Rapid Investigation of Oil Pollution in Water-Combined Induced Fluorescence and Random Sample Consensus Algorithm
Abstract
:1. Introduction
- Introduces an innovative analytical approach by integrating Laser-Induced Fluorescence (LIF) with the Random Sample Consensus (RANSAC) algorithm.
- Overcomes the constraints associated with lower oil concentration scales ranging from 0 to 10 mg/L, expanding the traditional oil-in-water detection dataset.
- Exhibits exceptional sensitivity, enabling precise detection of diverse oil spills within the concentration range of 0~1000 mg/L in water bodies.
2. Materials and Methods
2.1. Experimental Samples
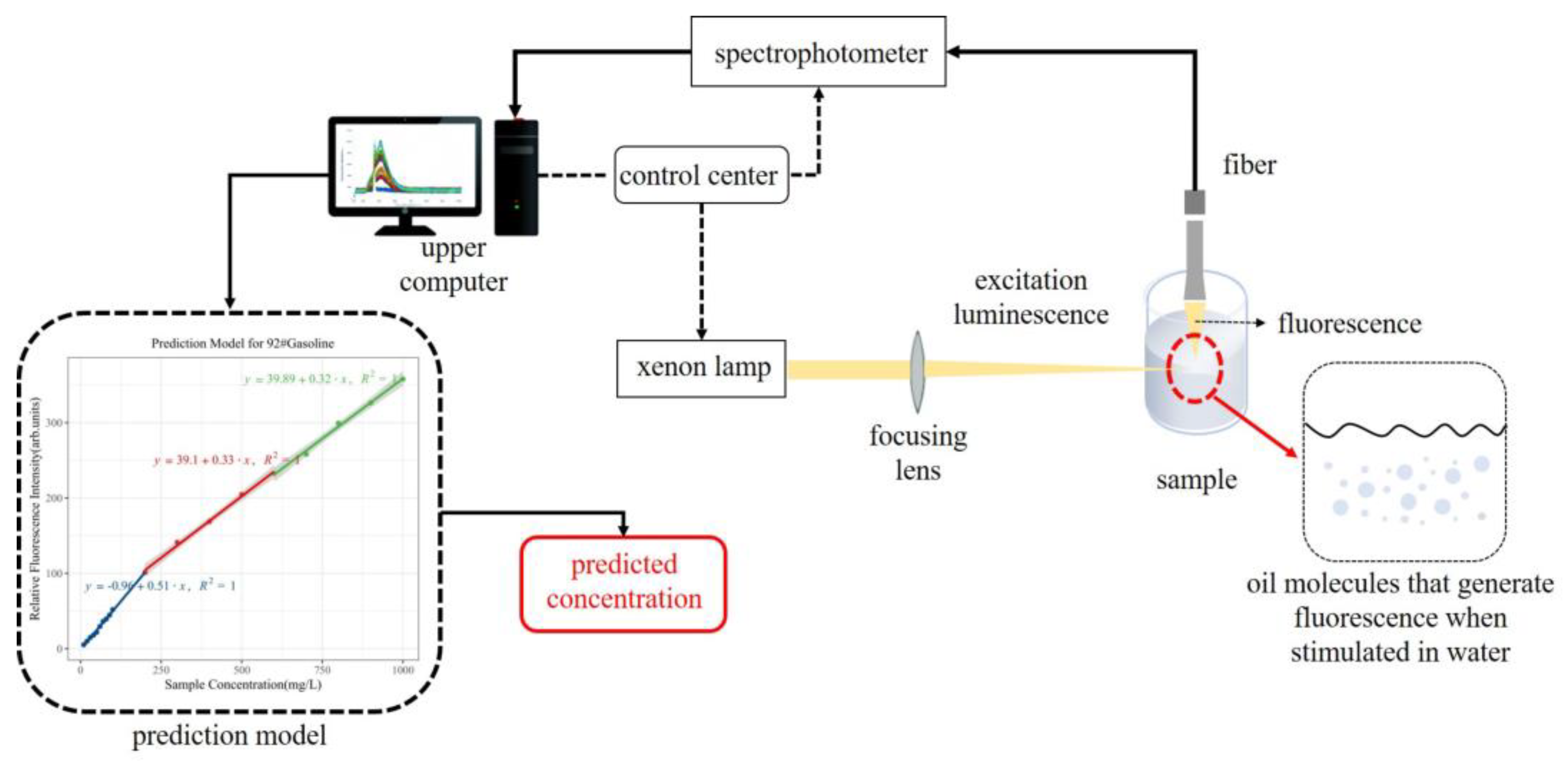
2.2. Experimental Setup
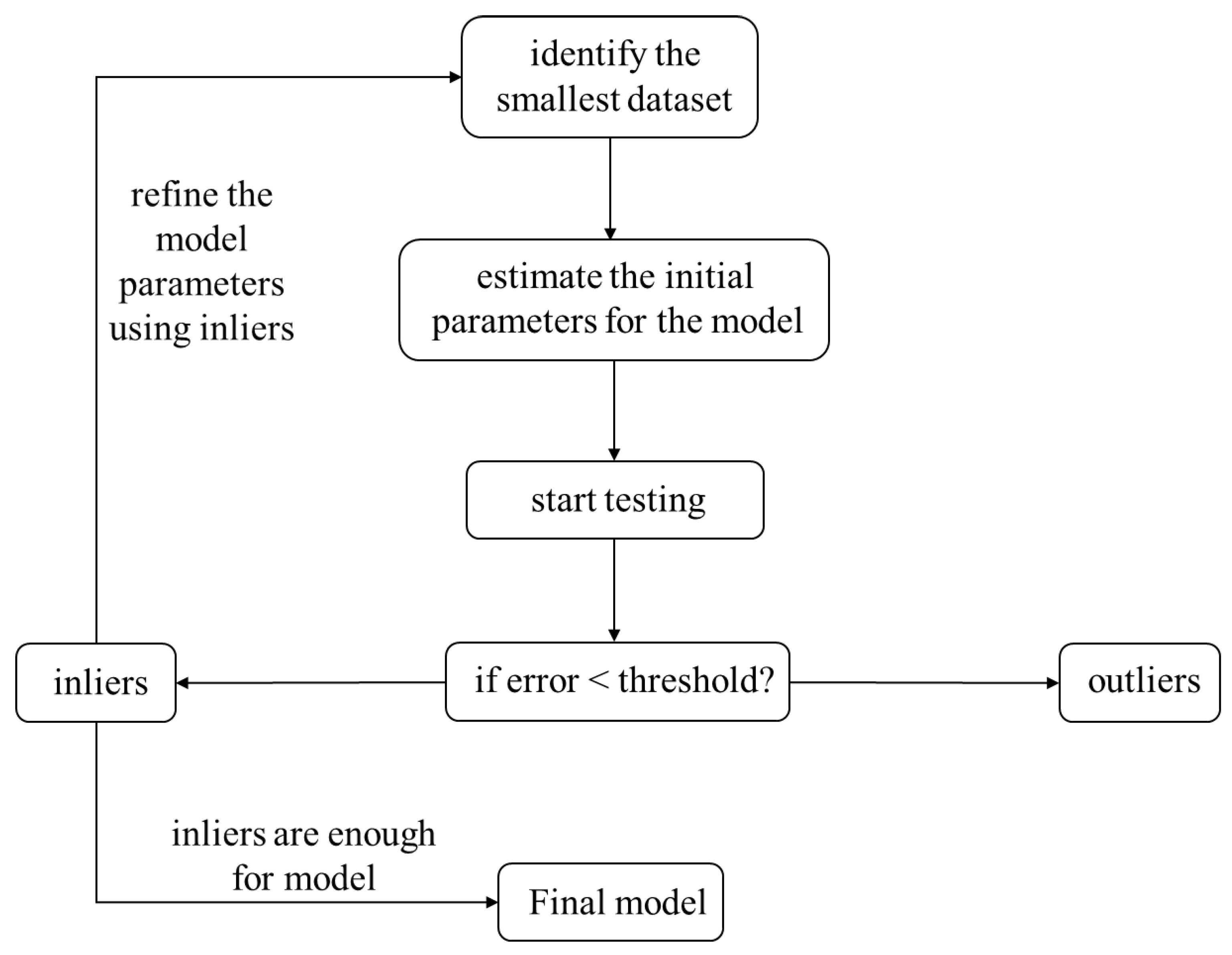
2.3. Principles of the RANSAC Algorithm
3. Results and Discussion
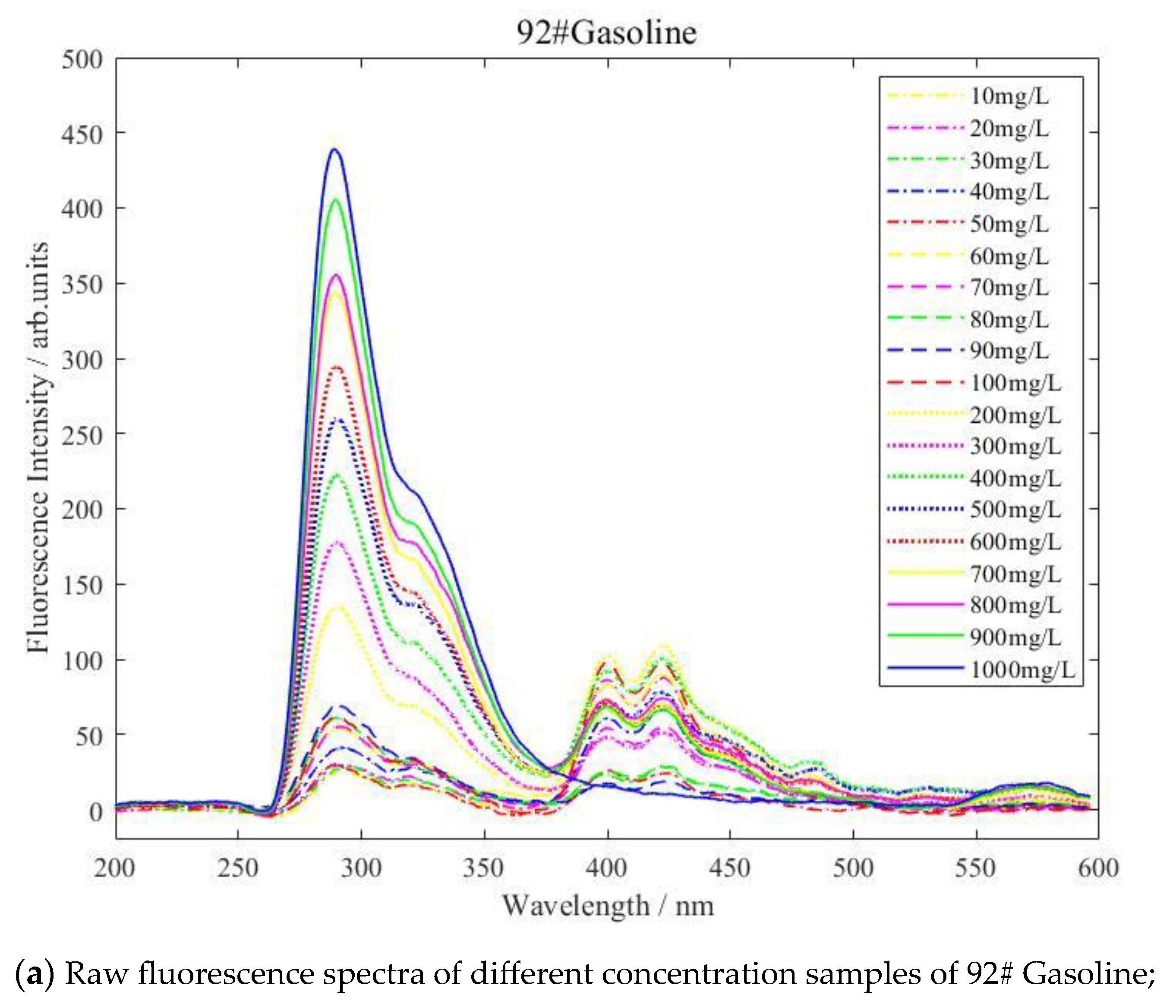
3.1. Fluorescence Spectral Analysis
3.2. Concentration Prediction Modeling
3.3. Detection Limits of Oil Pollutants
3.4. Predictive Model Evaluation Indicators
3.5. Analysis of Forecast Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elsheref, M.; Messina, L.; Tarr, M.A. Photochemistry of oil in marine systems: Developments since the Deepwater Horizon spill. Environ. Sci.-Process. Impacts 2023, 25, 1878–1908. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.W.; Zhang, H.J.; Wang, X.; Lian, K.; Li, X.; Chen, B.; Wang, H.; Niu, X. Ultraviolet-induced fluorescence of oil spill recognition using a semi-supervised algorithm based on thickness and mixing proportion-emission matrices. Anal. Methods 2023, 15, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- French-McCay, D.P.; Parkerton, T.F.; de Jourdan, B. Bridging the lab to field divide: Advancing oil spill biological effects models requires revisiting aquatic toxicity testing. Aquat. Toxicol. 2023, 256, 106389. [Google Scholar] [CrossRef] [PubMed]
- Nilén, G.; Larsson, M.; Hyötyläinen, T.; Keiter, S.H. A complex mixture of polycyclic aromatic compounds causes embryotoxic, behavioral, and molecular effects in zebrafish larvae (Danio rerio), and in vitro bioassays. Sci. Total Environ. 2024, 906, 167307. [Google Scholar] [CrossRef] [PubMed]
- Asif, Z.; Chen, Z.; An, C.; Dong, J. Environmental Impacts and Challenges Associated with Oil Spills on Shorelines. J. Mar. Sci. Eng. 2022, 10, 762. [Google Scholar] [CrossRef]
- Cui, Y.Y.; Kong, D.M.; Kong, L.; Wang, S. Excitation emission matrix fluorescence spectroscopy and parallel factor framework-clustering analysis for oil pollutants identification. Spectrochim. Acta Part A Biomol. Spectrosc. 2021, 253, 119586. [Google Scholar] [CrossRef] [PubMed]
- Jaén-González, L.; Aliaño-González, M.J.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. A Novel Method Based on Headspace-Ion Mobility Spectrometry for the Detection and Discrimination of Different Petroleum Derived Products in Seawater. Sensors 2021, 21, 2151. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Yan, L.; Jiang, X.; Ding, J.; Zhang, F.; Jiang, K.; Shang, K. Exploring the Potential of Optical Polarization Remote Sensing for Oil Spill Detection: A Case Study of Deepwater Horizon. Remote Sens. 2022, 14, 2398. [Google Scholar] [CrossRef]
- Jiao, J.N.; Lu, Y.C.; Hu, C.; Shi, J.; Sun, S.; Liu, Y. Quantifying ocean surface oil thickness using thermal remote sensing. Remote Sens. Environ. 2021, 261, 112513. [Google Scholar] [CrossRef]
- Arslan, N.; Nezhad, M.M.; Heydari, A.; Garcia, D.A.; Sylaios, G. A Principal Component Analysis Methodology of Oil Spill Detection and Monitoring Using Satellite Remote Sensing Sensors. Remote Sens. 2023, 15, 1460. [Google Scholar] [CrossRef]
- Guo, G.; Liu, B.X.; Liu, C. Thermal Infrared Spectral Characteristics of Bunker Fuel Oil to Determine Oil-Film Thickness and API. J. Mar. Sci. Eng. 2020, 8, 135. [Google Scholar] [CrossRef]
- Dala, A.; Arslan, T. In Situ Sensor for the Detection of Oil Spill in Seawater Using Microwave Techniques. Micromachines 2022, 13, 536. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Tian, Q.J.; Li, X. The remote sensing inversion theory of offshore oil slick thickness based on a two-beam interference model. Sci. China-Earth Sci. 2011, 54, 678–685. [Google Scholar] [CrossRef]
- Fingas, M. The Challenges of Remotely Measuring Oil Slick Thickness. Remote Sens. 2018, 10, 319. [Google Scholar] [CrossRef]
- Jafarzadeh, H.; Mahdianpari, M.; Homayouni, S.; Mohammadimanesh, F.; Dabboor, M. Oil spill detection from Synthetic Aperture Radar Earth observations: A meta-analysis and comprehensive review. GI Sci. Remote Sens. 2021, 58, 1022–1051. [Google Scholar] [CrossRef]
- Fingas, M.; Brown, C.E. A Review of Oil Spill Remote Sensing. Sensors 2018, 18, 91. [Google Scholar] [CrossRef]
- Lennon, M.; Babichenko, S.; Thomas, N.; Mariette, V.; Mercier, G.; Lisin, A. Detection and mapping of oil slicks in the sea by combined use of hyperspectral imagery and laser-induced fluorescence. EARSeL eProceedings 2006, 5, 120. [Google Scholar]
- Rayner, D.M.; Szabo, A.G. Time-resolved Laser Fluorosensors-Laboratory Study of Their Potential in the Remote Characterization of Oil. Appl. Opt. 1978, 17, 1624–1630. [Google Scholar] [CrossRef] [PubMed]
- Alaruri, S.D. Multiwavelength laser induced fluorescence (LIF) LIDAR system for remote detection and identification of oil spills. Optik 2019, 181, 239–245. [Google Scholar] [CrossRef]
- Cui, Y.Q.; Kong, D.M.; Ma, Q.; Xie, B.; Zhang, X.; Kong, D.; Kong, L. Algorithm Research on Inversion Thickness of Oil Spill on the Sea Surface Using Raman Scattering and Fluorescence Signal. Spectrosc. Spectr. Anal. 2022, 42, 104–109. [Google Scholar]
- Hoge, F.E. Oil film thickness using airborne laser-induced oil fluorescence backscatter. Appl. Opt. 1983, 22, 3316–3318. [Google Scholar] [CrossRef]
- Chen, Z.K.; Guo, R.; Cheng, P.F. Application of LIF Technology-Based Spectral Feature Extraction in Oil Detection. Laser Optoelectron. Prog. 2020, 57, 133002. [Google Scholar] [CrossRef]
- Hong, J.J.; Wang, Z.H.; Li, J.; Xu, Y.; Xin, H. Effect of Interface Structure and Behavior on the Fluid Flow Characteristics and Phase Interaction in the Petroleum Industry: State of the Art Review and Outlook. Energy Fuels 2023, 37, 9914–9937. [Google Scholar] [CrossRef]
- Köpplin, J.; Bednarz, L.; Hagemeier, T.; Thévenin, D. Fluorescence Imaging Methodology for Oil-in-Water Concentration Measurements. Chem. Eng. Technol. 2021, 44, 1343–1349. [Google Scholar] [CrossRef]
- Fischler, M.A.; Bolles, R.C. Random sample consensus: A paradigm for model fitting with applications to image analysis and automated cartography. Commun. ACM 1981, 24, 381–395. [Google Scholar] [CrossRef]
- Zhou, C.L.; Zhu, H.H.; Li, X. Research and application of robust plane fitting algorithm with RANSAC. Comput. Eng. Appl. 2011, 47, 177–179, 182. [Google Scholar]
- Xie, S.S.; Wang, Z.Q.; Huang, H. Applications of random sample consistency algorithm on laser spectroscopy. Laser Technol. 2017, 41, 133–137. [Google Scholar]
- Raguram, R.; Chum, O.; Pollefeys, M.; Matas, J.; Frahm, J.M. USAC: A Universal Framework for Random Sample Consensus. IEEE Trans. Pattern Anal. Mach. Intell. 2013, 35, 2022–2038. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.Q.; Chen, Z.L.; Song, S. CORE-Sketch: On Exact Computation of Median Absolute Deviation with Limited Space. Proc. Vldb Endwoment 2023, 16, 2832–2844. [Google Scholar] [CrossRef]
- Cheng, P.F.; Wang, Y.T.; Chen, Z.-K.; Yang, Z.; Cao, L.-F. The Fluorescence Detection of Oil Pollutants Based on Self-Weighted Alternating Trilinear Decomposition. Spectrosc. Spectr. Anal. 2016, 36, 2162–2168. [Google Scholar]
- Zheng, P.; Li, S.X.; Takeda, T.; Xu, J.; Takahashi, K.; Tian, R.; Wei, R.; Wang, L.; Zhou, T.-L.; Hirosaki, N.; et al. Unraveling the Luminescence Quenching of Phosphors under High-Power-Density Excitation. Acta Mater. 2021, 209, 116813. [Google Scholar] [CrossRef]
- Li, B.N.; Wang, H.X.; Zhao, Q.; Ouyang, J.; Wu, Y. Rapid detection of authenticity and adulteration of walnut oil by FTIR and fluorescence spectroscopy: A comparative study. Food Chem. 2015, 181, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Uhrovcík, J. Strategy for determination of LOD and LOQ values—Some basic aspects. Talanta 2014, 119, 178–180. [Google Scholar] [CrossRef] [PubMed]
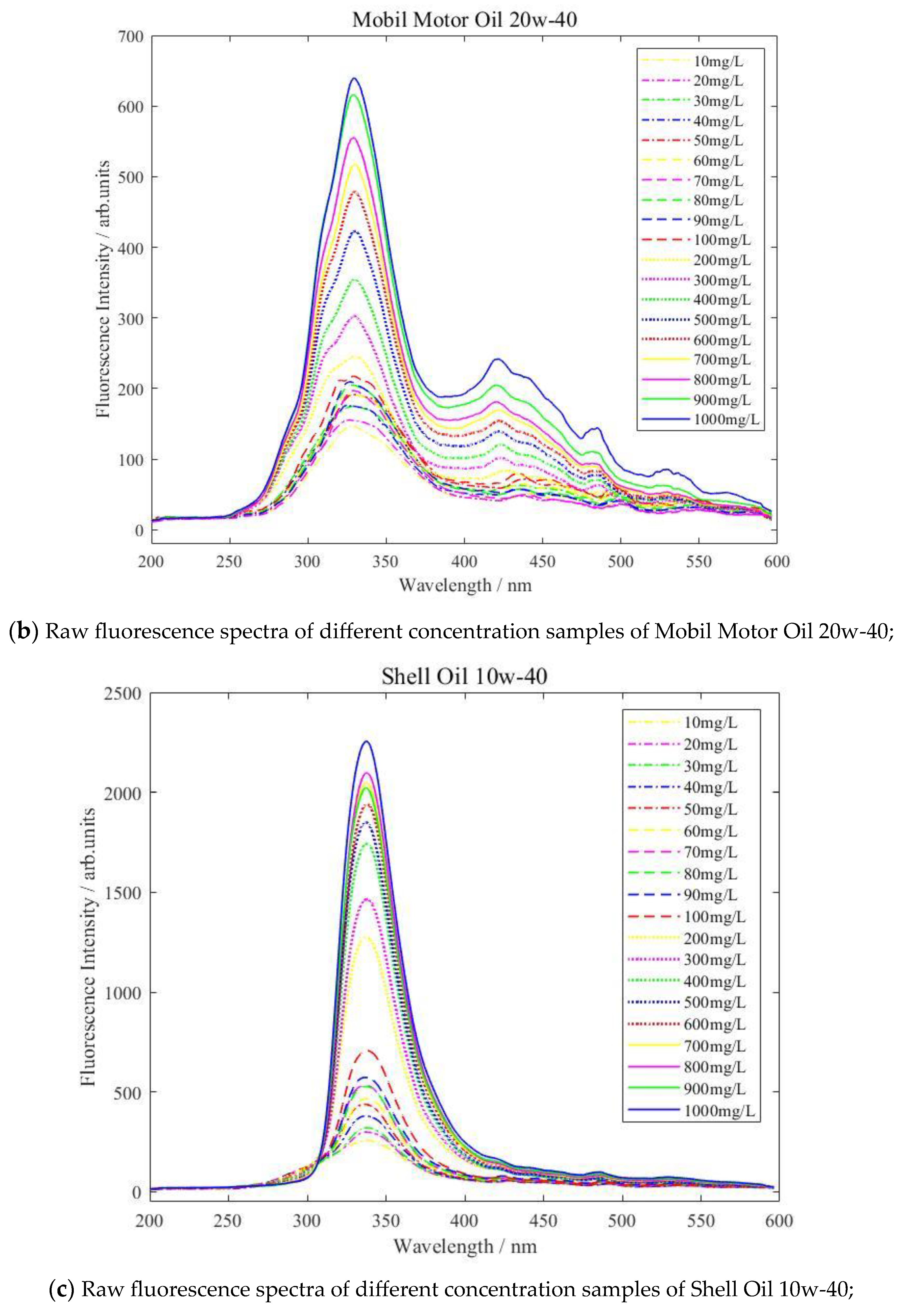
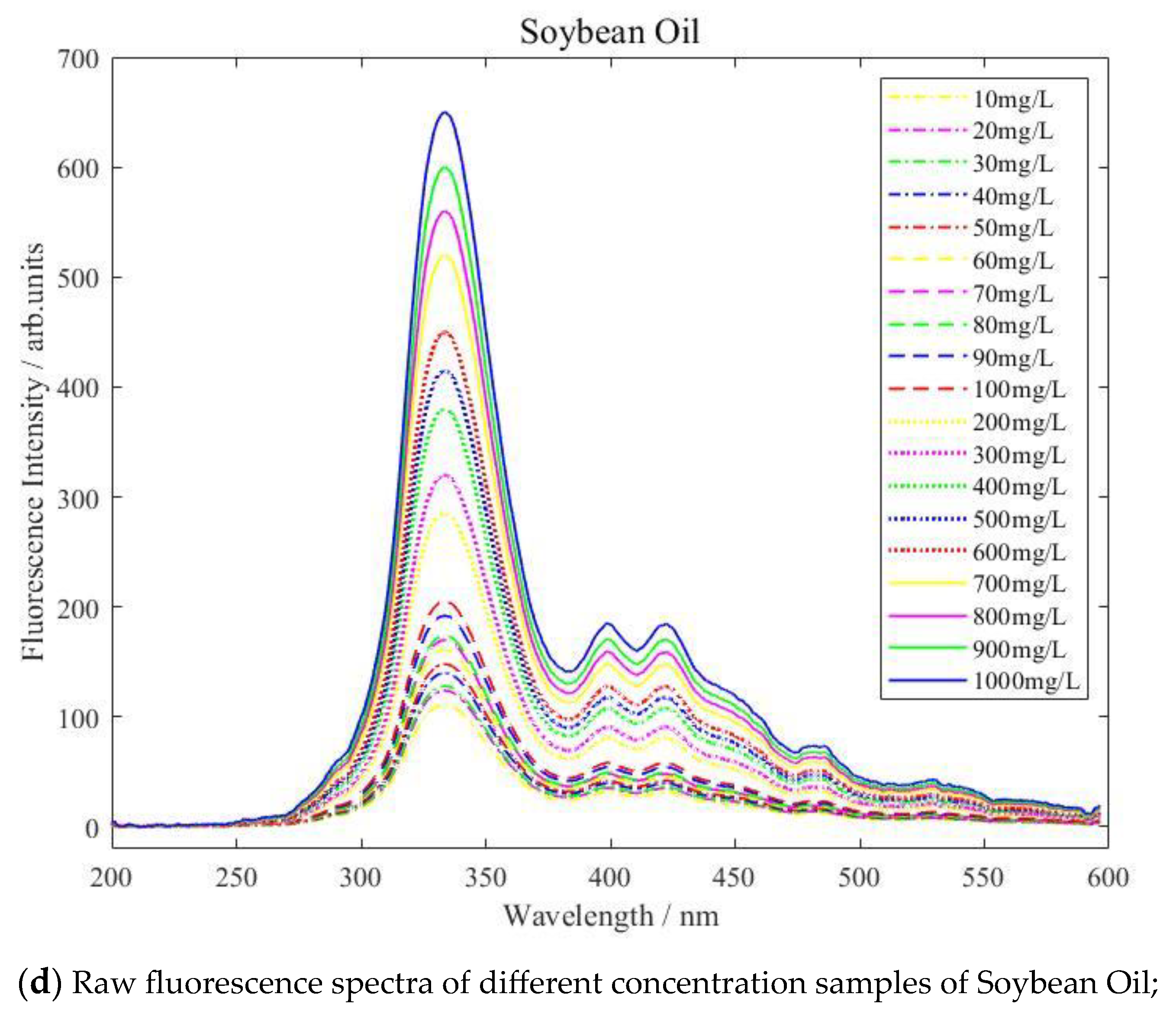
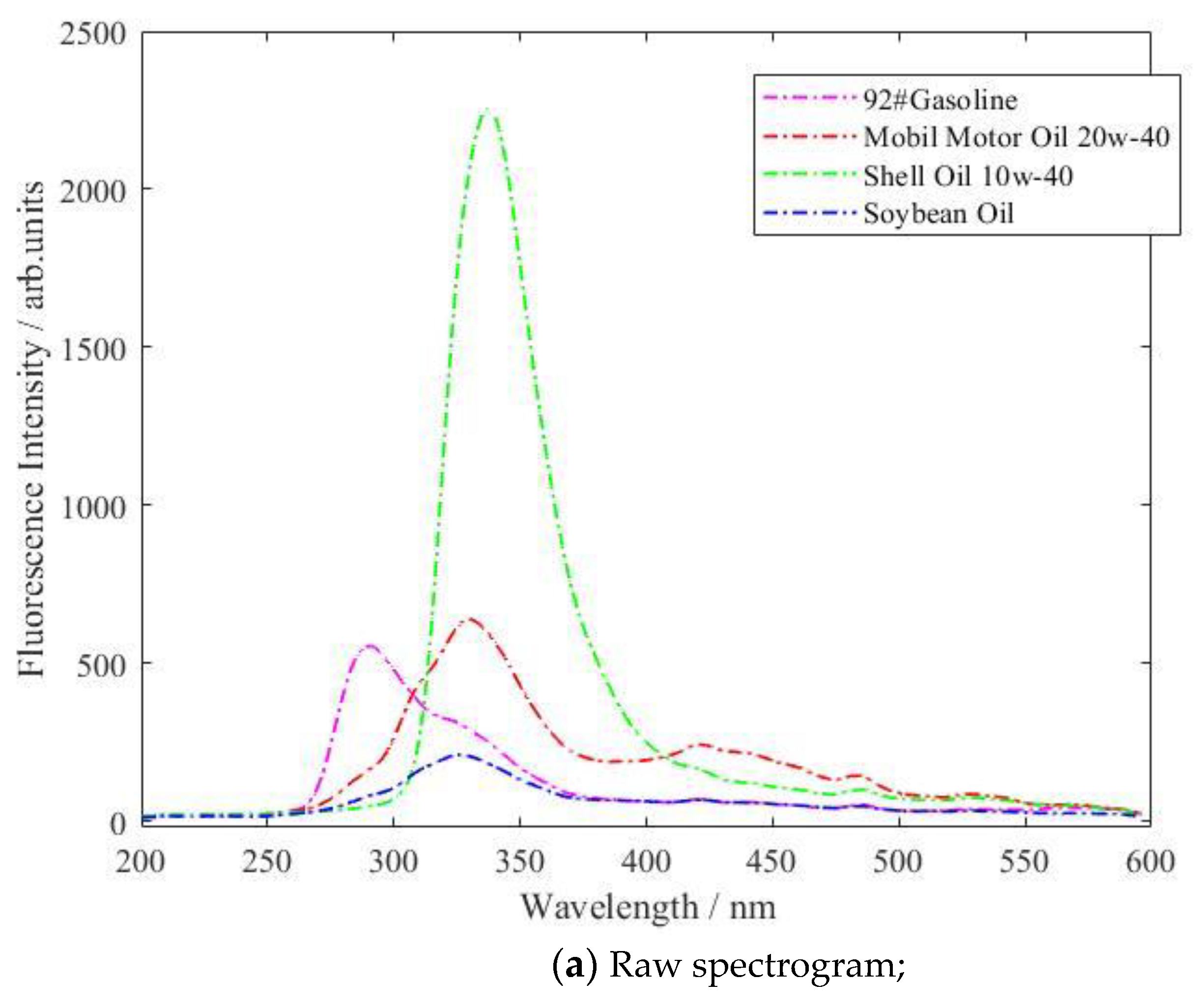
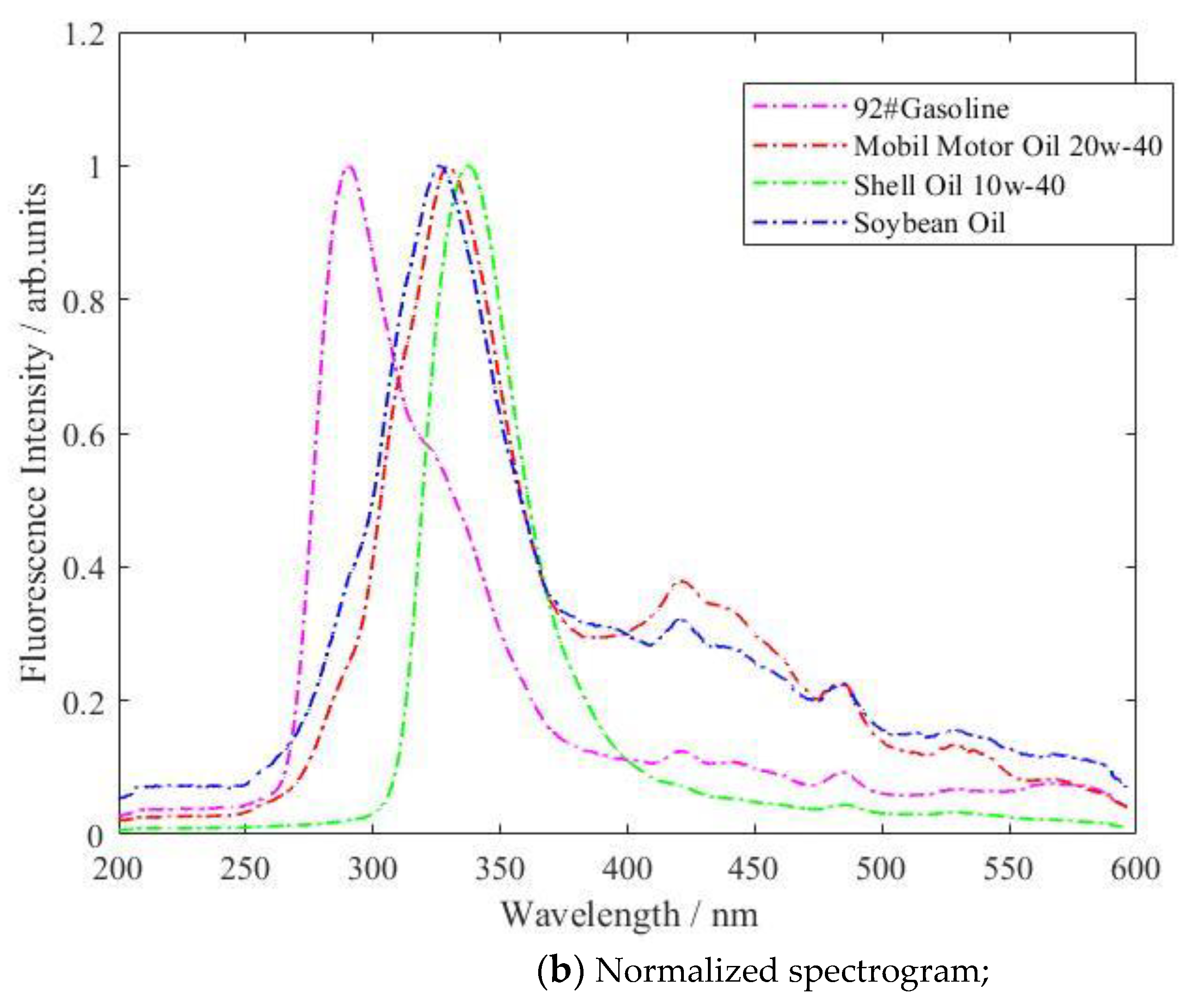
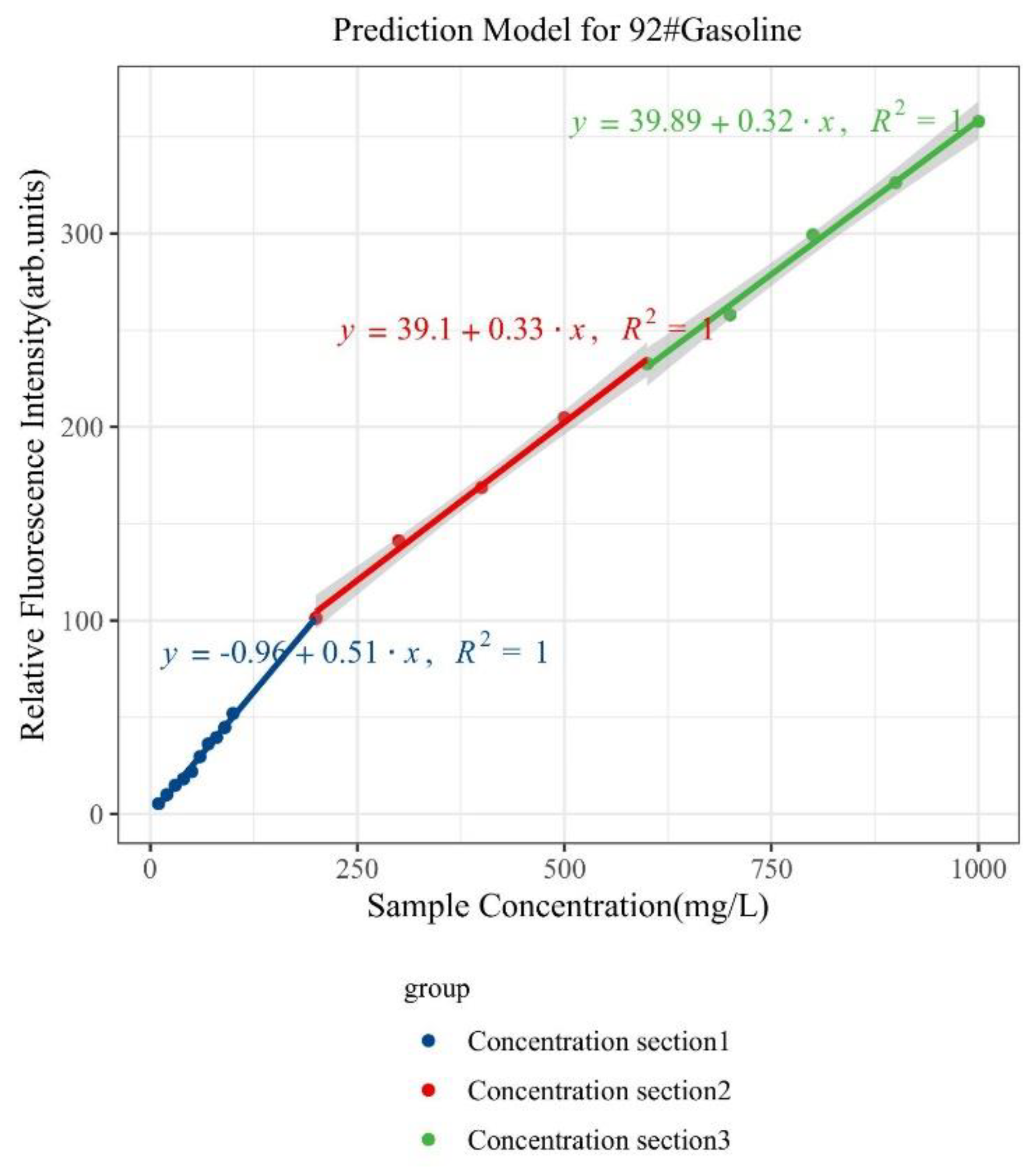
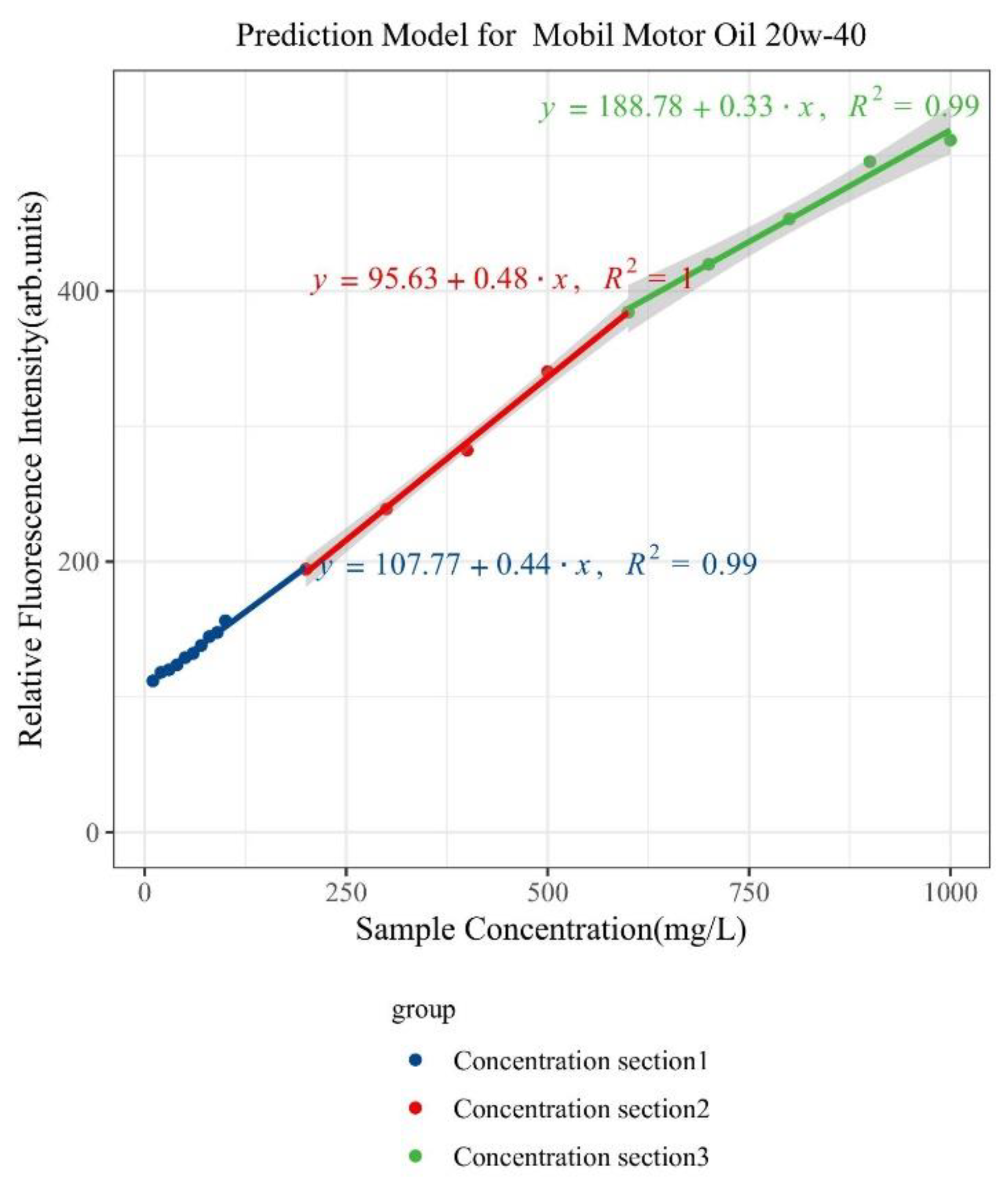

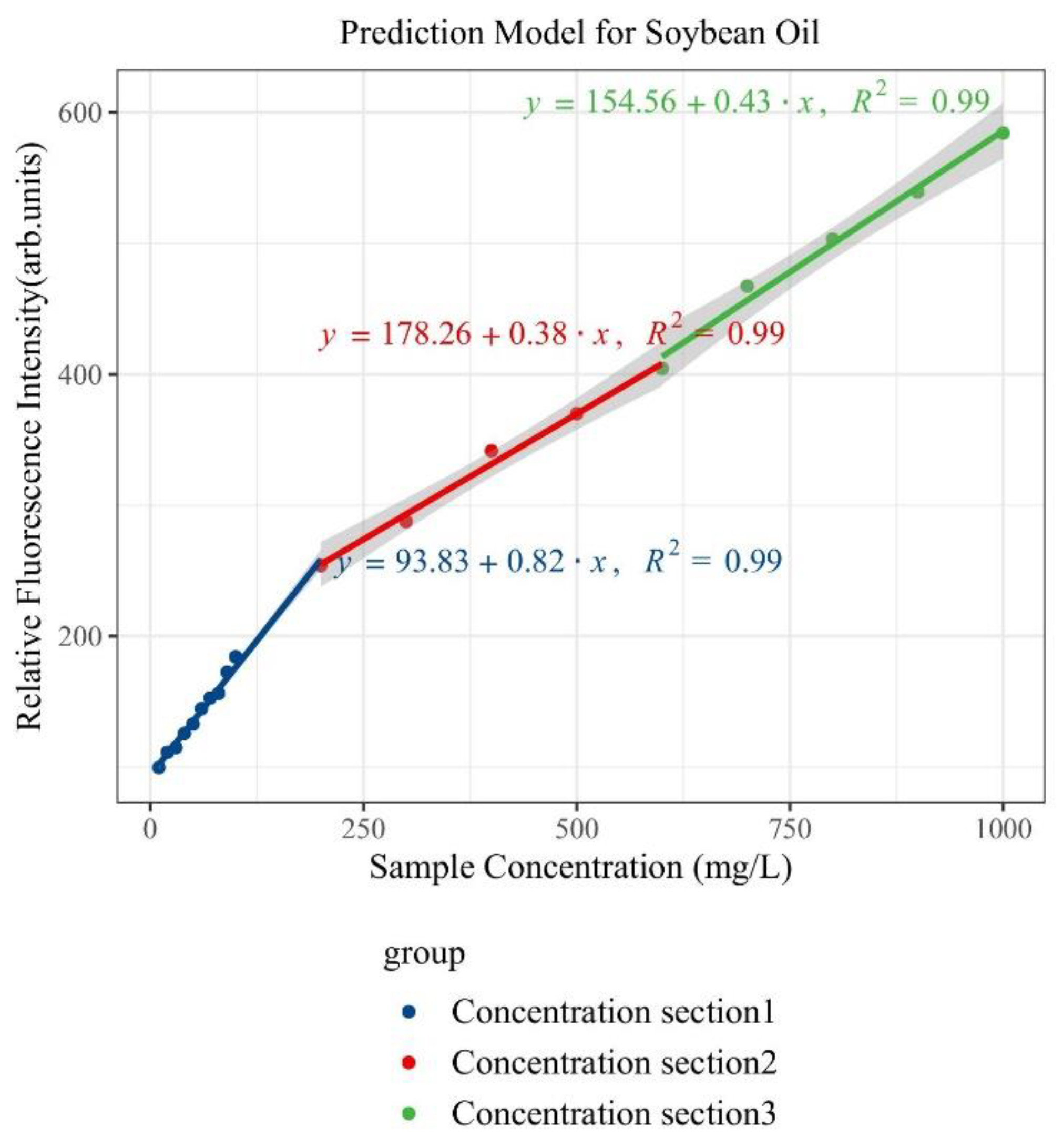
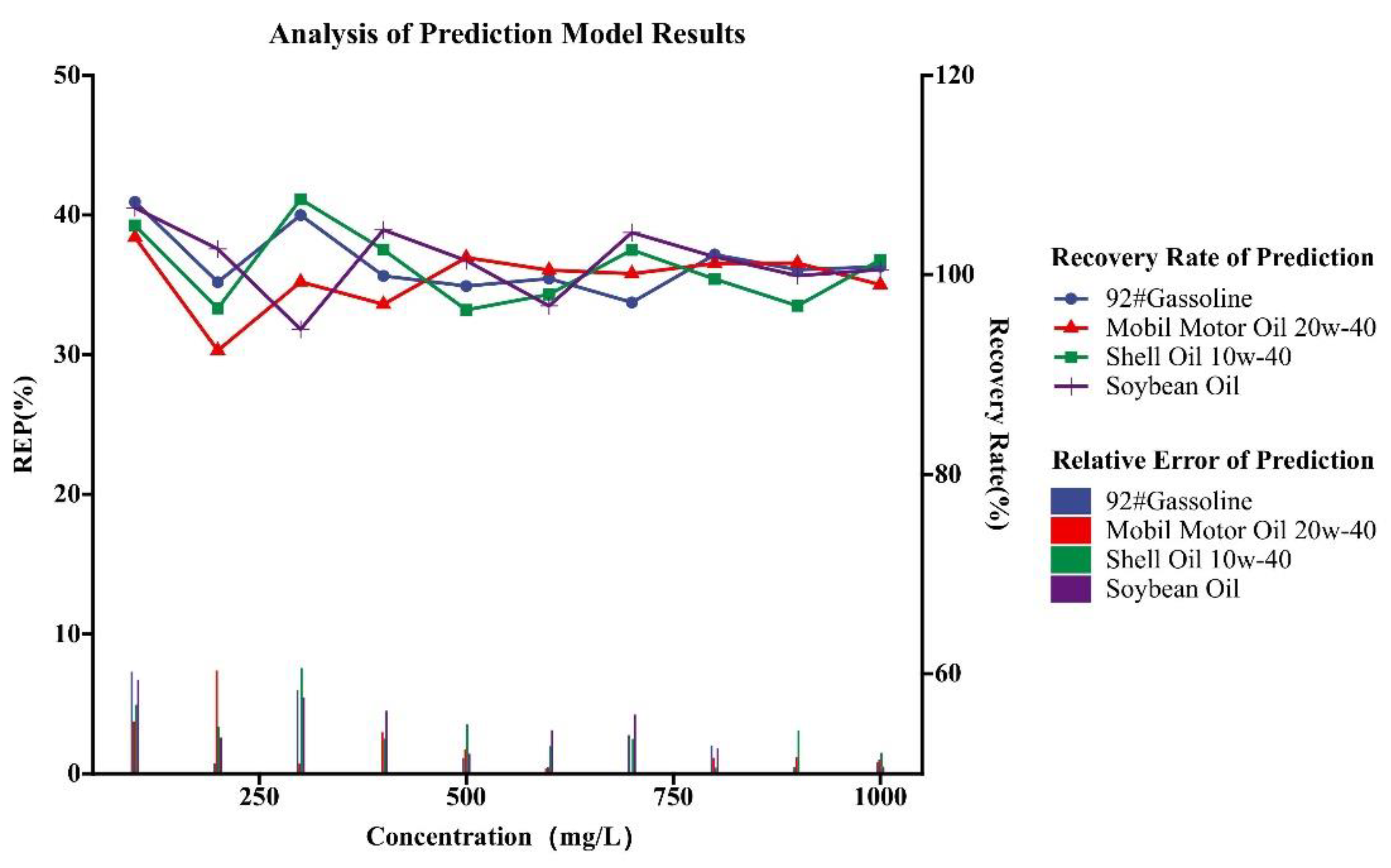
| Type of Oil | Detection Concentration (mg/L) | Number of Data Samples |
|---|---|---|
| 92# Gasoline | 0, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 200, 300, 400, 500, 600, 700, 800, 900, 1000 | 200 |
| Mobil Motor Oil 20w-40 | 0, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 200, 300, 400, 500, 600, 700, 800, 900, 1000 | 200 |
| Shell Oil 10W-40 | 0, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 200, 300, 400, 500, 600, 700, 800, 900, 1000 | 200 |
| Soybean Oil | 0, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 200, 300, 400, 500, 600, 700, 800, 900, 1000 | 200 |
| Type of Oil | Concentration | Linear Regression | Correlation Coefficient r | LOD (mg/L) |
|---|---|---|---|---|
| 92# Gasoline | 0~1000 | Y = 0.51x − 0.96 | >0.99 | 0.8 |
| Mobil Motor Oil 20w-40 | 0~1000 | Y = 0.44x + 107.77 | 0.99 | 0.9 |
| Shell Oil 10W-40 | 0~1000 | Y = 5.64x + 118.85 | 0.99 | 0.07 |
| Soybean Oil | 0~1000 | Y = 0.82x + 93.83 | 0.99 | 0.5 |
| Type of Oil | Add Concentration (mg/L) | Predict Concentration (mg/L) |
|---|---|---|
| 92# Gasoline | 90 | 90.3509 |
| 400 | 399.5246 | |
| 600 | 597.7125 | |
| 900 | 902.4779 | |
| 1000 | 1004.0943 | |
| Mobil Motor Oil 20w-40 | 10 | 9.1160 |
| 300 | 297.8706 | |
| 600 | 602.7507 | |
| 700 | 700.8044 | |
| 800 | 804.1076 | |
| Shell Oil 10w-40 | 90 | 90.7007 |
| 100 | 102.9365 | |
| 200 | 196.2613 | |
| 400 | 404.0503 | |
| 800 | 798.5954 | |
| Soybean Oil | 40 | 40.3556 |
| 200 | 203.1754 | |
| 500 | 504.1037 | |
| 900 | 899.1573 | |
| 1000 | 1003.1176 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Wang, Z.; Zhao, Y. Rapid Investigation of Oil Pollution in Water-Combined Induced Fluorescence and Random Sample Consensus Algorithm. Sustainability 2024, 16, 3930. https://doi.org/10.3390/su16103930
Wu H, Wang Z, Zhao Y. Rapid Investigation of Oil Pollution in Water-Combined Induced Fluorescence and Random Sample Consensus Algorithm. Sustainability. 2024; 16(10):3930. https://doi.org/10.3390/su16103930
Chicago/Turabian StyleWu, Hui, Ziyi Wang, and Youquan Zhao. 2024. "Rapid Investigation of Oil Pollution in Water-Combined Induced Fluorescence and Random Sample Consensus Algorithm" Sustainability 16, no. 10: 3930. https://doi.org/10.3390/su16103930




