; 356p. [Google Scholar]
- Souza, E.D.; Lima-Primo, H.E. BRS Dourada e BRS Gema de Ovo: Cultivares de Mandioca de Mesa Biofortificadas para Roraima; Comunicado técnico 91. 1ed.; Embrapa Roraima: Boa Vista, RR, Brazil, 2021; 8p. [Google Scholar]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo, 3rd ed.; Embrapa Solos: Rio de Janeiro, RJ, Brazil, 2017; 573p. [Google Scholar]
- Cruz, C.D. Genes Software—Extended and integrated with the R, Matlab and Selegen. Acta Sci. Agron. 2016, 38, 547–552. [Google Scholar]
- Orek, C. A review of the functions of transcription factors and related genes involved in cassava (Manihot esculenta Crantz) response to drought stress. Trop. Plants 2023, 2, 14. [Google Scholar] [CrossRef]
- Baía, D.C.; Olivares, F.L.; Zandonadi, D.B.; de Paula Soares, C.; Spaccini, R.; Canellas, L.P. Humic acids trigger the weak acids stress response in maize seedlings. Chem. Biol. Technol. Agric. 2020, 7, 31. [Google Scholar] [CrossRef]
- Silva, R.M.; Peres, A.N.A.; Peres, L.E.P.; Olivares, F.L.; Sangi, S.; Canellas, N.A.; Spaccini, R.; Cangemi, S.; Canellas, L.P. Humic substances isolated from recycled biomass trigger jasmonic acid biosynthesis and signalling. Plants 2023, 12, 3148. [Google Scholar] [CrossRef]
- Fageria, N.K.; Filho, M.B.; Moreira, A.; Guimarães, C.M. Foliar fertilization of crop plants. J. Plant Nutr. 2009, 32, 1044–1064. [Google Scholar] [CrossRef]

| pH (water) | P | K+ | Na+ | H+Al3+ | Al3+ | Ca2+ | Mg2+ | SB | CEC | OM |
|---|---|---|---|---|---|---|---|---|---|---|
| mg dm3 | cmolc dm3 | g kg−1 | ||||||||
| 6.36 | 34.09 | 30.52 | 0.02 | 2.64 | 0.05 | 2.30 | 3.20 | 5.60 | 8.24 | 27.69 |
| Variables | Untreated | Treated | p-Value |
|---|---|---|---|
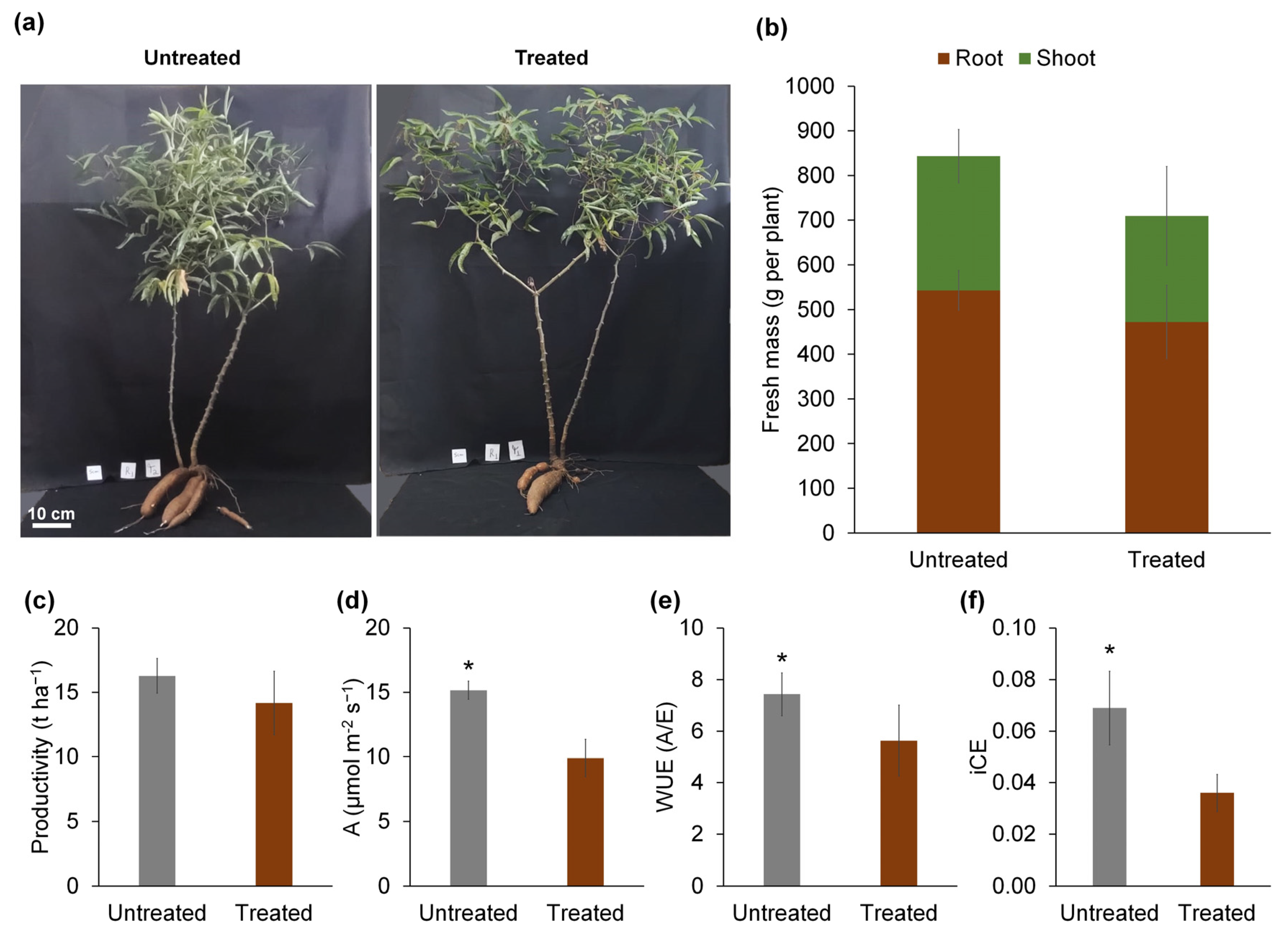
| Plant height (cm) | 65.03 ± 7.87 | 57.14 ± 2.66 | 0.13 ns |
| Main stem diameter (cm) | 1.21 ± 0.20 | 1.14 ± 0.11 | 0.49 ns |
| Number of roots per plant | 4.68 ± 0.67 | 4.13 ± 1.06 | 0.36 ns |
| Number of leaves per plant | 73.64 ± 15.64 | 52.67 ± 11.60 | 0.17 ns |
| Root mass (g per plant) | 542.87 ± 44.64 | 472.44 ± 82.20 | 0.19 ns |
| Shoot mass (g per plant) | 300.96 ± 59.36 | 237.24 ± 110.84 | 0.50 ns |
| Total plant mass (g per plant) | 843.83 ± 87.59 | 709.67 ± 171.33 | 0.31 ns |
| Average root mass (g per root) | 133.43 ± 31.75 | 128.63 ± 29.22 | 0.85 ns |
| Shoot/root ratio | 0.55 ± 0.10 | 0.49 ± 0.19 | 0.70 ns |
| Leaf area per plant (m2) | 6598.61 ± 2395.78 | 11,664.05 ± 9326.61 | 0.26 ns |
| E (mmol H2O m−2 s−1) | 2.05 ± 0.16 | 1.81 ± 0.32 | 0.10 ns |
| gs (mol H2O m−2 s−1) | 0.21 ± 0.04 | 0.18 ± 0.02 | 0.18 ns |
| Chl a index | 34.74 ± 3.25 | 37.68 ± 1.60 | 0.07 ns |
| Chl b index | 10.63 ± 2.17 | 11.81 ± 1.05 | 0.27 ns |
| Total Chl index | 45.36 ± 5.37 | 49.49 ± 2.59 | 0.11 ns |
| Mineral | Untreated | Treated | p-Value | |
|---|---|---|---|---|
| Soil | Mg (g/kg) | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.18 ns |
| Ca (g/kg) | 0.94 ± 0.07 | 1.04 ± 0.21 | 0.07 ns | |
| Na (g/kg) | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.00 * | |
| K (g/kg) | 0.10 ± 0.01 | 0.15 ± 0.03 | 0.00 * | |
| Zn (mg/kg) | 6.77 ± 1.61 | 7.45 ± 1.98 | 0.01 * | |
| Fe (mg/kg) | 100.90 ± 12.85 | 84.45 ± 14.86 | 0.00 * | |
| Mn (mg/kg) | 11.75 ± 1.06 | 14.07 ± 1.99 | 0.00 * | |
| Root | Mg (g/kg) | 0.80 ± 0.20 | 0.81 ± 0.07 | 0.84 ns |
| Ca (g/kg) | 0.79 ± 0.08 | 0.85 ± 0.33 | 0.25 ns | |
| Na (g/kg) | 0.50 ± 0.16 | 0.38 ± 0.21 | 0.00 * | |
| K (g/kg) | 8.38 ± 2.67 | 7.51 ± 2.13 | 0.28 ns | |
| Leaf | Mg (g/kg) | 3.59 ± 0.03 | 3.51 ± 0.03 | 0.10 ns |
| Ca (g/kg) | 10.63 ± 0.08 | 10.73 ± 0.02 | 0.19 ns | |
| Na (g/kg) | 0.12 ± 0.01 | 0.10 ± 0.01 | 0.07 ns | |
| K (g/kg) | 10.55 ± 0.13 | 9.44 ± 0.06 | 0.00 * | |
| Zn (mg/kg) | 46.59 ± 1.06 | 46.14 ± 0.75 | 0.13 ns | |
| Fe (mg/kg) | 208.84 ± 1.42 | 208.03 ± 0.84 | 0.42 ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, M.d.C.; Cavalcanti, M.T.; Pessoa, L.N.; Silva, Z.G.d.; da Silva, A.M.; Souza, T.; Henschel, J.M.; Pereira, E.M.; Diniz Neto, M.A.; Diniz, B.L.M.T. Exploring the Impact of Humic Biostimulants on Cassava Yield and Nutrition in Northeast Brazil. Sustainability 2024, 16, 4088. https://doi.org/10.3390/su16104088
Santos MdC, Cavalcanti MT, Pessoa LN, Silva ZGd, da Silva AM, Souza T, Henschel JM, Pereira EM, Diniz Neto MA, Diniz BLMT. Exploring the Impact of Humic Biostimulants on Cassava Yield and Nutrition in Northeast Brazil. Sustainability. 2024; 16(10):4088. https://doi.org/10.3390/su16104088
Chicago/Turabian StyleSantos, Maisa da Conceição, Mônica Tejo Cavalcanti, Larissa Nicácio Pessoa, Zenaide Gomes da Silva, Allisson Miguel da Silva, Tancredo Souza, Juliane Maciel Henschel, Emmanuel Moreira Pereira, Manoel Alexandre Diniz Neto, and Belísia Lúcia Moreira Toscano Diniz. 2024. "Exploring the Impact of Humic Biostimulants on Cassava Yield and Nutrition in Northeast Brazil" Sustainability 16, no. 10: 4088. https://doi.org/10.3390/su16104088






