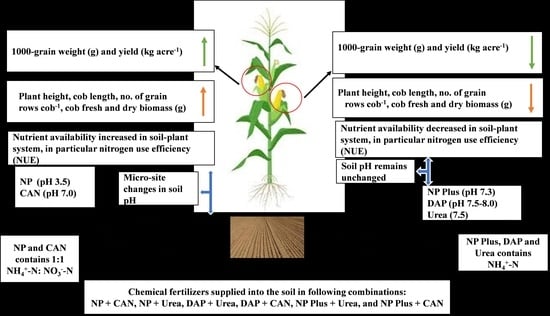
Fertilizers Containing Balanced Proportions of NH4+-N and NO3−-N Enhance Maize (Zea mays L.) Yield Due to Improved Nitrogen Recovery Efficiency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Experimentation
2.2. Plant Growth Conditions and Harvesting
2.3. Determination of Mineral N and Other N-Forms in Soils and Plant Tissues
2.4. Determination of NRE in Maize
- where
- Uptake efficiency = Nt/Ns;
- Utilization efficiency = Gw/Nt;
- Nt = Total N uptake by maize at harvesting (kg acre−1);
- Ns = Nitrogen supply rate (kg acre−1);
- Gw = Maize grain yield (kg acre−1).
2.5. Determination of Available Soil Phosphorus (P) and Plant Total P Concentrations
2.6. Determination of Potassium Concentrations in Soils and Leaf Tissues
2.7. Quantification of Net Return and Return on Investment (ROI)
2.8. Experimental Design and Data Analysis
3. Results
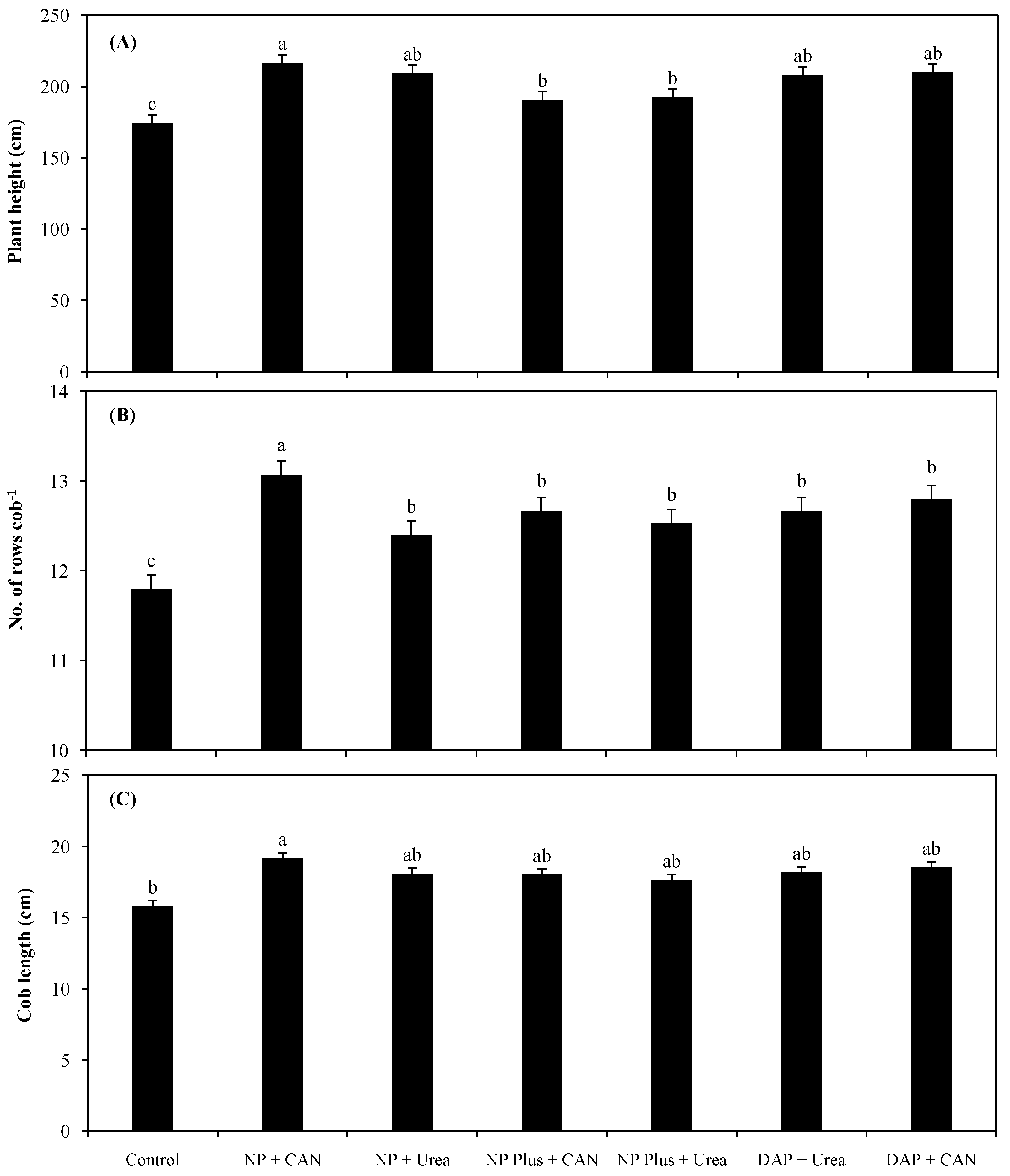
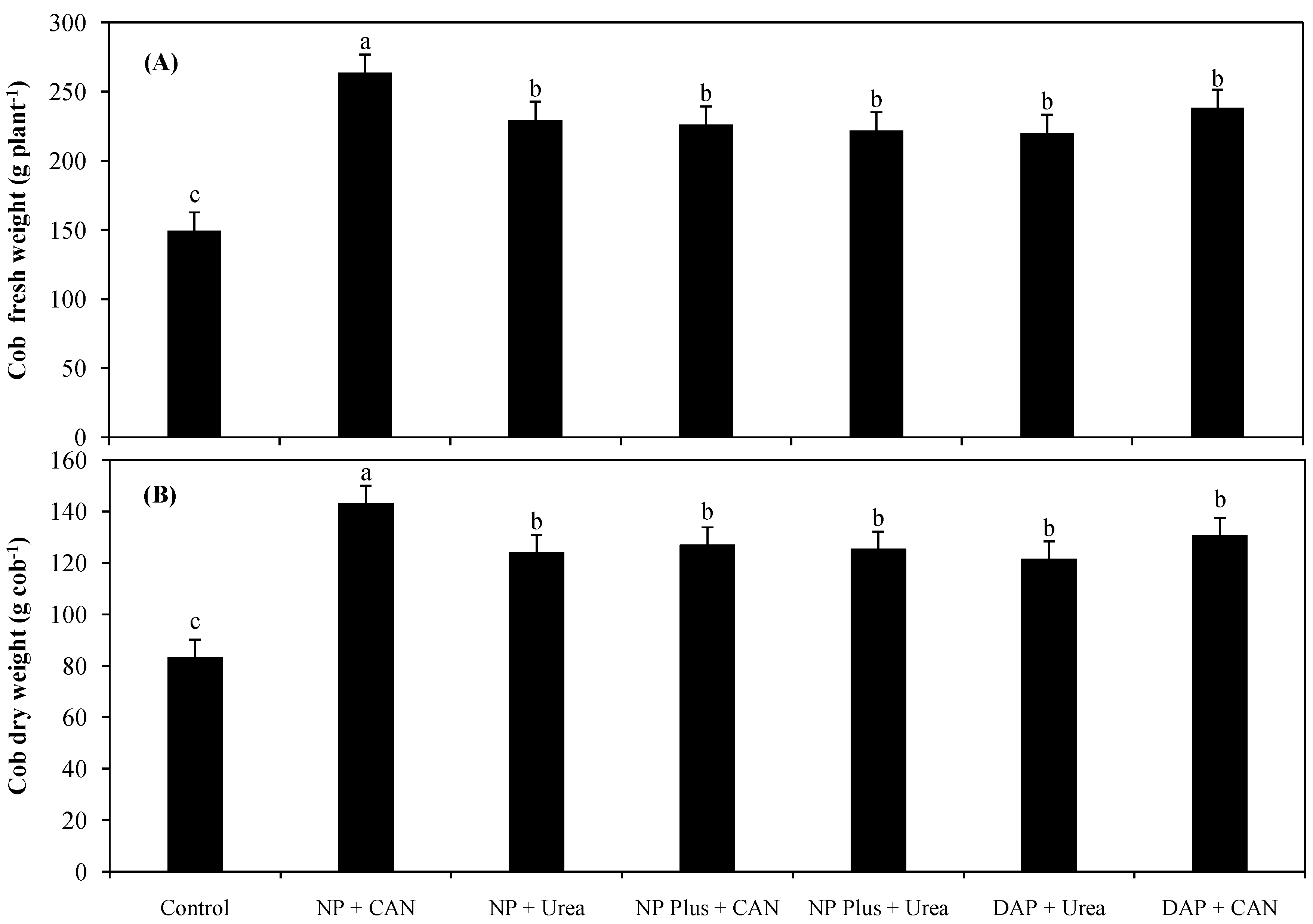
3.1. Plant Growth as Influenced by Inorganic Nitrogen Supplies
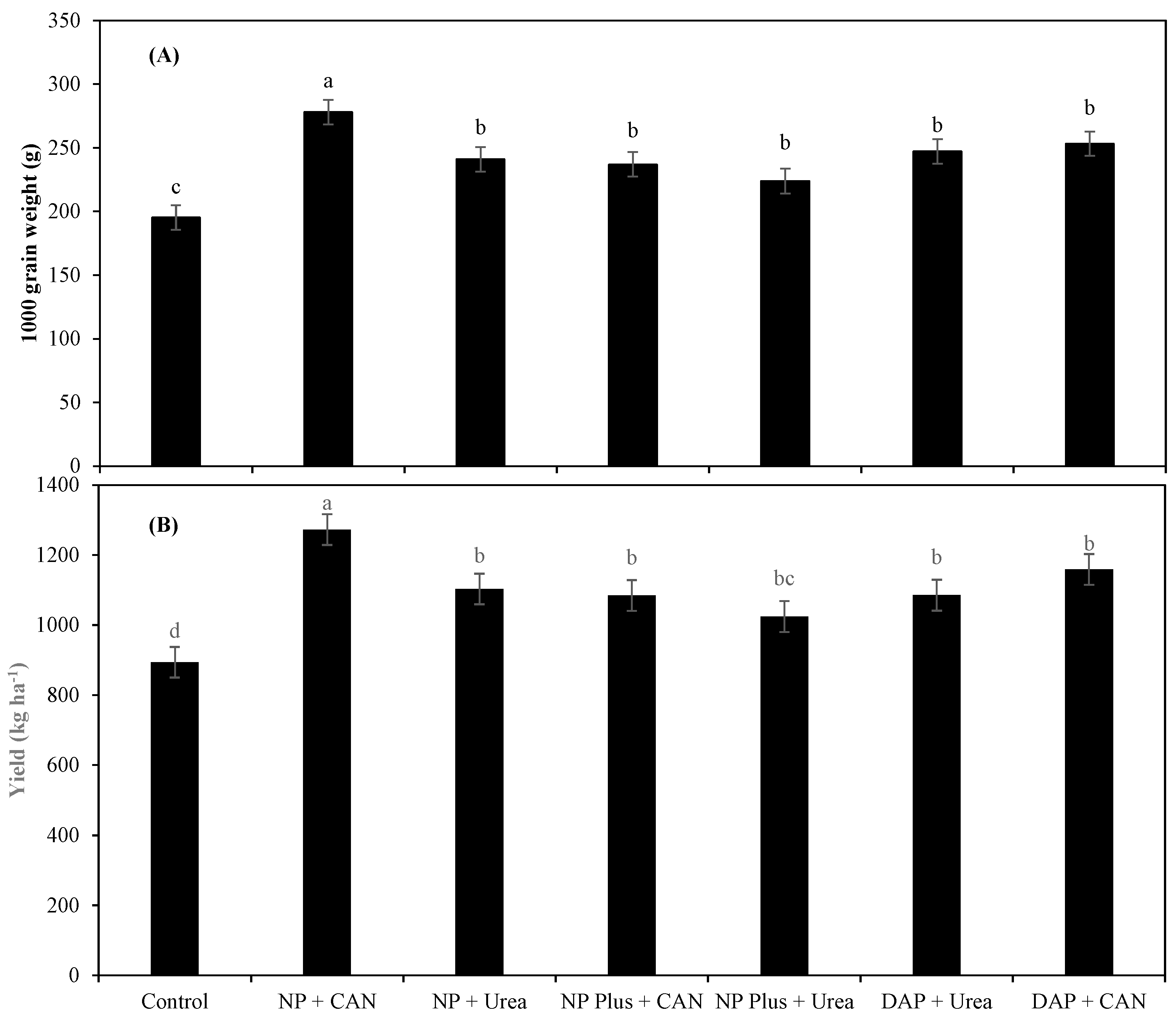
3.2. Maize Grain Yield as Influenced by Inorganic Nitrogen Supplies
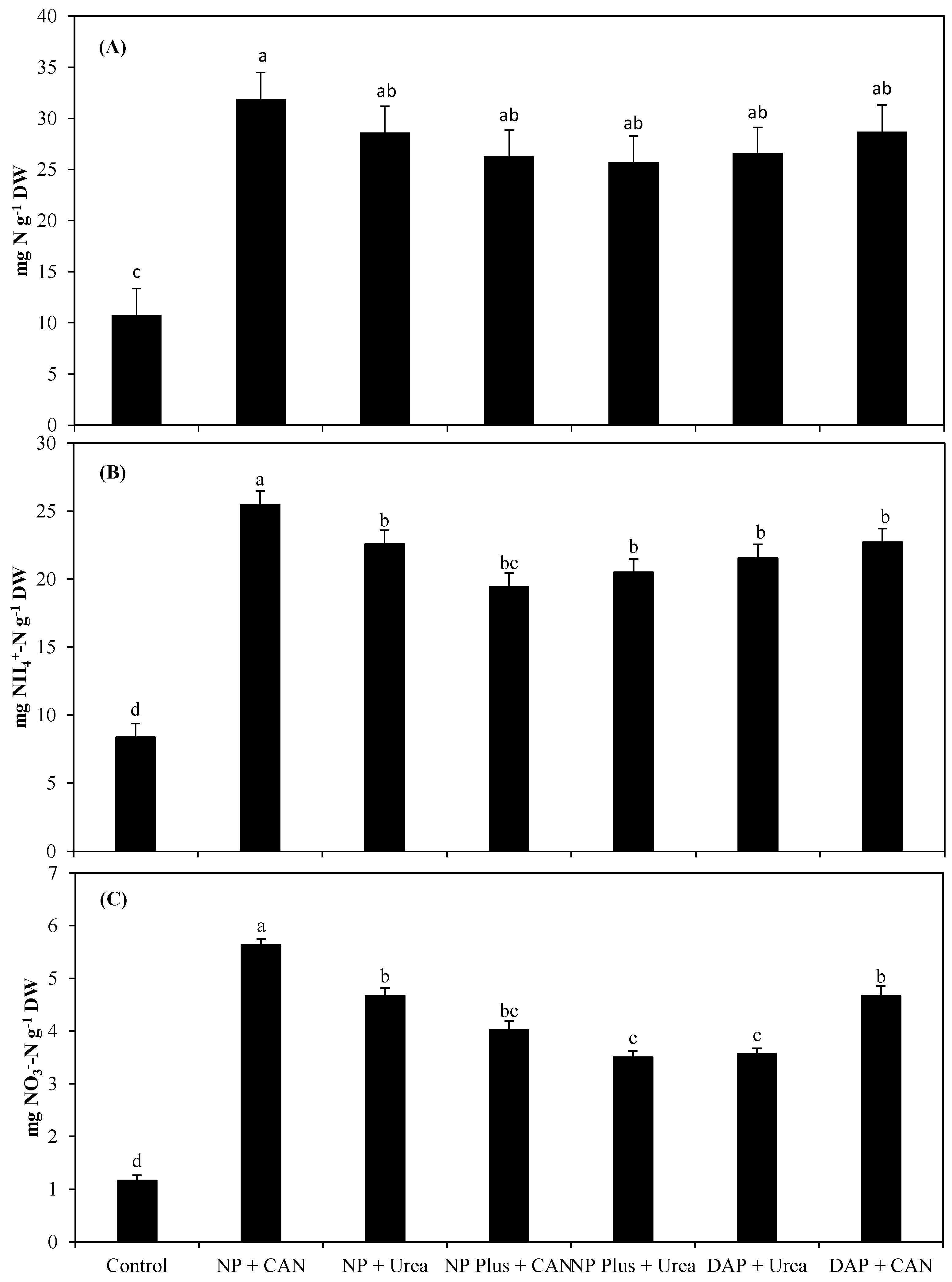
3.3. Total N, NH4+-N, and NO3−-N Concentrations (mg g−1 Dry Weight) and NRE of Maize as Influenced by Inorganic Nitrogen Supplies
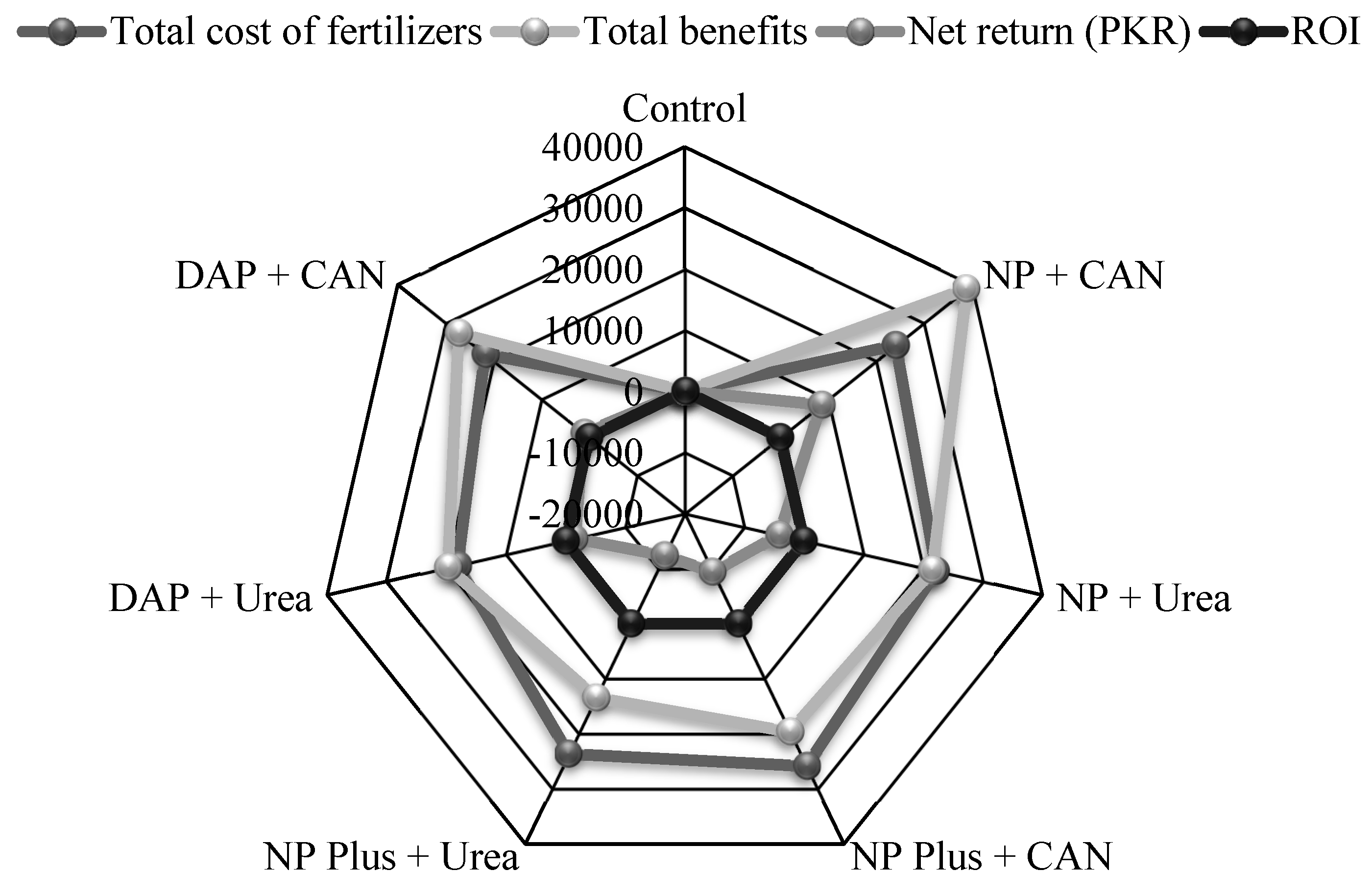
3.4. Economic Analysis of Various Inorganic Fertilizers Supplies
3.5. Effect of Balance Application of NH4+-N and NO3−-N on Soil Physicochemical Properties and Mineral Nutrient Concentrations
4. Discussion
4.1. The Improvement in Plant Growth and Grain Yield Was Attributed to Increased NH4+-N/NO3−-N Uptake as Well as NRE of Maize
4.2. Fertilizers Containing Balanced Proportions of NH4+-N and NO3−-N Increase Soil N Mobility and thus NRE of Maize
4.3. Net Return and Socioeconomic Impact of NP and CAN Fertilization
5. Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cameron, K.C.; Di, H.J.; Moir, J.L. Nitrogen losses from the soil/plant system: A review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef]
- Miller, A.J.; Cramer, M.D. Root Nitrogen Acquisition and Assimilation. Plant Soil 2004, 274, 1–36. [Google Scholar] [CrossRef]
- Kotur, Z.; Siddiqi, Y.M.; Glass, A.D.M. Characterization of nitrite uptake in Arabidopsis thaliana: Evidence for a nitrite-specific transporter. New Phytol. 2013, 200, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Tegeder, M.; Rentsch, D. Uptake and Partitioning of Amino Acids and Peptides. Mol. Plant 2010, 3, 997–1011. [Google Scholar] [CrossRef]
- Forde, B.G.; Lea, P.J. Glutamate in plants: Metabolism, regulation, and signaling. J. Exp. Bot. 2007, 58, 2339–2358. [Google Scholar] [CrossRef]
- Raven, J.A. Can plants rely on nitrate? Trends Plant Sci. 2003, 8, 314–315. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Hsu, P.-K.; Tsay, Y.-F. Uptake, allocation and signaling of nitrate. Trends Plant Sci. 2012, 17, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Yu, C.; Qiao, N.; Xu, X.; Tian, Y.; Ouyang, H. Plant preference for NH4+ versus NO3− At different growth stages in an alpine agroecosystem. Field Crops Res. 2017, 201, 192–199. [Google Scholar] [CrossRef]
- Neina, D. The Role of Soil pH in Plant Nutrition and Soil Remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- White, R.E. Principles and Practice of Soil Science: The Soil as a Natural Resource; Blackwell Publishing: Oxford, UK, 2006. [Google Scholar]
- Šimek, M.; Jíšová, L.; Hopkins, D.W. What is the so-called optimum pH for denitrification in soil? Soil Biol. Biochem. 2002, 34, 1227–1234. [Google Scholar] [CrossRef]
- Ali, A.; Noorka, I.R. Nitrogen and phosphorus management strategy for better growth and yield of sunflower (Helianthus annus L.). Soil Environ. 2013, 32, 44–48. [Google Scholar]
- Freney, J.R.; Simpson, J.R.; Denmead, O.T. Volatilization of Ammonia. In Gaseous Loss of Nitrogen from Plant-Soil Systems; Plant and Soil Science; Freney, J.R., Simpson, J.R., Eds.; Springer: Dordrecht, The Netherlands, 1983; Volume 9, pp. 1–32. [Google Scholar]
- Jordan, C. The effect of fertiliser type and application rate on denitrification losses from cut grassland in Northern Ireland. Fertil. Res. 1989, 19, 45–55. [Google Scholar] [CrossRef]
- Wasti, S.E. Economic Survey of Pakistan; Finance and Economic Affairs Division, Ministry of Finance, Govt. of Pakistan: Islamabad, Pakistan, 2013.
- Yara Fertilizer Handbook; Nitrate Fertilizers; Yara International ASA: Oslo, Norway, 2018; pp. 37–43.
- Masood, S.; Zhao, X.Q.; Shen, R.F. Bacillus pumilus promotes the growth and nitrogen uptake of tomato plants under nitrogen fertilization. Sci. Hortic. 2020, 272, 109581. [Google Scholar] [CrossRef]
- da Silva, N.F.; da Silva, E.C.; Muraoka, T.; Teixeira, M.B.; Soares, F.A.L.; Cunha, F.N.; Adu-Gyamfi, J.; Cavalcante, W.S.d.S. Nitrogen Utilization from Ammonium Nitrate and Urea Fertilizer by Irrigated Sugarcane in Brazilian Cerrado Oxisol. Agriculture 2020, 10, 323. [Google Scholar] [CrossRef]
- Sutton, M.A.; Howard, C.M.; Bleeker, A.; Datta, A. The global nutrient challenge: From science to public engagement. Environ. Dev. 2013, 6, 80–85. [Google Scholar] [CrossRef]
- Ghafoor, I.; Habib-Ur-Rahman, M.; Ali, M.; Afzal, M.; Ahmed, W.; Gaiser, T.; Ghaffar, A. Slow-release nitrogen fertilizers enhance growth, yield, NUE in wheat crop and reduce nitrogen losses under an arid environment. Environ. Sci. Pollut. Res. 2021, 28, 43528–43543. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.D.; Bloom, A.J.; Sukrapanna, S.S.; Ehleringer, J.R. Nitrogen isotope composition of tomato (Lycopersicon esculentum Mill. cv. T-5) grown under ammonium or nitrate nutrition. Plant Cell Environ. 1996, 19, 1317–1323. [Google Scholar] [CrossRef]
- Pritchard, E.S.; Guy, R.D. Nitrogen isotope discrimination in white spruce fed with low concentrations of ammonium and nitrate. Trees 2005, 19, 89–98. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. Ecological significance and complexity of N-source preference in plants. Ann. Bot. 2013, 112, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.-H.; Tsay, Y.-F. Nitrate, ammonium, and potassium sensing and signaling. Curr. Opin. Plant Biol. 2010, 13, 604–610. [Google Scholar] [CrossRef]
- Williams, K.; Percival, F.; Merino, J.; Mooney, H.A. Estimation of Tissue Construction Cost from Heat of Combustion and Organic Nitrogen Content. Plant Cell Environ. 1987, 10, 725–734. [Google Scholar] [CrossRef]
- Hachiya, T.; Sakakibara, H. Interactions between nitrate and ammonium in their uptake, allocation, assimilation, and signaling in plants. J. Exp. Bot. 2017, 68, 2501–2512. [Google Scholar] [CrossRef]
- Domínguez-Valdivia, M.D.; Aparicio-Tejo, P.M.; Lamsfus, C.; Cruz, C.; Martins-Loução, M.A.; Moran, J.F. Nitrogen nutrition and antioxidant metabolism in ammonium-tolerant and -sensitive plants. Physiol. Plant. 2008, 132, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Szczerba, M.W.; Britto, D.T.; Balkos, K.D.; Kronzucker, H.J. Alleviation of rapid, futile ammonium cycling at the plasma membrane by potassium reveals K+-sensitive and -insensitive components of NH4+ transport. J. Exp. Bot. 2008, 59, 303–313. [Google Scholar] [CrossRef]
- Errebhi, M.; Wilcox, G.E. Plant species response to ammonium-nitrate concentration ratios. J. Plant Nutr. 1990, 13, 1017–1029. [Google Scholar] [CrossRef]
- Poothong, S.; Reed, B.M. Optimizing shoot culture media for Rubus germplasm: The effects of NH4+, NO3−, and total nitrogen. Vitr. Cell. Dev. Biol. Plant 2016, 52, 265–275. [Google Scholar] [CrossRef]
- Gul, H.; Rahman, S.; Shahzad, A.; Gul, S.; Qian, M.; Xiao, Q.; Liu, Z. Maize (Zea mays L.) Productivity in Response to Nitrogen Management in Pakistan. Am. J. Plant Sci. 2021, 12, 1173–1179. [Google Scholar] [CrossRef]
- Amanullah; Iqbal, A.; Ali, A.; Fahad, S.; Parmar, B. Nitrogen Source and Rate Management Improve Maize Productivity of Smallholders under Semiarid Climates. Front. Plant Sci. 2016, 7, 1773. [Google Scholar] [CrossRef]
- Blandino, M.; Battisti, M.; Vanara, F.; Reyneri, A. The synergistic effect of nitrogen and phosphorus starter fertilization sub-surface banded at sowing on the early vigor, grain yield and quality of maize. Eur. J. Agron. 2022, 137, 126509. [Google Scholar] [CrossRef]
- Davies, B.; Coulter, J.A.; Pagliari, P.H. Timing and rate of nitrogen fertilization influence maize yield and nitrogen use efficiency. PLoS ONE 2020, 15, e0233674. [Google Scholar] [CrossRef]
- Gheith, E.M.S.; El-Badry, O.Z.; Lamlom, S.F.; Ali, H.M.; Siddiqui, M.H.; Ghareeb, R.Y.; El-Sheikh, M.H.; Jebril, J.; Abdelsalam, N.R.; Kandil, E.E. Maize (Zea mays L.) Productivity and Nitrogen Use Efficiency in Response to Nitrogen Application Levels and Time. Front. Plant Sci. 2022, 13, 941343. [Google Scholar] [CrossRef] [PubMed]
- Yuen, S.H.; Pollard, A.G. Determination of nitrogen in soil and plant materials: Use of boric acid in the micro-kjeldahl method. J. Sci. Food Agric. 1953, 4, 490–496. [Google Scholar] [CrossRef]
- Soltanpour, P.; Workman, S. Modification of the NH4 HCO3-DTPA soil test to omit carbon black. Commun. Soil Sci. Plant Anal. 1979, 10, 1411–1420. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis 21st Edition. 2019; Association of Official Analytical Chemists, AOAC Inc.: Arlington, VA, USA, 1995; p. 12. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice-Hall Inc.: Englewood Cliffs, NJ, USA, 1958; p. 498. [Google Scholar]
- Lüder, R.-M.H.; Qin, R.; Richner, W.; Stamp, P.; Streit, B.; Herrera, J.M.; Noulas, C. Small-Scale Variation in Nitrogen Use Efficiency Parameters in Winter Wheat as Affected by N Fertilization and Tillage Intensity. Sustainability 2020, 12, 3621. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; Circular of US Department of Agriculture: Washington, DC, USA, 1954.
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils; USDA Agric. Handbook 60; US Government Printing Office: Washington, DC, USA, 1954.
- Mohammed, Y.A.; Gesch, R.W.; Johnson, J.M.F.; Wagner, S.W. Agronomic and Economic Evaluations of N Fertilization in Maize under Recent Market Dynamics. Nitrogen 2022, 3, 514–527. [Google Scholar] [CrossRef]
- Peng, Y.; Niu, J.; Peng, Z.; Zhang, F.; Li, C. Shoot growth potential drives N uptake in maize plants and correlates with root growth in the soil. Field Crops Res. 2010, 115, 85–93. [Google Scholar] [CrossRef]
- Amin, M.E.-M.H. Effect of different nitrogen sources on growth, yield and quality of fodder maize (Zea mays L.). J. Saudi Soc. Agric. Sci. 2011, 10, 17–23. [Google Scholar] [CrossRef]
- Leila, H.; Soleymani, A. Effect of different amounts of nitrogen fertilizer on grain yield of forage corn cultivars in Isfahan. Int. J. Adv. Biol. Biomed. Res. 2014, 2, 608–614. [Google Scholar]
- Gasim, S.H. Effect of nitrogen, phosphorus and seed rate on growth, yield and quality of forage maize (Zea mays L.). Master’s Thesis, Faculty of Agriculture, University of Khartoum, Khartoum, Sudan, 2001. [Google Scholar]
- Asibi, A.E.; Chai, Q.; Coulter, J.A. Mechanisms of Nitrogen Use in Maize. Agronomy 2019, 9, 775. [Google Scholar] [CrossRef]
- Zhu, C.Q.; Zhang, J.H.; Zhu, L.F.; Abliz, B.; Zhong, C.; Bai, Z.G.; Hu, W.J.; Sajid, H.; James, A.B.; Cao, X.C.; et al. NH 4 + facilitates iron reutilization in the cell walls of rice (Oryza sativa) roots under iron-deficiency conditions. Environ. Exp. Bot. 2018, 151, 21–31. [Google Scholar] [CrossRef]
- Zhu, C.Q.; Zhu, X.F.; Hu, A.Y.; Wang, C.; Wang, B.; Dong, X.Y.; Shen, R.F. Differential effects of nitrogen forms on cell wall phosphorus remobilization in rice (Oryza sativa) are mediated by nitric oxide, pectin content and the expression of the phosphate transporter OsPT2. Plant Physiol. 2016, 171, 1407–1417. [Google Scholar] [CrossRef]
- Ciampitti, I.A.; Vyn, T.J. A comprehensive study of plant density consequences on nitrogen uptake dynamics of maize plants from vegetative to reproductive stages. Field Crops Res. 2011, 121, 2–18. [Google Scholar] [CrossRef]
- Ciampitti, I.A.; Vyn, T.J. Physiological perspectives of changes over time in maize yield dependency on nitrogen uptake and associated nitrogen efficiencies: A review. Field Crops Res. 2012, 133, 48–67. [Google Scholar] [CrossRef]
- Eid, M.A.M.; Abdel-Salam, A.A.; Salem, H.M.; Mahrous, S.E.; Seleiman, M.F.; Alsadon, A.A.; Solieman, T.H.I.; Ibrahim, A.A. Interaction Effects of Nitrogen Source and Irrigation Regime on Tuber Quality, Yield, and Water Use Efficiency of Solanum tuberosum L. Plants 2020, 9, 110. [Google Scholar] [CrossRef]
- Jensen, T.L. Soil pH and the Availability of Plant Nutrients. IPNI: International Plant Nutrition Institute. No. 2. 2010. Available online: www.ipni.net/pnt (accessed on 21 June 2023).
- Watson, C.J. The comparative effects of ammonium nitrate, urea or a combined ammonium nitrate/urea granular fertilizer on the efficiency of nitrogen recovery by perennial ryegrass. Fertil. Res. 1987, 11, 69–78. [Google Scholar] [CrossRef]
- Trenkel, M.E. Slow and Controlled-Release and Stabilized Fertilizers: An Option for Enhancing Nutrient Use Efficiency in Agriculture; International Fertilizer Association: Paris, France, 2010; Available online: www.fertilizer.org (accessed on 21 June 2023).
- Naku, M.; Kambizi, L.; Matimati, I. Functional roles of ammonium (NH4+) and nitrate (NO3−) in regulation of day- and night-time transpiration in Phaseolus vulgaris. Funct. Plant Biol. 2019, 46, 806. [Google Scholar] [CrossRef] [PubMed]
- Plett, D.C.; Ranathunge, K.; Melino, V.J.; Kuya, N.; Uga, Y.; Kronzucker, H.J. The intersection of nitrogen nutrition and water use in plants: New paths toward improved crop productivity. J. Exp. Bot. 2020, 71, 4452–4468. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Bedi, S. Nitrogen use Efficiency and Source-sink Relations in Maize. J. Plant Sci. Res. 2012, 28, 219–228. [Google Scholar]
- Kelly, J.T.; Bacon, R.K.; Wells, B.R. Genetic variability in nitrogen utilization at four growth stages in soft red winter wheat. J. Plant Nutr. 1995, 18, 969–982. [Google Scholar] [CrossRef]
- Richardson, A.E.; Barea, J.-M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Robinson, N.; Brackin, R.; Vinall, K.; Soper, F.; Holst, J.; Gamage, H.; Paungfoo-Lonhienne, C.; Rennenberg, H.; Lakshmanan, P.; Schmidt, S. Nitrate Paradigm Does Not Hold Up for Sugarcane. PLoS ONE 2011, 6, e19045. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, M.A.; Awan, U.K.; Aziz, T.; Arshad, H.; Ashraf, N.; Ali, M. Nitrogen Management in Calcareous Soils: Problems and Solutions. Pak. J. Agric. Sci. 2016, 53, 79–95. [Google Scholar] [CrossRef]
- Maize Yield Competition; Government of the Punjab Commissioner’s Office Sahiwal: Punjab, Pakistan, 2018.

| Depth (cm) | Soil Texture | pH | EC (mS cm−1) | meqNa 100 g−1 Dry Soil | meq Ca + Mg100 g−1 Dry Soil | SAR | SOM (%) | Total Soil N (mg kg−1 Dry Soil) | mg NH4+ -N kg−1 Dry Soil | mg NO3−-N kg−1 Dry Soil | Available P (mg kg−1 Dry Soil) | Extracatable K (mg kg−1 Dry Soil) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–15 | Loam | 8.18 ± 0.11 | 0.44 ± 0.03 | 9.19 ± 0.22 | 344 ± 11.21 | 0.77 ± 0.03 | 0.75 ± 0.03 | 573 ± 23.36 | 95 ± 4.17 | 197 ± 8.81 | 5.53 ± 0.16 | 107 ± 4.80 |
| 15–30 | Loam | 8.44 ± 0.03 | 0.47 ± 0.03 | 11.13 ± 0.36 | 356 ± 12.34 | 0.83 ± 0.04 | 0.53 ± 0.03 | 491 ± 21.77 | 78 ± 3.56 | 230 ± 10.63 | 5.15 ± 0.12 | 100 ± 4.03 |
| Treatments | pH | EC (dSm−1) | Sodium Adsorption Ratio | SOM (%) | Total Soil N (mg kg−1 Dry Soil) | mg NH4+-N kg−1 Dry Soil | mg NO3−-N kg−1 Dry Soil | Available P (mg kg−1 Dry Soil) | Extractable K (mg kg−1 Dry Soil |
|---|---|---|---|---|---|---|---|---|---|
| Control | 8.38 ± 0.11 ns | 0.42 ± 0.06 ab | 0.6 ± 0.02 ns | 0.57 ± 0.05 b | 410.91 ± 17.22 c | 150.93 ± 7.08 d | 354.18 ± 10.29 d | 4.19 ± 0.18 b | 88.54 ± 3.29 ab |
| NP + CAN | 8.27 ± 0.10 ns | 0.51 ± 0.09 a | 0.7 ± 0.03 ns | 0.82 ± 0.05 a | 505.33 ± 18.17 a | 240.93 ± 6.50 a | 513.72 ± 14.78 a | 13.07 ± 0.28 a | 99.38 ± 5.03 a |
| NP + Urea | 8.26 ± 0.08 ns | 0.46 ± 0.07 a | 0.7 ± 0.08 ns | 0.87 ± 0.06 a | 420.76 ± 14.48 c | 206.77 ± 7.36 b | 471.04 ± 11.85 ab | 10.38 ± 0.37 b | 100.92 ± 4.85 a |
| NP plus + CAN | 8.28 ± 0.03 ns | 0.40 ± 0.02 ab | 0.7 ± 0.01 ns | 0.65 ± 0.05 b | 393.02 ± 12.76 cd | 176.19 ± 5.02 c | 430.12 ± 16.03 c | 10.50 ± 0.43 b | 102.47 ± 4.88 a |
| NP plus + Urea | 8.23 ± 0.10 ns | 0.50 ± 0.09 a | 0.7 ± 0.01 ns | 0.81 ± 0.03 a | 407.77 ± 14.17 c | 183.56 ± 4.98 c | 450.35 ± 17.11 bc | 11.43 ± 0.42 ab | 100.92 ± 3.31 a |
| DAP + Urea | 8.30 ± 0.06 ns | 0.55 ± 0.02 a | 0.6 ± 0.01 ns | 0.82 ± 0.07 a | 416.80 ± 7.37 c | 190.45 ± 4.38 c | 452.23 ± 14.36 bc | 10.38 ± 0.14 b | 94.73 ± 3.80 ab |
| DAP + CAN | 8.26 ± 0.06 ns | 0.40 ± 0.04 ab | 0.6 ± 0.01 ns | 0.80 ± 0.07 a | 446.39 ± 14.69 b | 209.54 ± 6.56 b | 477.79 ± 13.19 ab | 11.37 ± 0.54 ab | 90.09 ± 2.29 ab |
| Plant Height | Cob Length | No. of Rows Cob-1 | Cob FW | Cob DW | 1000-Grain wt. | Grain Yield | Leaf K | Leaf P | Total Leaf N | Leaf NH4−N | Leaf NO3−N | NUE | Soil pH | Soil EC | SAR | SOM | Soil P | Soil K | Total Soil N | Soil NO3−N | Soil NH4−N | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant height | 1 | ** | * | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ns | ns | ns | ** | ns | ns | ** | ** | ** |
| Cob length | 0.683 | 1 | ns | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ns | ns | ns | * | ns | ns | ** | ** | ** |
| No. of rows Cob−1 | 0.514 | 0.47 | 1 | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ns | ns | ns | ns | ns | ns | ns | ** | ** |
| Cob FW | 0.745 | 0.846 | 0.638 | 1 | ** | ** | ** | ** | ** | ** | ** | ** | ** | * | ns | ns | * | * | ns | ** | ** | ** |
| Cob DW | 0.675 | 0.852 | 0.616 | 0.901 | 1 | ** | ** | ** | ** | ** | ** | ** | ** | ns | ns | ns | * | ns | ns | ** | ** | ** |
| 1000-grain wt. | 0.76 | 0.873 | 0.61 | 0.828 | 0.818 | 1 | ** | ** | ** | ** | ** | ** | ** | ns | ns | ns | * | ns | ns | ** | ** | ** |
| Grain yield | 0.865 | 0.754 | 0.806 | 0.911 | 0.878 | 0.843 | 1 | ** | ** | ** | ** | ** | ** | ns | ns | ns | * | ns | ns | ** | ** | ** |
| Leaf K | 0.634 | 0.732 | 0.563 | 0.798 | 0.837 | 0.676 | 0.704 | 1 | ** | ** | ** | ** | ** | * | ns | ns | ** | ns | ns | * | ** | ** |
| Leaf P | 0.751 | 0.786 | 0.637 | 0.881 | 0.901 | 0.865 | 0.834 | 0.878 | 1 | ** | ** | ** | ** | ns | ns | ns | ** | ns | ns | ** | ** | ** |
| Total leaf N | 0.697 | 0.805 | 0.605 | 0.906 | 0.928 | 0.863 | 0.772 | 0.905 | 0.978 | 1 | ** | ** | ** | * | ns | ns | ** | ** | ns | ** | ** | ** |
| Leaf NH4−N | 0.754 | 0.803 | 0.6 | 0.893 | 0.92 | 0.837 | 0.818 | 0.927 | 0.975 | 0.997 | 1 | ** | ** | * | ns | ns | ** | ns | ns | ** | ** | ** |
| Leaf NO3−N | 0.798 | 0.762 | 0.59 | 0.898 | 0.9 | 0.848 | 0.796 | 0.762 | 0.926 | 0.951 | 0.922 | 1 | ** | * | ns | ns | ** | ns | ns | ** | ** | ** |
| NUE | 0.754 | 0.826 | 0.599 | 0.866 | 0.852 | 0.992 | 0.891 | 0.668 | 0.861 | 0.863 | 0.841 | 0.895 | 1 | ns | ns | ns | ns | ** | ns | ** | ** | ** |
| Soil pH | −0.423 | −0.287 | −0.483 | −0.524 | −0.39 | −0.369 | −0.41 | −0.466 | −0.423 | −0.46 | −0.455 | −0.447 | −0.42 | 1 | ns | ns | ns | ns | ns | ns | ns | ns |
| Soil EC | 0.167 | 0.136 | 0.204 | 0.185 | 0.152 | 0.194 | 0.203 | 0.22 | 0.229 | 0.229 | 0.241 | 0.168 | 0.141 | −0.17 | 1 | ns | ns | ns | ns | ns | ns | ns |
| SAR | 0.065 | 0.212 | 0.059 | 0.188 | 0.259 | 0.05 | 0.044 | 0.281 | 0.209 | 0.226 | 0.242 | 0.149 | 0.066 | 0.098 | 0.068 | 1 | ns | ns | ** | ns | ns | ns |
| SOM | 0.802 | 0.491 | 0.417 | 0.538 | 0.473 | 0.462 | 0.523 | 0.601 | 0.563 | 0.602 | 0.604 | 0.559 | 0.415 | −0.46 | 0.337 | 0.24 | 1 | ns | ns | * | * | ** |
| Soil P | 0.089 | 0.394 | 0.201 | 0.454 | 0.333 | 0.31 | 0.331 | 0.151 | 0.249 | 0.847 | 0.264 | 0.321 | 0.711 | 0.005 | 0.349 | 0.21 | 0.138 | 1 | ns | ** | * | * |
| Soil K | 0.045 | 0.085 | 0.358 | 0.229 | 0.292 | 0.086 | 0.092 | 0.285 | 0.27 | 0.282 | 0.291 | 0.229 | 0.133 | −0.16 | 0.267 | 0.61 | 0.093 | 0.140 | 1 | ns | ns | ns |
| Total soil N | 0.697 | 0.718 | 0.363 | 0.75 | 0.696 | 0.821 | 0.868 | 0.505 | 0.677 | 0.697 | 0.676 | 0.736 | 0.821 | −0.25 | 0.293 | 0.12 | 0.51 | 0.734 | 0.033 | 1 | ** | ** |
| Soil NO3−N | 0.711 | 0.764 | 0.607 | 0.851 | 0.857 | 0.849 | 0.798 | 0.763 | 0.883 | 0.896 | 0.89 | 0.865 | 0.857 | −0.28 | 0.331 | 0.21 | 0.518 | 0.522 | 0.183 | 0.813 | 1 | ** |
| Soil NH4−N | 0.775 | 0.727 | 0.64 | 0.82 | 0.765 | 0.876 | 0.807 | 0.629 | 0.835 | 0.832 | 0.806 | 0.881 | 0.901 | −0.4 | 0.34 | 0.08 | 0.573 | 0.456 | 0.258 | 0.839 | 0.865 | 1 |
| Sr. # | Farmer | Address with GPS Coordinates | Crop | Yield (kg Acre−1) | Reference |
|---|---|---|---|---|---|
| 1 | Faisal Ali | 34/12 L Chichawatni Sahiwal, Punjab 30°28′48″ N, 72°43′11.9994″ E | Autumn Maize | 3840 | Maize Yield Competition [64], Government of Punjab, Pakistan |
| 2 | Ashfaq Ahmed Joyia | MouzaJoyia Tehsil Okara, Punjab 31°0′36″ N, 73°30′0″ E | Autumn Maize | 3680 | - |
| 3 | Malik Ali Gohar | Chak# 96/D Tehsil Pakpattan, Sahiwal, Punjab 30°28′11.9994″ N, 73°10′12″ E | Autumn Maize | 3280 | - |
| 4 | Ghulam Mujtaba | Chak# 50/SP Pakpattan, Arifwala, Sahiwal, Punjab 30°25′47.9994″ N, 73°10′12″ E | Autumn Maize | 3280 | - |
| 5 | Muhammad Sharif | 33/12 L Chichawatni Sahiwal, Punjab 30°27′36″ N, 72°42′35.9994″ E | Autumn Maize | 3160 | - |
| 6 | Abid Rasheed | Chak# 4/AL RenalaKhurd, Okara, Punjab 30°56′24″ N, 73°42′0″ E | Autumn Maize | 3280 | - |
| 7 | Masood Saeed | Chak# 56/5-L Tehsil Sahiwal, Punjab 30°42′36″ N, 73°15′36″ E | Autumn Maize | 3160 | - |
| 8 | Muhamamd Arshad | Tehsil Sahiwal, Punjab 30°38′24″ N, 73°8′24″ E | Autumn Maize | 3160 | - |
| 9 | Nawab Khan | Chak# 3/EB Jiwan Shan Pakpattan, Punjab 30°18′35.9994″ N, 73°12′0″ E | Autumn Maize | 3240 | - |
| 10 | Muhammad Asif | BalaraLakhoKa Tehsil Arifwala, Punjab 30°17′23.9994″ N, 73°4′11.9994″ E | Autumn Maize | 3200 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masood, S.; Suleman, M.; Hussain, S.; Jamil, M.; Ashraf, M.; Siddiqui, M.H.; Nazar, R.; Khan, N.; Jehan, S.; Khan, K.S.; et al. Fertilizers Containing Balanced Proportions of NH4+-N and NO3−-N Enhance Maize (Zea mays L.) Yield Due to Improved Nitrogen Recovery Efficiency. Sustainability 2023, 15, 12547. https://doi.org/10.3390/su151612547
Masood S, Suleman M, Hussain S, Jamil M, Ashraf M, Siddiqui MH, Nazar R, Khan N, Jehan S, Khan KS, et al. Fertilizers Containing Balanced Proportions of NH4+-N and NO3−-N Enhance Maize (Zea mays L.) Yield Due to Improved Nitrogen Recovery Efficiency. Sustainability. 2023; 15(16):12547. https://doi.org/10.3390/su151612547
Chicago/Turabian StyleMasood, Sajid, Muhammad Suleman, Sajid Hussain, Muhammad Jamil, Muhammad Ashraf, Manzer H. Siddiqui, Rizwan Nazar, Naseerullah Khan, Sarvet Jehan, Khalid Saifullah Khan, and et al. 2023. "Fertilizers Containing Balanced Proportions of NH4+-N and NO3−-N Enhance Maize (Zea mays L.) Yield Due to Improved Nitrogen Recovery Efficiency" Sustainability 15, no. 16: 12547. https://doi.org/10.3390/su151612547