Copper Contamination Affects the Biogeochemical Cycling of Nitrogen in Freshwater Sediment Mesocosms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sediment and Water Sampling
2.1.1. Sediment Characterisation
2.1.2. Sediment Treatment
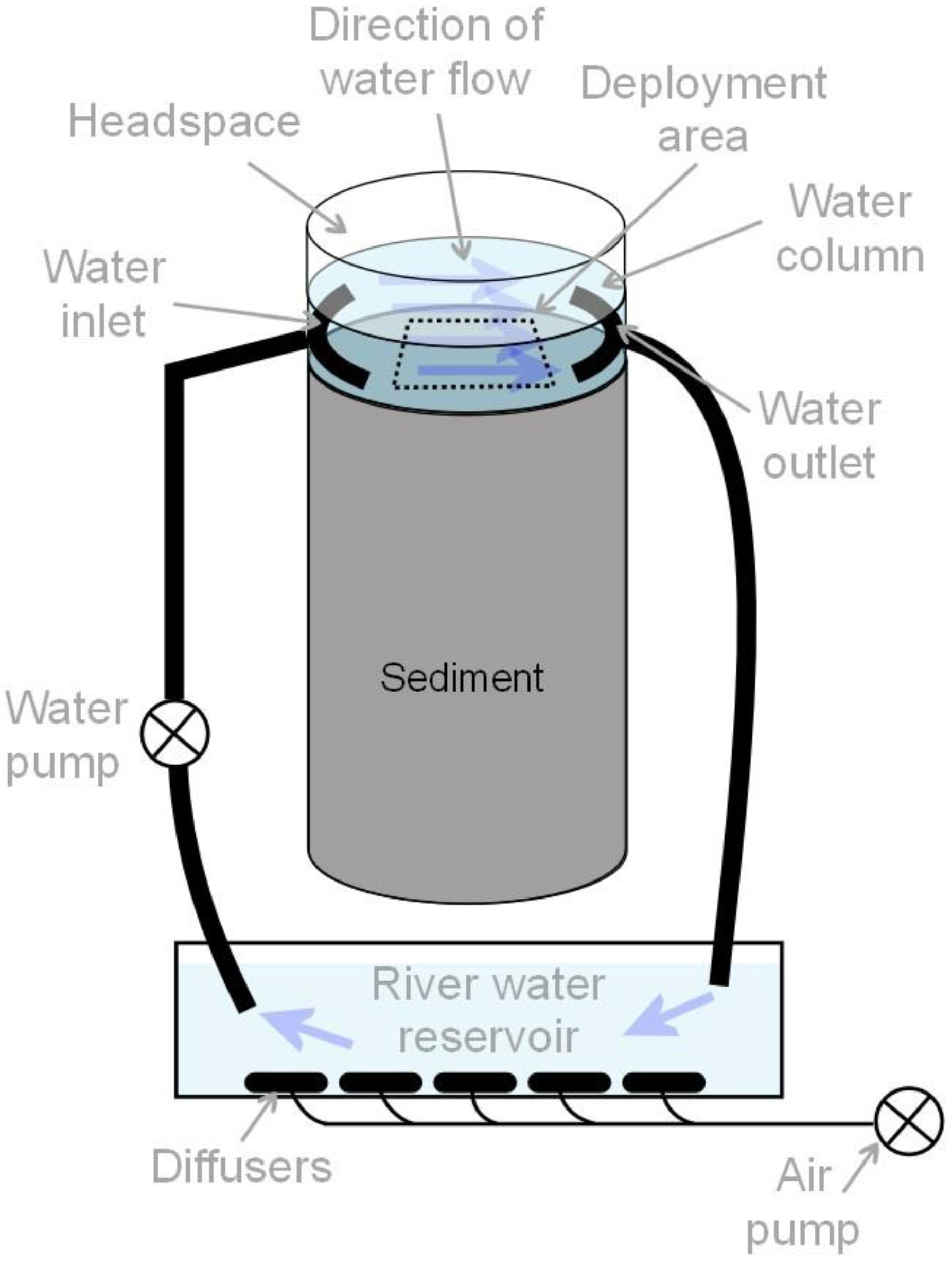
2.2. The Mesocosm Experiment
2.3. Physical and Chemical Characteristics of the Circulating Water
2.4. Nitrous Oxide Sampling
2.5. DGT and DET Preparation and Measurements
2.6. Trace Element Solid-Phase Speciation
2.7. Trace Element and Nutrient Analyses, and Quality Assurance
2.8. Statistical Analyses
3. Results
3.1. Water and Sediment Physical and Chemical Characteristics
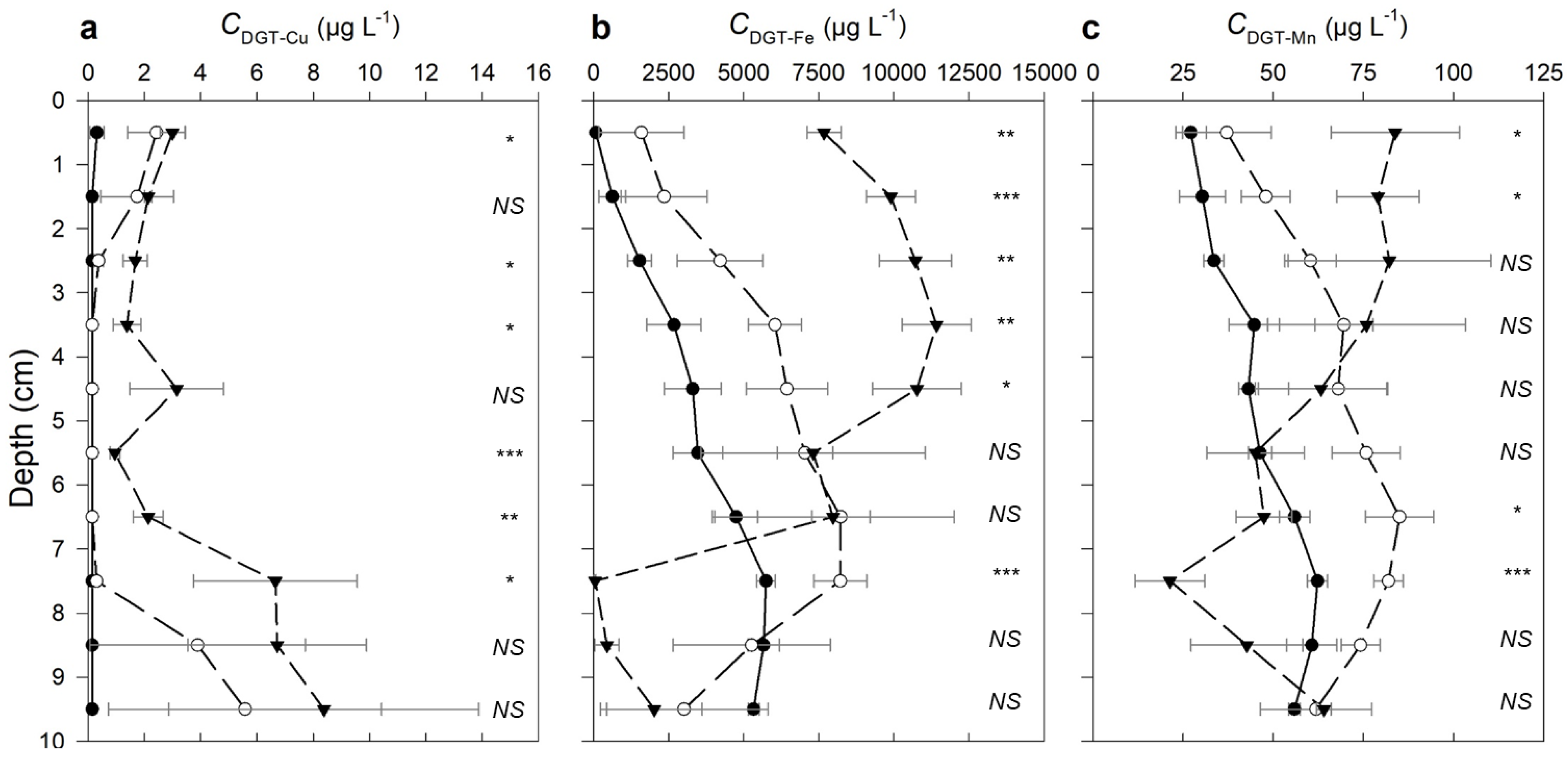
3.2. Trace Element Concentration Depth Profiles
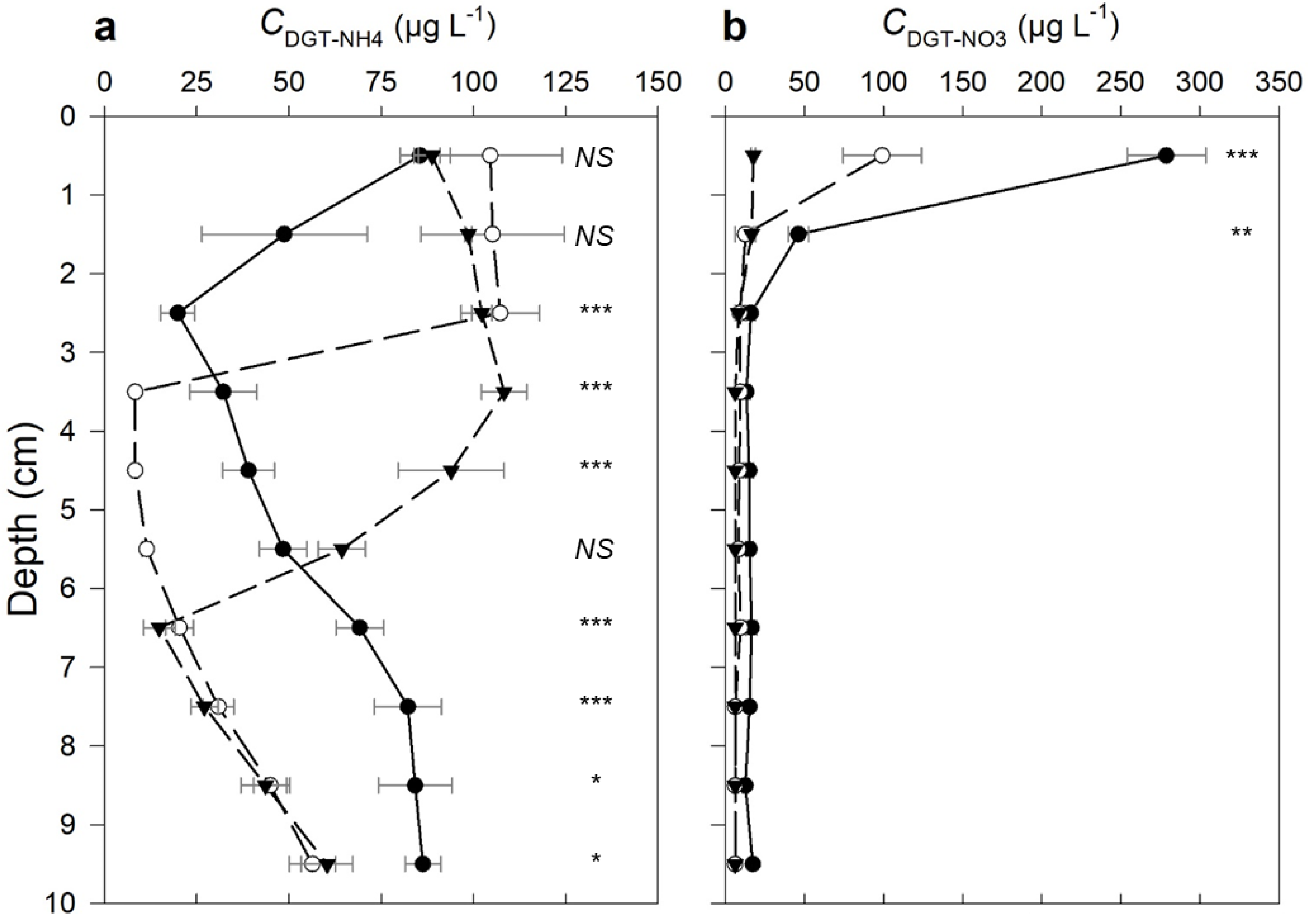
3.3. Nitrogen Species in the Sediment and the Mesocosm Headspace
4. Discussion
4.1. Cycling of Nitrogen Species under Increasing Copper Concentrations
4.2. Iron and Manganese Mobilisation in the Mesocosms
4.3. Mesocosm Experiments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meybeck, M. Global analysis of river systems: From Earth system controls to Anthropocene syndromes. Philos. Trans. R. Soc. B Biol. Sci. 2003, 358, 1935–1955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miralles-Wilhelm, F.; Matthews, J.H.; Karres, N.; Abell, R.; Dalton, J.; Kang, S.-T.; Liu, J.; Maendly, R.; Matthews, N.; McDonald, R.; et al. Emerging themes and future directions in watershed resilience research. Water Secur. 2023, 18, 100132. [Google Scholar] [CrossRef]
- Gaillardet, J.; Viers, J.; Dupré, B. Trace Elements in River Waters. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2003; Volume 5, pp. 225–272. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, E.; Zhang, B.; Bai, X.; Lei, P.; Qiao, X.; Li, Y.-F.; Li, B.; Wu, G.; Gao, Y. Pollution characteristics and ecological risks associated with heavy metals in the Fuyang river system in North China. Environ. Pollut. 2021, 281, 116994. [Google Scholar] [CrossRef] [PubMed]
- Nystrand, M.I.; Österholm, P.; Nyberg, M.E.; Gustafsson, J.P. Metal speciation in rivers affected by enhanced soil erosion and acidity. Appl. Geochem. 2012, 27, 906–916. [Google Scholar] [CrossRef]
- Loureiro, R.C.; Calisto, J.F.F.; Magro, J.D.; Restello, R.M.; Hepp, L.U. The influence of the environment in the incorporation of copper and cadmium in scraper insects. Environ. Monit. Assess. 2021, 193, 215. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Erisman, J.W.; Sutton, M.A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 2008, 1, 636–639. [Google Scholar] [CrossRef]
- Galloway, J.N.; Aber, J.D.; Erisman, J.W.; Seitzinger, S.P.; Howarth, R.W.; Cowling, E.B.; Cosby, B.J. The Nitrogen Cascade. BioScience 2003, 53, 341–356. [Google Scholar] [CrossRef]
- Thamdrup, B. New Pathways and Processes in the Global Nitrogen Cycle. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 407–428. [Google Scholar] [CrossRef]
- Quick, A.M.; Reeder, W.J.; Farrell, T.B.; Tonina, D.; Feris, K.P.; Benner, S.G. Nitrous oxide from streams and rivers: A review of primary biogeochemical pathways and environmental variables. Earth-Sci. Rev. 2019, 191, 224–262. [Google Scholar] [CrossRef]
- Roberto, A.A.; Van Gray, J.B.; Leff, L.G. Sediment bacteria in an urban stream: Spatiotemporal patterns in community composition. Water Res. 2018, 134, 353–369. [Google Scholar] [CrossRef]
- Seeley, M.E.; Song, B.; Passie, R.; Hale, R.C. Microplastics affect sedimentary microbial communities and nitrogen cycling. Nat. Commun. 2020, 11, 2372. [Google Scholar] [CrossRef]
- Rother, J.A.; Millbank, J.W.; Thornton, I. Effects of heavy-metal additions on ammonification and nitrification in soils contaminated with cadmium, lead and zinc. Plant Soil 1982, 69, 239–258. [Google Scholar] [CrossRef]
- Li, C.; Quan, Q.; Gan, Y.; Dong, J.; Fang, J.; Wang, L.; Liu, J. Effects of heavy metals on microbial communities in sediments and establishment of bioindicators based on microbial taxa and function for environmental monitoring and management. Sci. Total. Environ. 2020, 749, 141555. [Google Scholar] [CrossRef]
- Babich, H.; Stotzky, G. Heavy metal toxicity to microbe-mediated ecologic processes: A review and potential application to regulatory policies. Environ. Res. 1985, 36, 111–137. [Google Scholar] [CrossRef]
- Hoostal, M.J.; Bidart-Bouzat, M.G.; Bouzat, J.L. Local adaptation of microbial communities to heavy metal stress in polluted sediments of Lake Erie. FEMS Microbiol. Ecol. 2008, 65, 156–168. [Google Scholar] [CrossRef] [Green Version]
- Flemming, C.A.; Trevors, J.T. Copper toxicity and chemistry in the environment: A review. Water Air Soil Pollut. 1989, 44, 143–158. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef]
- He, Z.L.; Yang, X.E.; Stoffella, P.J. Trace elements in agroecosystems and impacts on the environment. J. Trace Elem. Med. Biol. 2005, 19, 125–140. [Google Scholar] [CrossRef]
- Morgan, R.K.; Taylor, E. Copper Accumulation in Vineyard Soils in New Zealand. Environ. Sci. 2004, 1, 139–167. [Google Scholar] [CrossRef] [Green Version]
- Bolan, N.S.; Khan, M.A.; Donaldson, J.; Adriano, D.C.; Matthew, C. Distribution and bioavailability of copper in farm effluent. Sci. Total Environ. 2003, 309, 225–236. [Google Scholar] [CrossRef]
- Fernández, D.; Voss, K.; Bundschuh, M.; Zubrod, J.P.; Schäfer, R.B. Effects of fungicides on decomposer communities and litter decomposition in vineyard streams. Sci. Total. Environ. 2015, 533, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Bereswill, R.; Golla, B.; Streloke, M.; Schulz, R. Entry and toxicity of organic pesticides and copper in vineyard streams: Erosion rills jeopardise the efficiency of riparian buffer strips. Agric. Ecosyst. Environ. 2012, 146, 81–92. [Google Scholar] [CrossRef]
- Pennington, S.L.; Webster-Brown, J.G. Stormwater runoff quality from copper roofing, Auckland, New Zealand. N. Z. J. Marit. Freshw. Res. 2008, 42, 99–108. [Google Scholar] [CrossRef] [Green Version]
- ANZECC/ARMCANZ. Australian and New Zealand Guidelines for Fresh and Marine Water Quality; Australian and New Zealand Environment and Conservation Council and Agriculture and Resource Management Council of Australia and New Zealand: Canberra, Australia, 2000; Volume 1, pp. 1–314. [Google Scholar]
- Ahlf, W.; Drost, W.; Heise, S. Incorporation of metal bioavailability into regulatory frameworks—Metal exposure in water and sediment. J. Soils Sediments 2009, 9, 411–419. [Google Scholar] [CrossRef]
- Magalhães, C.; Costa, J.; Teixeira, C.; Bordalo, A.A. Impact of trace metals on denitrification in estuarine sediments of the Douro River estuary, Portugal. Mar. Chem. 2007, 107, 332–341. [Google Scholar] [CrossRef]
- Sakadevan, K.; Zheng, H.; Bavor, H. Impact of heavy metals on denitrification in surface wetland sediments receiving wastewater. Water Sci. Technol. 1999, 40, 349–355. [Google Scholar] [CrossRef]
- Vázquez-Blanco, R.; Arias-Estévez, M.; Bååth, E.; Fernández-Calviño, D. Comparison of Cu salts and commercial Cu based fungicides on toxicity towards microorganisms in soil. Environ. Pollut. 2019, 257, 113585. [Google Scholar] [CrossRef]
- Sutcliffe, B.; Hose, G.; Harford, A.; Midgley, D.; Greenfield, P.; Paulsen, I.; Chariton, A. Microbial communities are sensitive indicators for freshwater sediment copper contamination. Environ. Pollut. 2019, 247, 1028–1038. [Google Scholar] [CrossRef]
- Froelich, P.; Klinkhammer, G.; Bender, M.; Luedtke, N.; Heath, G.; Cullen, D.; Dauphin, P.; Hammond, D.; Hartman, B.; Maynard, V. Early oxidation of organic matter in pelagic sediments of the eastern equatorial Atlantic—Suboxic diagenesis. Geochim. Cosmochim. Acta 1979, 43, 1075–1090. [Google Scholar] [CrossRef]
- Chen, J.; Erler, D.V.; Wells, N.S.; Huang, J.; Welsh, D.T.; Eyre, B.D. Denitrification, anammox, and dissimilatory nitrate reduction to ammonium across a mosaic of estuarine benthic habitats. Limnol. Oceanogr. 2021, 66, 1281–1297. [Google Scholar] [CrossRef]
- Morgan, T.K.K.B. Waiora and Cultural Identity: Water quality assessment using the Mauri Model. Altern. Int. J. Indig. Peoples 2006, 3, 42–67. [Google Scholar] [CrossRef]
- Land, Air, Water, Aotearoa (LAWA) (n.d.). LII Stream at Pannetts Road Bridge. Available online: https://www.lawa.org.nz/explore-data/canterbury-region/river-quality/ellesmere-waihora-catchment/lii-stream-at-pannetts-road-bridge/ (accessed on 25 March 2021).
- Simmler, M.; Ciadamidaro, L.; Schulin, R.; Madejón, P.; Reiser, R.; Clucas, L.; Weber, P.; Robinson, B. Lignite Reduces the Solubility and Plant Uptake of Cadmium in Pasturelands. Environ. Sci. Technol. 2013, 47, 4497–4504. [Google Scholar] [CrossRef]
- Clough, T.J.; Addy, K.; Kellogg, D.Q.; Nowicki, B.L.; Gold, A.J.; Groffman, P.M. Dynamics of nitrous oxide in groundwater at the aquatic-terrestrial interface. Glob. Chang. Biol. 2007, 13, 1528–1537. [Google Scholar] [CrossRef]
- Clough, T.; Kelliher, F.; Wang, Y.; Sherlock, R. Diffusion of 15N-labelled N2O into soil columns: A promising method to examine the fate of N2O in subsoils. Soil Biol. Biochem. 2006, 38, 1462–1468. [Google Scholar] [CrossRef]
- Zhang, H.; Davison, W.; Miller, S.; Tych, W. In situ high resolution measurements of fluxes of Ni, Cu, Fe, and Mn and concentrations of Zn and Cd in porewaters by DGT. Geochim. Cosmochim. Acta 1995, 59, 4181–4192. [Google Scholar] [CrossRef]
- Huang, J.; Franklin, H.; Teasdale, P.R.; Burford, M.A.; Kankanamge, N.R.; Bennett, W.W.; Welsh, D.T. Comparison of DET, DGT and conventional porewater extractions for determining nutrient profiles and cycling in stream sediments. Environ. Sci. Process. Impacts 2019, 21, 2128–2140. [Google Scholar] [CrossRef]
- Jolley, D.F.; Mason, S.; Gao, Y.; Zhang, H. Practicalities of working with DGT. Diffusive Gradients in Thin Films for Environmental Measurements. In Diffusive Gradients in Thin-Films for Environmental Measurements; Davison, W., Ed.; Cambridge University Press: Cambridge, UK, 2016; pp. 263–290. [Google Scholar]
- Huang, J.; Bennett, W.W.; Welsh, D.T.; Li, T.; Teasdale, P.R. Development and evaluation of a diffusive gradients in a thin film technique for measuring ammonium in freshwaters. Anal. Chim. Acta 2016, 904, 83–91. [Google Scholar] [CrossRef]
- Huang, J.; Bennett, W.W.; Teasdale, P.R.; Kankanamge, N.R.; Welsh, D.T. A modified DGT technique for the simultaneous measurement of dissolved inorganic nitrogen and phosphorus in freshwaters. Anal. Chim. Acta 2017, 988, 17–26. [Google Scholar] [CrossRef]
- Garmo, A.; Røyset, O.; Steinnes, E.; Flaten, T.P. Performance Study of Diffusive Gradients in Thin Films for 55 Elements. Anal. Chem. 2003, 75, 3573–3580. [Google Scholar] [CrossRef]
- Lehto, N.J. Principles and Application in Soils and Sediments. In Diffusive Gradients in Thin-Films for Environmental Measurements; Davison, W., Ed.; Cambridge University Press: Cambridge, UK, 2016; pp. 146–173. [Google Scholar]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Hantush, M.M. Modeling nitrogen-carbon cycling and oxygen consumption in bottom sediments. Adv. Water Resour. 2007, 30, 59–79. [Google Scholar] [CrossRef]
- Magalhães, C.M.; Machado, A.; Matos, P.; Bordalo, A.A. Impact of copper on the diversity, abundance and transcription of nitrite and nitrous oxide reductase genes in an urban European estuary. FEMS Microbiol. Ecol. 2011, 77, 274–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holtan-Hartwig, L.; Bechmann, M.; Høyås, T.R.; Linjordet, R.; Bakken, L.R. Heavy metals tolerance of soil denitrifying communities: N2O dynamics. Soil Biol. Biochem. 2002, 34, 1181–1190. [Google Scholar] [CrossRef]
- Guo, Q.; Li, N.; Bing, Y.; Chen, S.; Zhang, Z.; Chang, S.; Chen, Y.; Xie, S. Denitrifier communities impacted by heavy metal contamination in freshwater sediment. Environ. Pollut. 2018, 242, 426–432. [Google Scholar] [CrossRef]
- Felgate, H.; Giannopoulos, G.; Sullivan, M.J.; Gates, A.J.; Clarke, T.A.; Baggs, E.; Rowley, G.; Richardson, D.J. The impact of copper, nitrate and carbon status on the emission of nitrous oxide by two species of bacteria with biochemically distinct denitrification pathways. Environ. Microbiol. 2012, 14, 1788–1800. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Gates, A.J.; Appia-Ayme, C.; Rowley, G.; Richardson, D.J. Copper control of bacterial nitrous oxide emission and its impact on vitamin B12-dependent metabolism. Proc. Natl. Acad. Sci. USA 2013, 110, 19926–19931. [Google Scholar] [CrossRef] [Green Version]
- Giannopoulos, G.; Hartop, K.R.; Brown, B.L.; Song, B.; Elsgaard, L.; Franklin, R.B. Trace Metal Availability Affects Greenhouse Gas Emissions and Microbial Functional Group Abundance in Freshwater Wetland Sediments. Front. Microbiol. 2020, 11, 560861. [Google Scholar] [CrossRef]
- Bakker, E.A.H.; Vizza, C.; Arango, C.P.; Roley, S.S. Nitrogen fixation rates in forested mountain streams: Are sediment microbes more important than previously thought? Freshw. Biol. 2022, 67, 1395–1410. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Niu, L.; Zhang, W.; Zhang, H.; Wang, L.; Wang, P. Response of ammonia oxidizing archaea and bacteria to decabromodiphenyl ether and copper contamination in river sediments. Chemosphere 2018, 191, 858–867. [Google Scholar] [CrossRef]
- Schaedler, F.; Lockwood, C.; Lueder, U.; Glombitza, C.; Kappler, A.; Schmidt, C. Microbially Mediated Coupling of Fe and N Cycles by Nitrate-Reducing Fe(II)-Oxidizing Bacteria in Littoral Freshwater Sediments. Appl. Environ. Microbiol. 2018, 84, e02013-17. [Google Scholar] [CrossRef] [Green Version]
- Ottley, C.; Davison, W.; Edmunds, W. Chemical catalysis of nitrate reduction by iron (II). Geochim. Cosmochim. Acta 1997, 61, 1819–1828. [Google Scholar] [CrossRef]
- Picardal, F. Abiotic and microbial interactions during anaerobic transformations of Fe (II) and NOx. Front. Microbiol. 2012, 3, 112. [Google Scholar] [CrossRef] [Green Version]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Tai, Y.-L.; Dempsey, B.A. Nitrite reduction with hydrous ferric oxide and Fe(II): Stoichiometry, rate, and mechanism. Water Res. 2009, 43, 546–552. [Google Scholar] [CrossRef]
- Li, X.; Sardans, J.; Hou, L.; Gao, D.; Liu, M.; Peñuelas, J. Dissimilatory Nitrate/Nitrite Reduction Processes in River Sediments Across Climatic Gradient: Influences of Biogeochemical Controls and Climatic Temperature Regime. J. Geophys. Res. Biogeosciences 2019, 124, 2305–2320. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Yuan, X.; Zhao, W.; Luo, X.; Li, F.; Liu, T. Chemodenitrification by Fe(II) and nitrite: pH effect, mineralization and kinetic modeling. Chem. Geol. 2020, 541, 119586. [Google Scholar] [CrossRef]
- Jones, L.C.; Peters, B.; Pacheco, J.S.L.; Casciotti, K.L.; Fendorf, S. Stable Isotopes and Iron Oxide Mineral Products as Markers of Chemodenitrification. Environ. Sci. Technol. 2015, 49, 3444–3452. [Google Scholar] [CrossRef]
- Woollard-Shore, J.G.; Holland, J.P.; Jones, M.W.; Dilworth, J.R. Nitrite reduction by copper complexes. Dalton Trans. 2010, 39, 1576–1585. [Google Scholar] [CrossRef]
- Mondal, A.; Reddy, K.P.; Bertke, J.A.; Kundu, S. Phenol Reduces Nitrite to NO at Copper(II): Role of a Proton-Responsive Outer Coordination Sphere in Phenol Oxidation. J. Am. Chem. Soc. 2020, 142, 1726–1730. [Google Scholar] [CrossRef]
- Tipping, E.; Lofts, S.; Sonke, J. Humic Ion-Binding Model VII: A revised parameterisation of cation-binding by humic substances. Environ. Chem. 2011, 8, 225–235. [Google Scholar] [CrossRef] [Green Version]
- Burgin, A.J.; Hamilton, S.K. Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Front. Ecol. Environ. 2007, 5, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.F.; Smith, C.J.; Papaspyrou, S.; Stott, A.; Osborn, A.M.; Nedwell, D.B. Changes in Benthic Denitrification, Nitrate Ammonification, and Anammox Process Rates and Nitrate and Nitrite Reductase Gene Abundances along an Estuarine Nutrient Gradient (the Colne Estuary, United Kingdom). Appl. Environ. Microbiol. 2009, 75, 3171–3179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, E.K.; Thamdrup, B. The fate of nitrogen is linked to iron(II) availability in a freshwater lake sediment. Geochim. Et Cosmochim. Acta 2017, 205, 84–99. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.-Q.; Yang, G.-F.; Wang, H.; Wu, K.; Jin, R.-C.; Zheng, P. Estimating the recovery of ANAMMOX performance from inhibition by copper (II) and oxytetracycline (OTC). Sep. Purif. Technol. 2013, 113, 90–103. [Google Scholar] [CrossRef]
- Mak, C.; Lin, J.; Bashir, M. An overview of the effects of heavy metals content in wastewater on anammox bacteria. Adv. Environ. Stud. 2018, 2, 61–70. [Google Scholar]
- Zhang, Z.-Z.; Deng, R.; Cheng, Y.-F.; Zhou, Y.-H.; Buayi, X.; Zhang, X.; Wang, H.-Z.; Jin, R.-C. Behavior and fate of copper ions in an anammox granular sludge reactor and strategies for remediation. J. Hazard. Mater. 2015, 300, 838–846. [Google Scholar] [CrossRef]
- Wan, L.; Liu, H.; Wang, X. Anaerobic ammonium oxidation coupled to Fe(III) reduction: Discovery, mechanism and application prospects in wastewater treatment. Sci. Total. Environ. 2021, 818, 151687. [Google Scholar] [CrossRef]
- Davison, W. Iron and manganese in lakes. Earth-Sci. Rev. 1993, 34, 119–163. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Yao, Z.; Wang, F.; Wang, C.; Xu, H.; Jiang, H. Anaerobic ammonium oxidation coupled to ferric iron reduction in the sediment of a eutrophic lake. Environ. Sci. Pollut. Res. 2019, 26, 15084–15094. [Google Scholar] [CrossRef]
- Huang, S.; Chen, C.; Peng, X.; Jaffé, P.R. Environmental factors affecting the presence of Acidimicrobiaceae and ammonium removal under iron-reducing conditions in soil environments. Soil Biol. Biochem. 2016, 98, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Yang, F.; Gong, Z.; Meng, F.; Chen, H.; Xue, Y.; Furukawa, K. Application of anaerobic ammonium-oxidizing consortium to achieve completely autotrophic ammonium and sulfate removal. Bioresour. Technol. 2008, 99, 6817–6825. [Google Scholar] [CrossRef]
- Rios-del Toro, E.E.; Cervantes, F.J. Coupling between anammox and autotrophic denitrification for simultaneous removal of ammonium and sulfide by enriched marine sediments. Biodegradation 2016, 27, 107–118. [Google Scholar] [CrossRef]
- Rios-del Toro, E.E.; Valenzuela, E.I.; López-Lozano, N.E.; Cortés-Martínez, M.G.; Sánchez-Rodríguez, M.A.; Calvario-Martínez, O.; Sánchez-Carrillo, S.; Cervantes, F.J. Anaerobic ammonium oxidation linked to sulfate and ferric iron reduction fuels nitrogen loss in marine sediments. Biodegradation 2018, 29, 429–442. [Google Scholar] [CrossRef]
- Morse, J.W.; Luther, G., III. Chemical influences on trace metal-sulfide interactions in anoxic sediments. Geochim. Cosmochim. Acta 1999, 63, 3373–3378. [Google Scholar] [CrossRef]
- Lehto, N.J.; Glud, R.N.; Norði, G.; Zhang, H.; Davison, W. Anoxic microniches in marine sediments induced by aggregate settlement: Biogeochemical dynamics and implications. Biogeochemistry 2014, 119, 307–327. [Google Scholar] [CrossRef]
- Clough, T.J.; Kelliher, F.M. Nitrous Oxide Emission from Waterways. New Zealand Ministry for Primary Industries Technical Paper No: 2013/05; 2013. Available online: https://www.mpi.govt.nz/dmsdocument/2960/direct (accessed on 25 March 2021).
- Woodward, K.B.; Fellows, C.S.; Conway, C.L.; Hunter, H.M. Nitrate removal, denitrification and nitrous oxide production in the riparian zone of an ephemeral stream. Soil Biol. Biochem. 2009, 41, 671–680. [Google Scholar] [CrossRef]
- Santner, J.; Williams, P.N. Measurement at High Spatial Resolution. In Diffusive Gradients in Thin-Films for Environmental Measurements; Davison, W., Ed.; Cambridge University Press: Cambridge, UK, 2016; pp. 174–215. [Google Scholar] [CrossRef]
- Lehto, N.J.; Davison, W.; Zhang, H. The use of ultra-thin diffusive gradients in thin-films (DGT) devices for the analysis of trace metal dynamics in soils and sediments: A measurement and modelling approach. Environ. Chem. 2012, 9, 415–423. [Google Scholar] [CrossRef]

| Sediment Treatment * | |||
|---|---|---|---|
| Tc | T5 | T20 | |
| Moisture (%) | 40 ± 0 | - | - |
| Organic matter (%) | 1.61 ± 0.39 | - | - |
| Total carbon (%) | 0.93 ± 0.22 | - | - |
| Total nitrogen (%) | 0.41 ± 0.01 | - | - |
| P (mg kg−1) | 475.58 ± 9.10 | - | - |
| S (mg kg−1) | 349.18 ± 26.18 | - | - |
| Exchangeable pH | 6.67 ± 0.02 a | 6.43 ± 0.13 a | 6.57 ± 0.16 a |
| Cu (mg kg−1) | 6.22 ± 0.26 c | 33.14 ± 1.35 b | 119.66 ± 1.40 a |
| Fe (mg kg−1) | 13 235 ± 345 a | 13 261 ± 41 a | 13 169 ± 85 a |
| Mn (mg kg−1) | 153.17 ± 1.28 a | 151.18 ± 0.17 a | 148 ± 3.19 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomoiye, T.; Huang, J.; Lehto, N.J. Copper Contamination Affects the Biogeochemical Cycling of Nitrogen in Freshwater Sediment Mesocosms. Sustainability 2023, 15, 9958. https://doi.org/10.3390/su15139958
Tomoiye T, Huang J, Lehto NJ. Copper Contamination Affects the Biogeochemical Cycling of Nitrogen in Freshwater Sediment Mesocosms. Sustainability. 2023; 15(13):9958. https://doi.org/10.3390/su15139958
Chicago/Turabian StyleTomoiye, Tomson, Jianyin Huang, and Niklas J. Lehto. 2023. "Copper Contamination Affects the Biogeochemical Cycling of Nitrogen in Freshwater Sediment Mesocosms" Sustainability 15, no. 13: 9958. https://doi.org/10.3390/su15139958






