Determination of Nitrate Migration and Distribution through Eutric Cambisols in an Area without Anthropogenic Sources of Nitrate (Velika Gorica Well Field, Croatia)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Data Collection and Sampling
2.3. Laboratory Measurements
2.4. Statistical Analysis
3. Results
3.1. Precipitation and Air Temperature
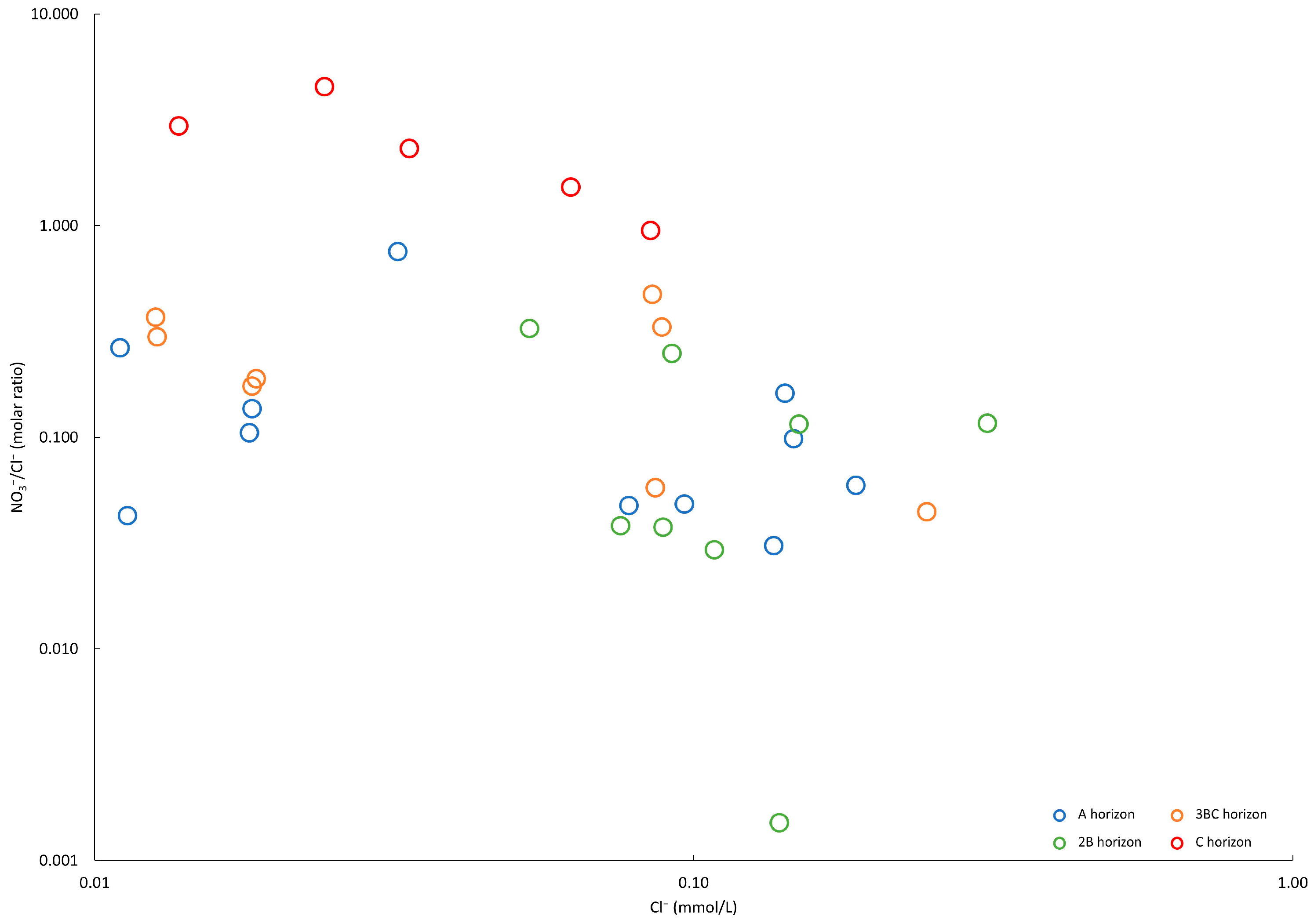
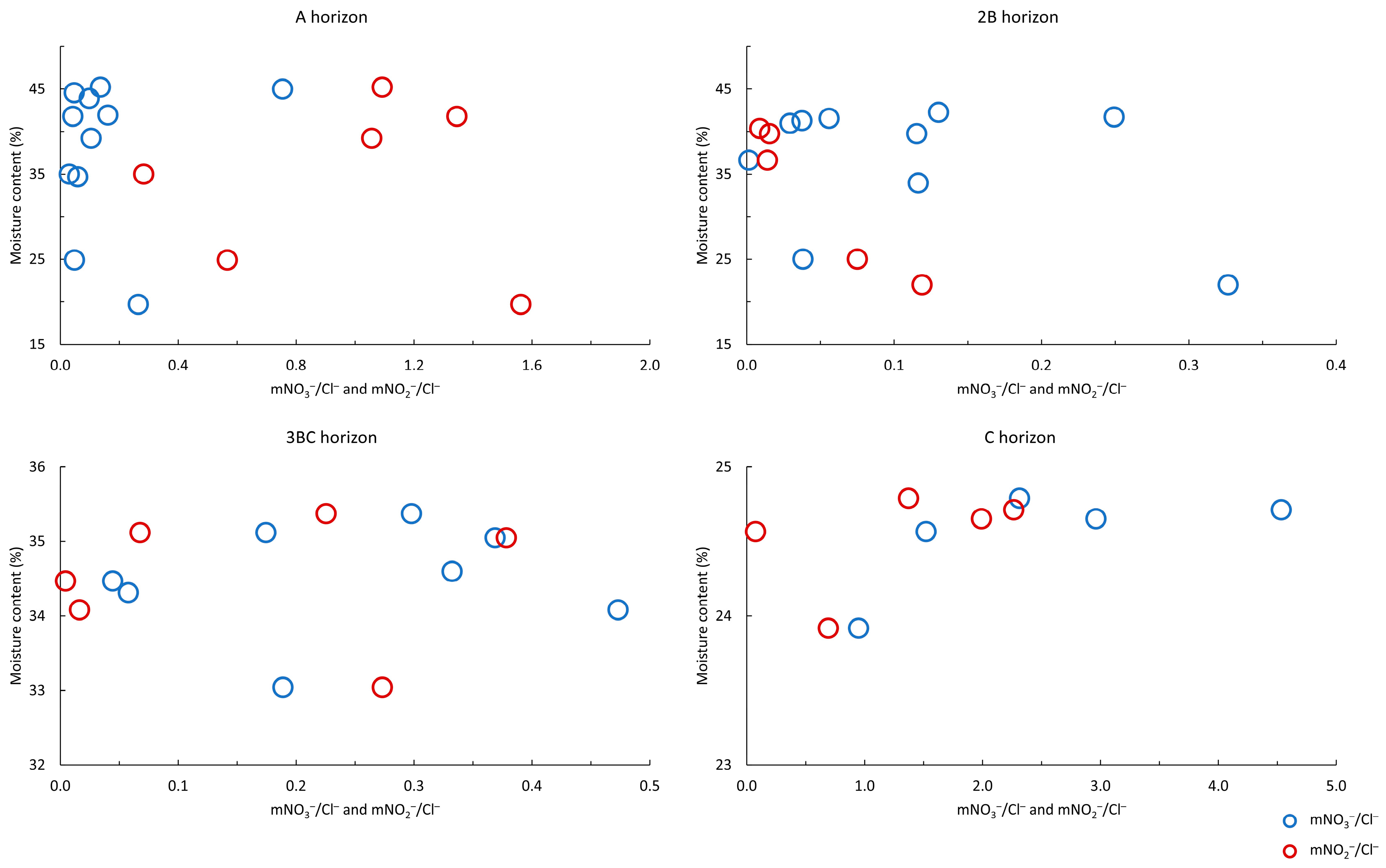
3.2. Characteristics of Soil Water
3.3. Analysis of Variance (ANOVA) and Kruskal-Wallis Test
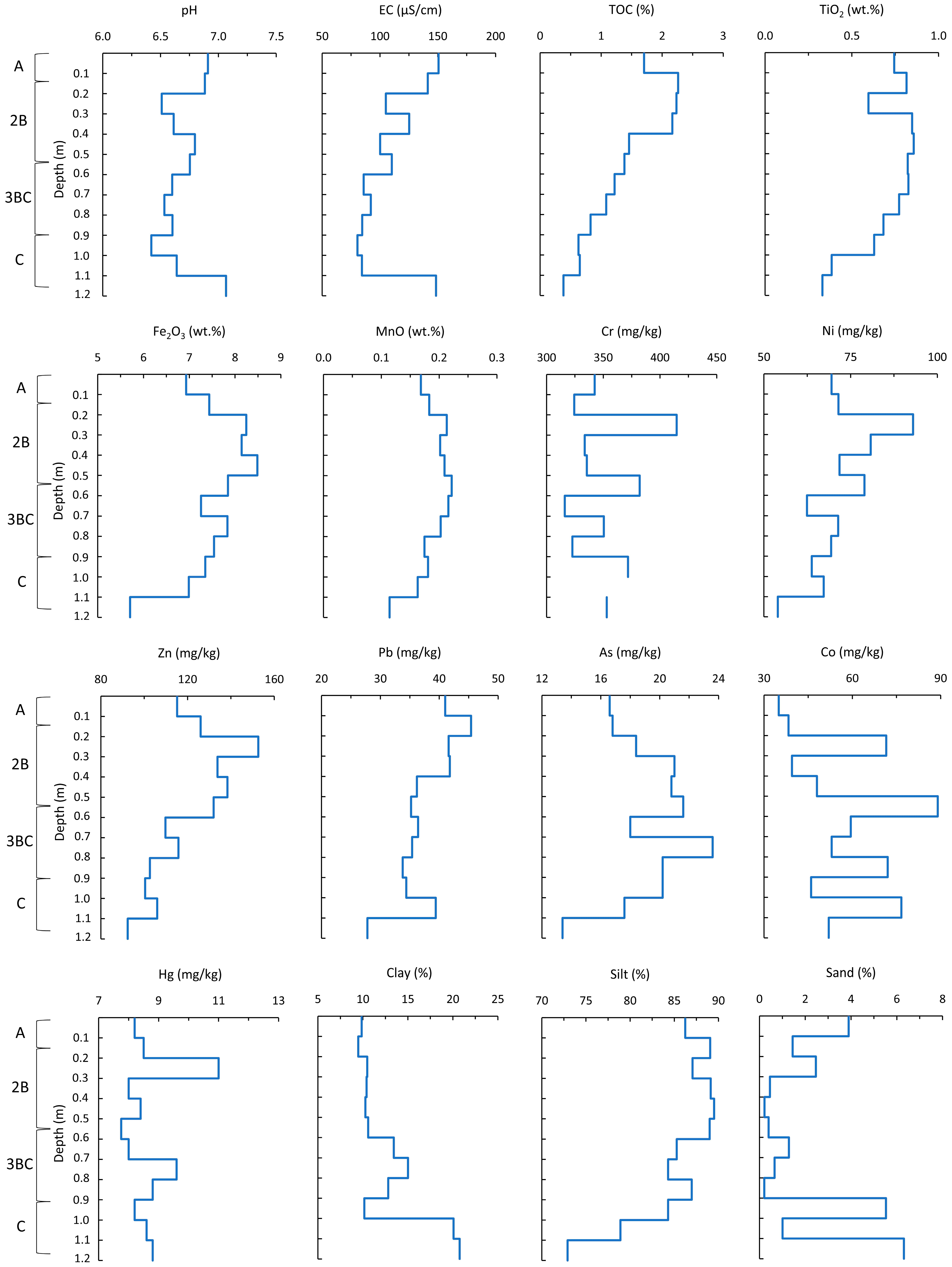
3.4. Characteristics of Soil
4. Discussion
4.1. Nitrogen Species Distribution within the Soil Zone
4.2. Variability of Soil Water Parameters within the Soil Zone
4.3. Factors Influencing NO3− Distribution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, K.S.; Bong, Y.S.; Lee, D.; Kim, Y.; Kim, K. Tracing the Sources of Nitrate in the Han River Watershed in Korea, Using δ15N-NO3− and δ18O-NO3− Values. Sci. Total Environ. 2008, 395, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xiao, Q.; Miao, Y.; Wang, Z.; Wang, Q. Sources and Transformations of Nitrate Constrained by Nitrate Isotopes and Bayesian Model in Karst Surface Water, Guilin, Southwest China. Environ. Sci. Pollut. Res. 2020, 27, 21299–21310. [Google Scholar] [CrossRef] [PubMed]
- Amoo, A.E.; Babalola, O.O. Ammonia-Oxidizing Microorganisms: Key Players in the Promotion of Plant Growth. J. Soil Sci. Plant Nutr. 2017, 17, 935–947. [Google Scholar] [CrossRef]
- Mayer, B.; Boyer, E.W.; Goodale, C.; Jaworski, N.A.; Van Breemen, N.; Howarth, R.W.; Seitzinger, S.; Billen, G.; Lajtha, K.; Nadelhoffer, K.; et al. Sources of Nitrate in Rivers Draining Sixteenwatersheds in the Northeastern U.S.: Isotopic Constraints. Biogeochemistry 2002, 57, 171–197. [Google Scholar] [CrossRef]
- Torres-Martínez, J.A.; Mora, A.; Knappett, P.S.K.; Ornelas-Soto, N.; Mahlknecht, J. Tracking Nitrate and Sulfate Sources in Groundwater of an Urbanized Valley Using a Multi-Tracer Approach Combined with a Bayesian Isotope Mixing Model. Water Res. 2020, 182, 115962. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Kim, J.M.; Lee, D.R.; Kim, Y.; Lee, D. Analysis of water movement through an unsaturated soil zone in Jeju Island, Korea using stable oxygen and hydrogen isotopes. J. Hydrol. 2007, 345, 199–211. [Google Scholar] [CrossRef]
- Spalding, R.F.; Exner, M.E. Occurrence of Nitrate in Groundwater—A Review. J. Environ. Qual. 1993, 22, 392–402. [Google Scholar] [CrossRef]
- Varnier, C.; Hirata, R.; Aravena, R. Examining Nitrogen Dynamics in the Unsaturated Zone under an Inactive Cesspit Using Chemical Tracers and Environmental Isotopes. Appl. Geochem. 2017, 78, 129–138. [Google Scholar] [CrossRef]
- Ayiti, O.E.; Babalola, O.O. Factors Influencing Soil Nitrification Process and the Effect on Environment and Health. Front. Sustain. Food Syst. 2022, 6, 821994. [Google Scholar] [CrossRef]
- Sepaskhah, A.R.; Yousefi, F. Effects of zeolite application on nitrate and ammonium retention of a loamy soil under saturated conditions. Soil Res. 2007, 45, 368–373. [Google Scholar] [CrossRef]
- Pastén-Zapata, E.; Ledesma-Ruiz, R.; Harter, T.; Ramírez, A.I.; Mahlknecht, J. Assessment of Sources and Fate of Nitrate in Shallow Groundwater of an Agricultural Area by Using a Multi-Tracer Approach. Sci. Total Environ. 2014, 470–471, 855–864. [Google Scholar] [CrossRef]
- Zeng, H.; Wu, J. Tracing the Nitrate Sources of the Yili River in the Taihu Lake Watershed: A Dual Isotope Approach. Water 2015, 7, 188–201. [Google Scholar] [CrossRef]
- Li, S.L.; Liu, C.Q.; Lang, Y.C.; Zhao, Z.Q.; Zhou, Z.H. Tracing the Sources of Nitrate in Karstic Groundwater in Zunyi, Southwest China: A Combined Nitrogen Isotope and Water Chemistry Approach. Environ. Earth Sci. 2010, 60, 1415–1423. [Google Scholar] [CrossRef]
- Liu, C.Q.; Li, S.A.; Lang, Y.C.; Xiao, H.Y. Using δ15N- and δ18O-Values to Identify Nitrate Sources in Karst Ground Water, Guiyang, Southwest China. Environ. Sci. Technol. 2006, 40, 6928–6933. [Google Scholar] [CrossRef] [PubMed]
- Kendall, C. Tracing Nitrogen Sources and Cycling in Catchments. In Isotope Tracers in Catchment Hydrology; Elsevier: Amsterdam, The Netherlands, 1998; pp. 519–576. [Google Scholar]
- Andersson, K.K.; Hooper, A.B. O2 and H2O Are Each the Source of One O in NO2− Produced from NH3 by Nitrosomonas: 15N-NMR Evidence. FEBS Lett. 1983, 164, 236–240. [Google Scholar] [CrossRef]
- Canfield, D.E.; Glazer, A.N.; Falkowski, P.G. The Evolution and Future of Earth’s Nitrogen Cycle. Science 2010, 330, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Leininger, S.; Urich, T.; Schloter, M.; Schwark, L.; Qi, J.; Nicol, G.W.; Prosser, J.I.; Schuster, S.C.; Schleper, C. Archaea Predominate among Ammonia-Oxidizing Prokaryotes in Soils. Nature 2006, 442, 806–809. [Google Scholar] [CrossRef] [PubMed]
- Kowalchuk, G.A.; Stephen, J.R. Ammonia-Oxidizing Bacteria: A Model for Molecular Microbial Ecology. Annu. Rev. Microbiol. 2001, 44, 485–529. [Google Scholar] [CrossRef]
- Xiao, R.; Ran, W.; Hu, S.; Guo, H. The Response of Ammonia Oxidizing Archaea and Bacteria in Relation to Heterotrophs under Different Carbon and Nitrogen Amendments in Two Agricultural Soils. Appl. Soil Ecol. 2021, 158, 103812. [Google Scholar] [CrossRef]
- Attard, E.; Poly, F.; Commeaux, C.; Laurent, F.; Terada, A.; Smets, B.F.; Recous, S.; Roux, X. Le Shifts between Nitrospira- and Nitrobacter-like Nitrite Oxidizers Underlie the Response of Soil Potential Nitrite Oxidation to Changes in Tillage Practices. Environ. Microbiol. 2010, 12, 315–326. [Google Scholar] [CrossRef]
- Freitag, T.E.; Chang, L.; Clegg, C.D.; Prosser, J.I. Influence of Inorganic Nitrogen Management Regime on the Diversity of Nitrite-Oxidizing Bacteria in Agricultural Grassland Soils. Appl. Environ. Microbiol. 2005, 71, 8323–8334. [Google Scholar] [CrossRef] [PubMed]
- Philips, S.; Wyffels, S.; Sprengers, R.; Verstraete, W. Oxygen-Limited Autotrophic Nitrification/Denitrification by Ammonia Oxidisers Enables Upward Motion towards More Favourable Conditions. Appl. Microbiol. Biotechnol. 2002, 59, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Buday, J.; Drtil, M.; Hutňan, M.; Derco, J. Substrate and Product Inhibition of Nitrification. Chem. Pap. 1999, 53, 379–383. [Google Scholar]
- Brandes, J.A.; Devol, A.H. Isotopic Fractionation of Oxygen and Nitrogen in Coastal Marine Sediments. Geochim. Cosmochim. Acta 1997, 61, 1793–1801. [Google Scholar] [CrossRef]
- Koba, K.; Naoko, T.; Wada, E.; Nakajima, T.; Iwatsubo, G. Intermittent Denitrification: The Application of a 15N Natural Abundance Method to a Forested Ecosystem. Geochim. Cosmochim. Acta 1997, 61, 5043–5050. [Google Scholar] [CrossRef]
- Rivett, M.O.; Buss, S.R.; Morgan, P.; Smith, J.W.N.; Bemment, C.D. Nitrate Attenuation in Groundwater: A Review of Biogeochemical Controlling Processes. Water Res. 2008, 42, 4215–4232. [Google Scholar] [CrossRef]
- Norton, J.M.; Stark, J.M. Chapter Fifteen-Regulation and Measurement of Nitrification in Terrestrial Systems. Methods Enzym. 2011, 486, 343–368. [Google Scholar] [CrossRef]
- Chen, F.; Jia, G.; Chen, J. Nitrate Sources and Watershed Denitrification Inferred from Nitrate Dual Isotopes in the Beijiang River, South China. Biogeochemistry 2009, 94, 163–174. [Google Scholar] [CrossRef]
- Zhang, L.M.; Hu, H.W.; Shen, J.P.; He, J.Z. Ammonia-Oxidizing Archaea Have More Important Role than Ammonia-Oxidizing Bacteria in Ammonia Oxidation of Strongly Acidic Soils. ISME J. 2012, 6, 1032–1045. [Google Scholar] [CrossRef]
- Prosser, J.I.; Nicol, G.W. Archaeal and Bacterial Ammonia-Oxidisers in Soil: The Quest for Niche Specialisation and Differentiation. Trends Microbiol. 2012, 20, 523–531. [Google Scholar] [CrossRef]
- Cui, L.; Li, D.; Wu, Z.; Xue, Y.; Song, Y.; Xiao, F.; Zhang, L.; Gong, P.; Zhang, K. Effects of Nitrification Inhibitors on Nitrogen Dynamics and Ammonia Oxidizers in Three Black Agricultural Soils. Agronomy 2022, 12, 294. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, Z.; Tian, D.; Wang, J.; Fu, Z.; Zhang, F.; Zhang, R.; Chen, W.; Luo, Y.; Niu, S. Global Patterns and Controlling Factors of Soil Nitrification Rate. Glob. Chang. Biol. 2020, 26, 4147–4157. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Campbell, C.D.; Qiao, X. Soil pH Controls Nitrification and Carbon Substrate Utilization More than Urea or Charcoal in Some Highly Acidic Soils. Biol. Fertil. Soils 2011, 47, 515–522. [Google Scholar] [CrossRef]
- Hong, S.; Gan, P.; Chen, A. Environmental Controls on Soil pH in Planted Forest and Its Response to Nitrogen Deposition. Environ. Res. 2019, 172, 159–165. [Google Scholar] [CrossRef]
- Krishnan, K.P.; Loka Bharathi, P.A. Organic Carbon and Iron Modulate Nitrification Rates in Mangrove Swamps of Goa, South West Coast of India. Estuar. Coast. Shelf Sci. 2009, 84, 419–426. [Google Scholar] [CrossRef]
- Hu, J.; Li, D.; Liu, Q.; Tao, Y.; He, X.; Wang, X.; Li, X.; Gao, P. Effect of Organic Carbon on Nitriffication Efficiency and Community Composition of Nitrifying Bioffilms. J. Environ. Sci. 2009, 21, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Liu, M.; Zhang, Y.; Zhang, W.; Wang, Z. Analyzing the Effect of Organic Carbon on Partial Nitrification-Anammox Process Based on Metagenomics and Quorum Sensing. Water Environ. Res. 2021, 93, 254–262. [Google Scholar] [CrossRef]
- Cassinari, C.; Manfredi, P.; Giupponi, L.; Trevisan, M.; Piccini, C. Relationship between Hydraulic Properties and Plant Coverage of the Closed-Landfill Soils in Piacenza (Po Valley, Italy). Solid Earth 2015, 6, 929–943. [Google Scholar] [CrossRef]
- Han, G.; Li, F.; Tang, Y. Variations in Soil Organic Carbon Contents and Isotopic Compositions under Different Land Uses in a Typical Karst Area in Southwest China. Geochem. J. 2015, 49, 63–71. [Google Scholar] [CrossRef]
- Simonin, M.; Martins, J.M.F.; Le Roux, X.; Uzu, G.; Calas, A.; Richaume, A. Toxicity of TiO2 Nanoparticles on Soil Nitrification at Environmentally Relevant Concentrations: Lack of Classical Dose–Response Relationships. Nanotoxicology 2017, 11, 247–255. [Google Scholar] [CrossRef]
- Huang, X.; Zhu-Barker, X.; Horwath, W.R.; Faeflen, S.J.; Luo, H.; Xin, X.; Jiang, X. Effect of Iron Oxide on Nitrification in Two Agricultural Soils with Different pH. Biogeosciences 2016, 13, 5609–5617. [Google Scholar] [CrossRef]
- Clément, J.C.; Shrestha, J.; Ehrenfeld, J.G.; Jaffé, P.R. Ammonium Oxidation Coupled to Dissimilatory Reduction of Iron under Anaerobic Conditions in Wetland Soils. Soil Biol. Biochem 2005, 37, 2323–2328. [Google Scholar] [CrossRef]
- Yang, W.H.; Weber, K.A.; Silver, W.L. Nitrogen Loss from Soil through Anaerobic Ammonium Oxidation Coupled to Iron Reduction. Nat. Geosci 2012, 5, 538–541. [Google Scholar] [CrossRef]
- Xin, X.P.; Wright, A.L.; He, Z.L.; Jiang, X.J. Manganese Oxide Affects Nitrification and N2O Emissions in a Subtropical Paddy Soil with Variable Water Regimes. Eur. J. Soil Sci. 2017, 68, 749–757. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, L.; Li, N.; Liu, B.; Meng, N.; Wang, M.; Chen, S.B. Toxicity Threshold of Lead (Pb) to Nitrifying Microorganisms in Soils Determined by Substrate-Induced Nitrification Assay and Prediction Model. J. Integr. Agric. 2017, 16, 1832–1840. [Google Scholar] [CrossRef]
- Hamsa, N.; Yogesh, G.S.; Koushik, U.; Patil, L. Nitrogen Transformation in Soil: Effect of Heavy Metals. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 816–832. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Radziemska, M. Effects of Chromium(III and VI) on Spring Barley and Maize Biomass Yield and Content of Nitrogenous Compounds. J. Toxicol. Environ. Health-Part A Curr. Issues 2010, 73, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chandran, K.; Grasso, D.; Smets, B.F. Effect of Nickel and Cadmium Speciation on Nitrification Inhibition. Environ. Sci. Technol. 2002, 36, 3074–3078. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Xia, L.; Ti, C.; Zhou, W.; Fountain, L.; Shan, J.; Yan, X. Oxytetracycline, Copper, and Zinc Effects on Nitrification Processes and Microbial Activity in Two Soil Types. Food Energy Secur. 2020, 9, e248. [Google Scholar] [CrossRef]
- Carboni, M.F.; Arriaga, S.; Lens, P.N.L. Effect of Copper, Arsenic and Nickel on Pyrite-Based Autotrophic Denitrification. Biodegradation 2023. [Google Scholar] [CrossRef]
- He, T.; Xie, D.; Ni, J.; Li, Z.; Li, Z. Effect of Cobalt, Cadmium and Manganese on Nitrogen Removal Capacity of Arthrobacter Arilaitensis Y-10. Water 2020, 12, 1701. [Google Scholar] [CrossRef]
- Mahbub, K.R.; Krishnan, K.; Megharaj, M.; Naidu, R. Mercury Inhibits Soil Enzyme Activity in a Lower Concentration than the Guideline Value. Bull. Environ. Contam. Toxicol. 2016, 96, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Dexter, A.R. Soil Physical Quality Part I. Theory, Effects of Soil Texture, Density, and Organic Matter, and Effects on Root Growth. Geoderma 2004, 120, 201–214. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization Mechanisms of Soil Organic Matter: Implications for C-Saturation of Soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Zhou, W.; Han, G.; Liu, M.; Li, X. Effects of Soil pH and Texture on Soil Carbon and Nitrogen in Soil Profiles under Different Land Uses in Mun River Basin, Northeast Thailand. PeerJ 2019, 2019, e7880. [Google Scholar] [CrossRef] [PubMed]
- Cellone, F.; Carol, E.; Pugliese, I.; Córdoba, J.; Butler, L.; Lamarche, L. Nitrate Pollution in Dairy Farms and Its Impact on Groundwater Quality in a Sector of the Pampas Plain, Argentina. Environ. Earth Sci. 2020, 79, 258. [Google Scholar] [CrossRef]
- Lu, X.; Vitousek, P.M.; Mao, Q.; Gilliam, F.S.; Luo, Y.; Zhou, G.; Zou, X.; Bai, E.; Scanlon, T.M.; Hou, E.; et al. Plant Acclimation to Long-Term High Nitrogen Deposition in an N-Rich Tropical Forest. Proc. Natl. Acad. Sci. USA 2018, 115, 5187–5192. [Google Scholar] [CrossRef]
- Tian, D.; Niu, S. A Global Analysis of Soil Acidification Caused by Nitrogen Addition. Environ. Res. Lett. 2015, 10, 024019. [Google Scholar] [CrossRef]
- Matschonat, G.; Matzner, E. Soil Chemical Properties Affecting NH4+ Sorption in Forest Soils. J. Plant Nutr. Soil Sci. 1996, 159, 505–511. [Google Scholar] [CrossRef]
- Qi, S.; Ma, J.; Ling, X.; Feng, Q.; He, J.; Shu, H.; Jia, B. NO3−Circulation and Associated Driving Factors in the Unsaturated Zone of Southwestern Tengger Desert, Northwestern China. Catena 2019, 173, 394–409. [Google Scholar] [CrossRef]
- Laura Bačani Teaching-Research Polygon, Velika Gorica. Available online: https://www.rgn.unizg.hr/en/studies/postgraduate-study/blog-en/2727-teaching-research-polygon-velika-gorica-en (accessed on 9 October 2023).
- Kovač, Z.; Nakić, Z.; Barešić, J.; Parlov, J. Nitrate Origin in the Zagreb Aquifer System. Geofluids 2018, 2018, 2789691. [Google Scholar] [CrossRef]
- Kovač, Z.; Nakić, Z.; Špoljarić, D.; Stanek, D.; Bačani, A. Estimation of Nitrate Trends in the Groundwater of the Zagreb Aquifer. Geosciences 2018, 8, 159. [Google Scholar] [CrossRef]
- Buškulić, P.; Parlov, J.; Kovač, Z.; Nakić, Z. Estimation of Nitrate Background Value in Groundwater under the Long-Term Human Impact. Hydrology 2023, 10, 63. [Google Scholar] [CrossRef]
- Ružičić, S.; Kovač, Z.; Perković, D.; Bačani, L.; Majhen, L. The Relationship between the Physicochemical Properties and Permeability of the Fluvisols and Eutric Cambisols in the Zagreb Aquifer, Croatia. Geosciences 2019, 9, 416. [Google Scholar] [CrossRef]
- Kovač, Z.; Ružičić, S.; Rubinić, V.; Nakić, Z.; Sertić, M. Sorption of Cadmium, Zinc and Copper in Dominant Soils of the Zagreb Aquifer System, Croatia. Geol. Croat. 2022, 75, 177–188. [Google Scholar] [CrossRef]
- Kovač, Z.; Krevh, V.; Filipović, L.; Defterdarović, J.; Buškulić, P.; Han, L.; Filipović, V. Utilizing stable water isotopes (δ2H and δ2O) to study soil-water origin in sloped vineyard: First results. Min.-Geol.-Pet. Eng. Bull. 2022, 59, 1–14. [Google Scholar] [CrossRef]
- Kovač, Z.; Bačani, L.; Ružičić, S.; Parlov, J.; Posavec, K.; Buškulić, P. Using Water Stable Isotopes and Cross-Correlation Analysis to Characterize Infiltration of Precipitation through Unsaturated Zone at the Velika Gorica Site of Zagreb Aquifer. J. Hydrol. Eng. 2023, 28, 04023002. [Google Scholar] [CrossRef]
- Bogunović, M.; Vidaček, Ž.; Husnjak, S.; Sraka, M. Inventory of Soils in Croatia. Agric. Conspec. Sci. 1998, 63, 105–112. [Google Scholar]
- Kukolja, A. Određivanje Hidrauličke Vodljivosti Metodom Permeametra sa Stalnom Razinom na Području Velike Gorice (Determination of Hydraulic Conductivity Using Constat Head Permeability Test Method in the Area of the Well Field Velika Gorica). Master’s Thesis, University of Zagreb, Faculty of Mining, Geology and Petroleum Engineering, Zagreb, Croatia, 2018. [Google Scholar]
- Michelsen, N.; van Geldern, R.; Roßmann, Y.; Bauer, I.; Schulz, S.; Barth, J.A.C.; Schüth, C. Comparison of Precipitation Collectors Used in Isotope Hydrology. Chem. Geol. 2018, 488, 171–179. [Google Scholar] [CrossRef]
- Coplen, T.B.; Wassenaar, L.I. LIMS for Lasers 2015 for Achieving Long-Term Accuracy and Precision of δ2H, δ17O, and δ18O of Waters Using Laser Absorption Spectrometry. Rapid Commun. Mass Spectrom. 2015, 29, 2122–2130. [Google Scholar] [CrossRef]
- Dansgaard, W. Stable isotopes in precipitation. Tellus 1964, 16, 436–468. [Google Scholar] [CrossRef]
- IUSS Working Group. WRB World Reference Base for Soil Resources 2014: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; FAO: Rome, Italy, 2015. [Google Scholar]
- Howladar, M.F.; Mustafizur Rahman, M. Characterization of Underground Tunnel Water Hydrochemical System and Uses through Multivariate Statistical Methods: A Case Study from Maddhapara Granite Mine, Dinajpur, Bangladesh. Environ. Earth Sci. 2016, 75, 1501. [Google Scholar] [CrossRef]
- Rahman, M.M.; Howladar, M.F.; Faruque, M.O. Assessment of Soil Quality for Agricultural Purposes around the Barapukuria Coal Mining Industrial Area, Bangladesh: Insights from Chemical and Multivariate Statistical Analysis. Environ. Syst. Res. 2017, 6, 24. [Google Scholar] [CrossRef]
- Das, S.; Sarkar, R. Impact of Brickfields on Soil Quality of Agricultural Land along the Bhagirathi-Hugli River Basin, West Bengal, India. Spat. Inf. Res. 2020, 28, 405–418. [Google Scholar] [CrossRef]
- Haidary, A.; Amiri, B.J.; Adamowski, J.; Fohrer, N.; Nakane, K. Assessing the Impacts of Four Land Use Types on the Water Quality of Wetlands in Japan. Water Resour. Manag. 2013, 27, 2217–2229. [Google Scholar] [CrossRef]
- Emoyan, O.O.; Akporido, S.O.; Agbaire, P.O. Effects of Soil pH, Total Organic Carbon and Texture on Fate of Polycyclic Aromatic Hydrocarbons (PAHs) in Soils. Glob. NEST J. 2018, 20, 181–187. [Google Scholar]
- Good, P.I.; Lunneborg, C. Limitations of the Analysis of Variance. J. Mod. Appl. Stat. Methods 2006, 5, 41–43. [Google Scholar] [CrossRef]
- Day, R.W.; Quinn, G.P. Comparisons of Treatments After an Analysis of Variance in Ecology. Ecol. Monogr. 1989, 59, 433–463. [Google Scholar] [CrossRef]
- Benjamini, Y.; Braun, H. John W. Tukey’s Contributions to Multiple Comparisons. Ann. Stat. 2002, 30, 1576–1594. [Google Scholar] [CrossRef]
- Shah Anuar, H.; Udin, Z.M. Post-Hoc Analysis on the R&D Capabilities of Chemical and Metallurgical Manufacturing. Manag. Inf. Syst. 2013, 8, 017–024. [Google Scholar]
- Sprenger, M.; Leistert, H.; Gimbel, K.; Weiler, M. Illuminating Hydrological Processes at the Soil-Vegetation-Atmosphere Interface with Water Stable Isotopes. Rev. Geophys. 2016, 54, 674–704. [Google Scholar] [CrossRef]
- Skierucha, W.; Wilczek, A.; Szypłowska, A.; Sławiński, C.; Lamorski, K. A TDR-Based Soil Moisture Monitoring System with Simultaneous Measurement of Soil Temperature and Electrical Conductivity. Sensors 2012, 12, 13545–13566. [Google Scholar] [CrossRef]
- Wen, T.; Wang, P.; Shao, L.; Guo, X. Experimental investigations of soil shrinkage characteristics and their effects on the soil water characteristic curve. Eng. Geol. 2021, 284, 106035. [Google Scholar] [CrossRef]
- Meng, Y.; He, Z.; Liu, B.; Chen, L.; Lin, P.; Luo, W. Soil Salinity and Moisture Control the Processes of Soil Nitrification and Denitrification in a Riparian Wetlands in an Extremely Arid Regions in Northwestern China. Water 2020, 12, 2815. [Google Scholar] [CrossRef]
- Zhao, Y.; Wen, T.; Shao, L.; Chen, R.; Sun, X.; Huang, L.; Chen, X. Predicting hysteresis loops of the soil water characteristic curve from initial drying. Soil Sci. Soc. Am. J. 2020, 84, 1642–1649. [Google Scholar] [CrossRef]
- Si, B.C.; Kachanoski, R.C. Unified solution for infiltration and drainage with hysteresis: Theory and field test. Soil Sci. Soc. Am. J. 2000, 64, 30–36. [Google Scholar] [CrossRef]
- Zhang, Q.; Werner, A.D.; Aviyanto, R.F.; Hutson, J.L. Influence of soil moisture hysteresis on the functioning of capillary barriers. Hydrol. Process. 2009, 23, 1369–1375. [Google Scholar] [CrossRef]
- Wen, T.; Shao, L.; Guo, X.; Zhao, Y. Experimental investigations of the soil water retention curve under multiple drying–wetting cycles. Acta Geotech. 2020, 15, 3321–3326. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buškulić, P.; Parlov, J.; Kovač, Z.; Brenko, T.; Pejić, M. Determination of Nitrate Migration and Distribution through Eutric Cambisols in an Area without Anthropogenic Sources of Nitrate (Velika Gorica Well Field, Croatia). Sustainability 2023, 15, 16529. https://doi.org/10.3390/su152316529
Buškulić P, Parlov J, Kovač Z, Brenko T, Pejić M. Determination of Nitrate Migration and Distribution through Eutric Cambisols in an Area without Anthropogenic Sources of Nitrate (Velika Gorica Well Field, Croatia). Sustainability. 2023; 15(23):16529. https://doi.org/10.3390/su152316529
Chicago/Turabian StyleBuškulić, Patricia, Jelena Parlov, Zoran Kovač, Tomislav Brenko, and Marija Pejić. 2023. "Determination of Nitrate Migration and Distribution through Eutric Cambisols in an Area without Anthropogenic Sources of Nitrate (Velika Gorica Well Field, Croatia)" Sustainability 15, no. 23: 16529. https://doi.org/10.3390/su152316529







