Investigation of the Main Parameters Influencing the Kinetics of an Ammonia Stripping Plant Treating Swine Digestate
Abstract
:1. Introduction
2. Materials and Methods
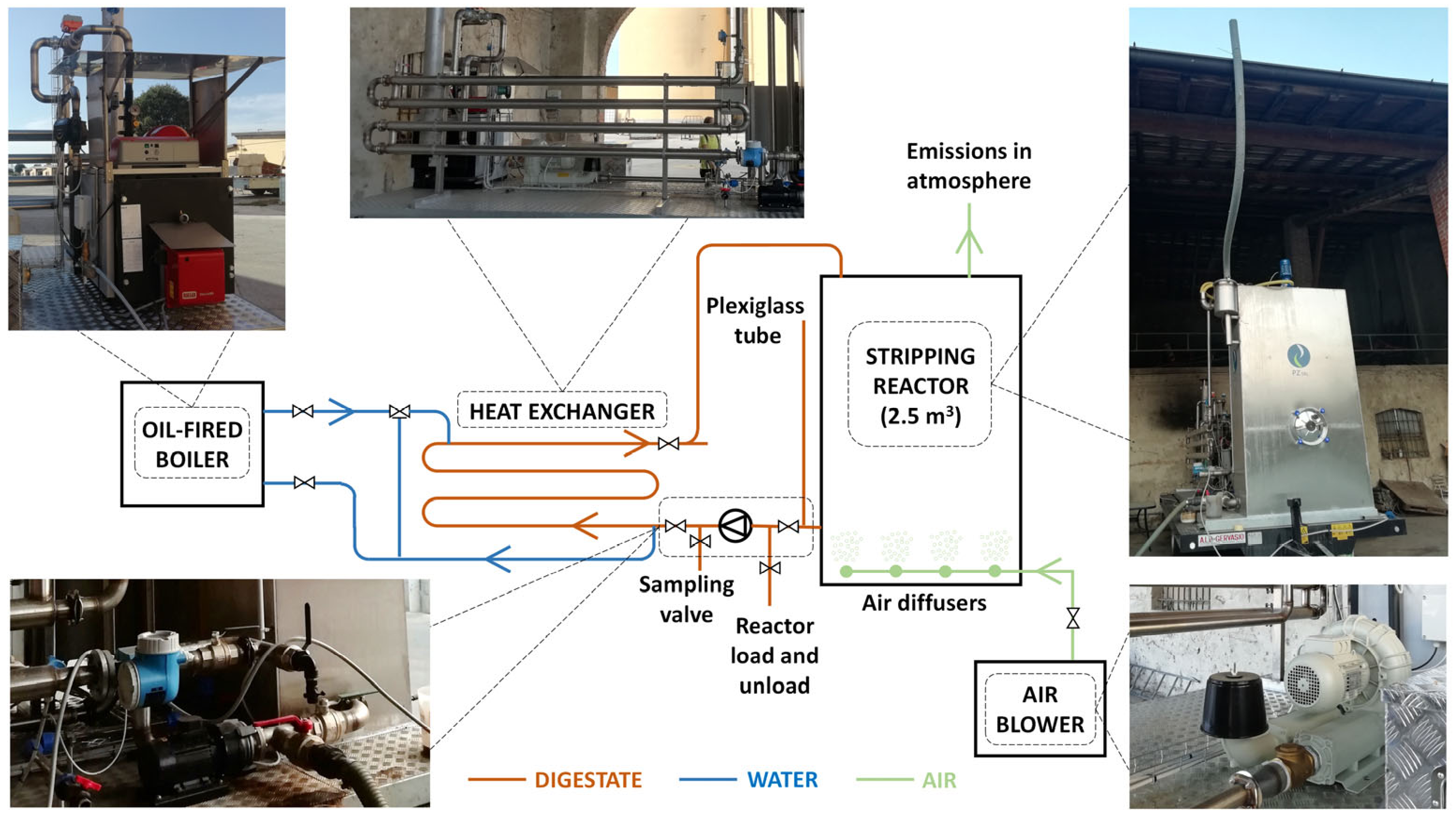
2.1. The Ammonia Stripping Pilot Plant
- -
- a stripping reactor;
- -
- an air blower;
- -
- a heating system (an oil-fired boiler and a tube-in-tube heat exchanger);
- -
- pipes and pumps for handling the digestate and heating water;
- -
- a process control and monitoring system.
2.2. Tested Conditions and Experimental Procedure
- the reactor was loaded with 1.5 m3 of digestate and the digestate level measured;
- the digestate circulation pump was switched on in order to homogenize the digestate content in the system;
- after 10 min, at time 0 h, one digestate sample was collected from the sampling valve;
- the test temperature and the airflow rate were set, and the boiler, water circulation pump and foam-breaking turbine were switched on;
- six digestate samples were collected from the tap at times: 0.5 h; 1 h; 2 h; 4 h; 6 h; 8 h; and 12 h. The temperature and flow rate of the water and digestate were monitored during the whole test;
- at the end of the test, the digestate level was measured again, to estimate the water loss from the system as a result of the release of water-saturated gas into the atmosphere.
2.3. Ammonia Stripping Efficiency
2.4. Modelling the Ammonia Decay
3. Results
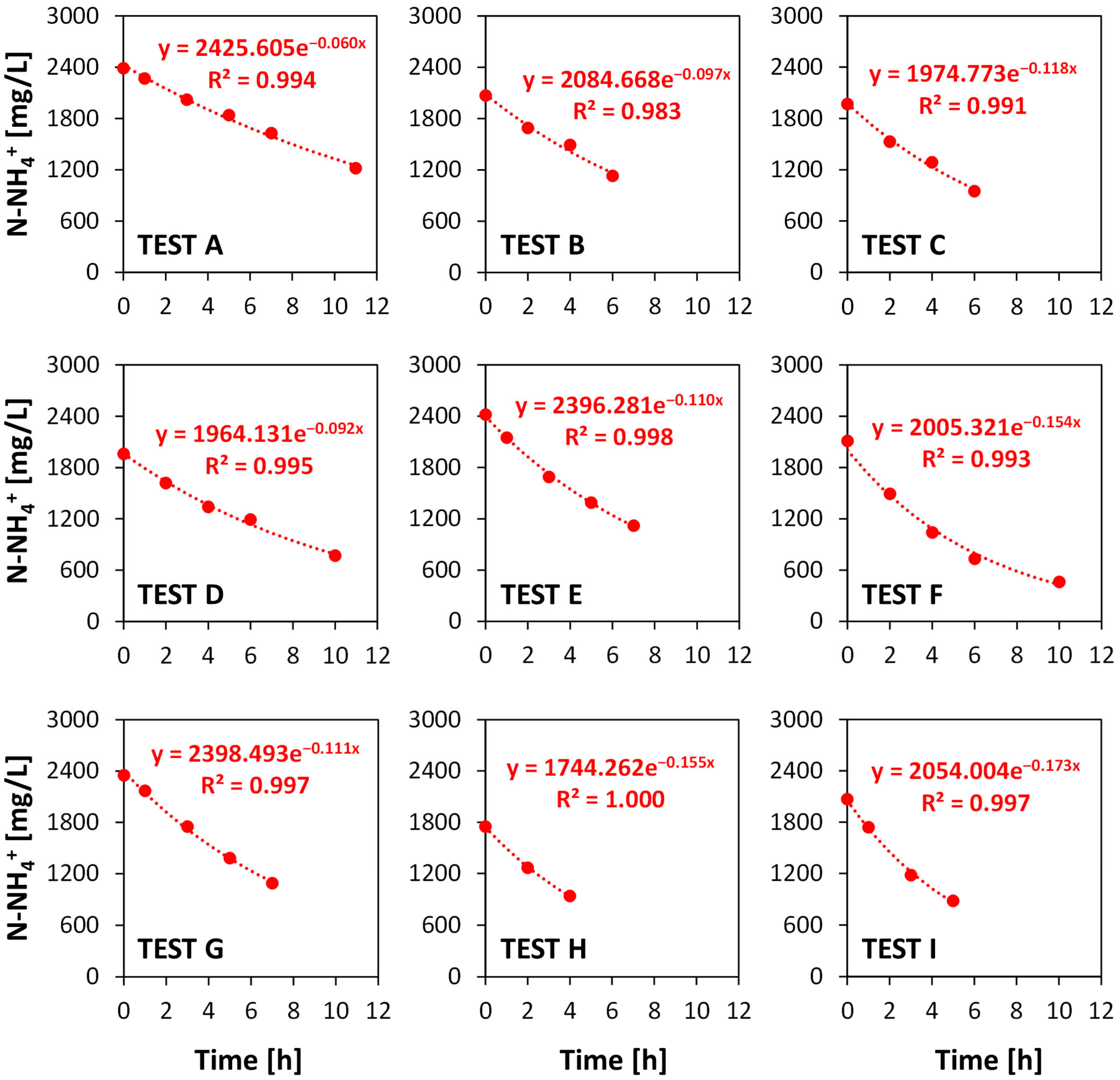
3.1. The Experimental Study
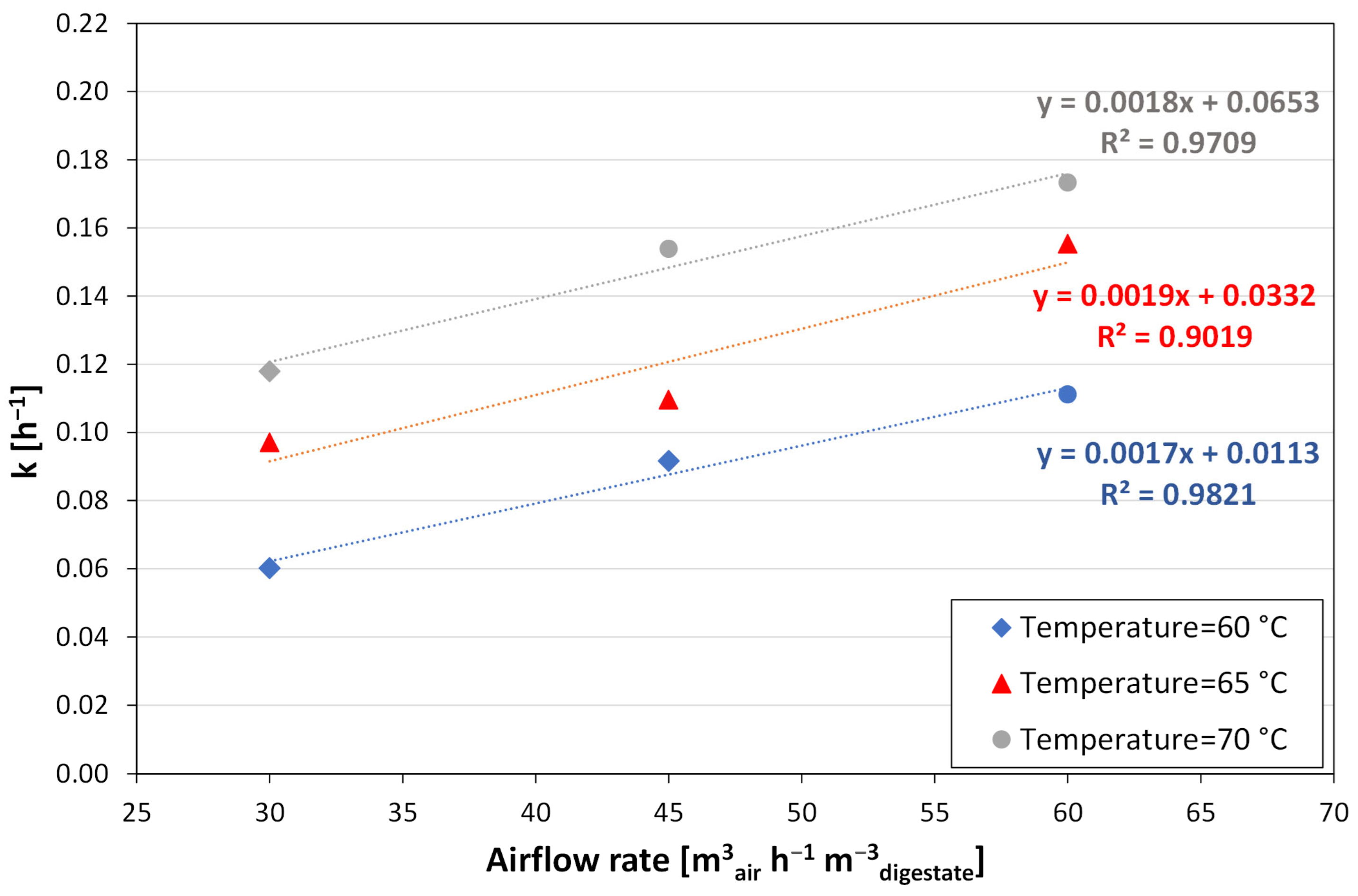
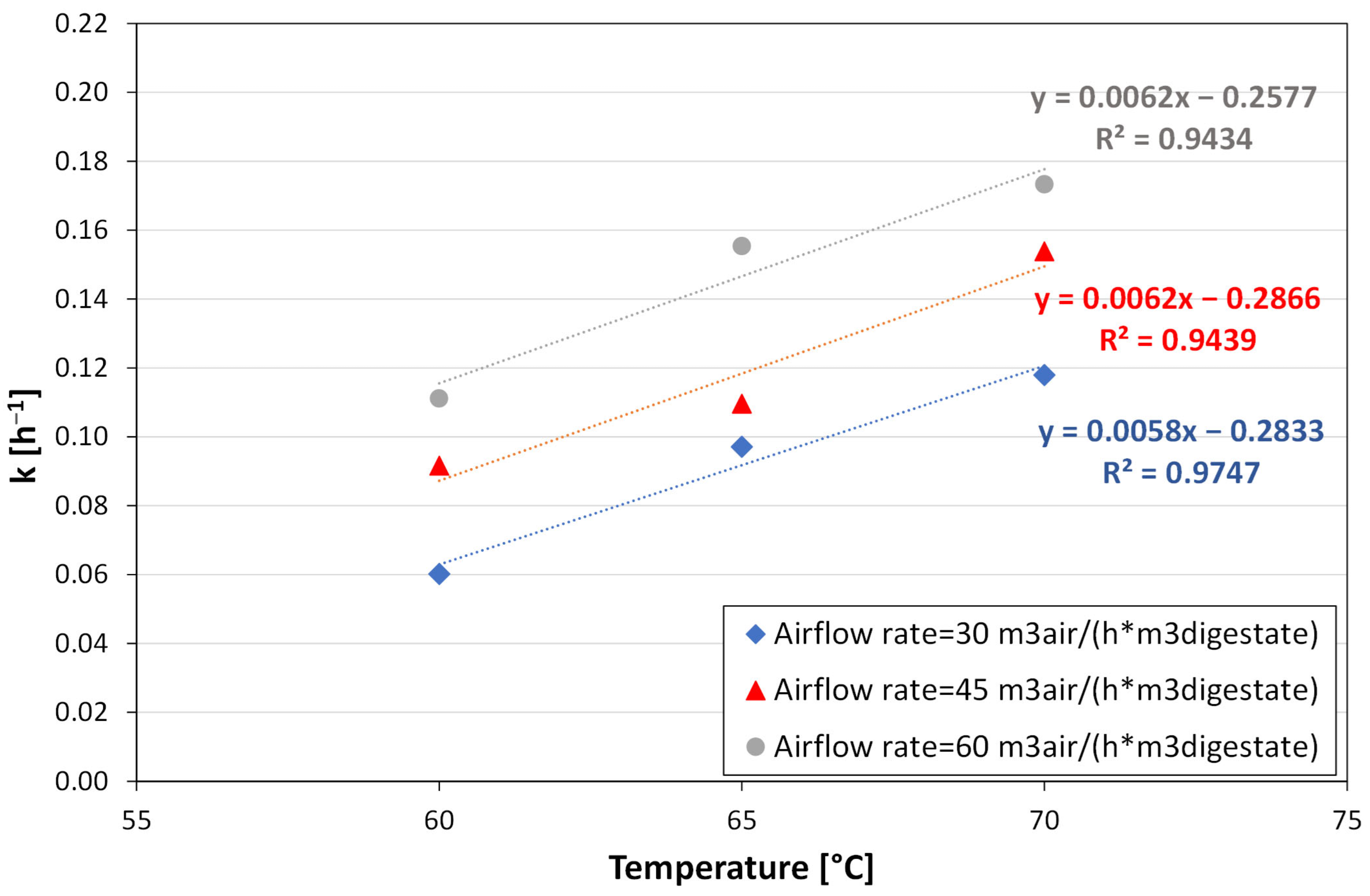
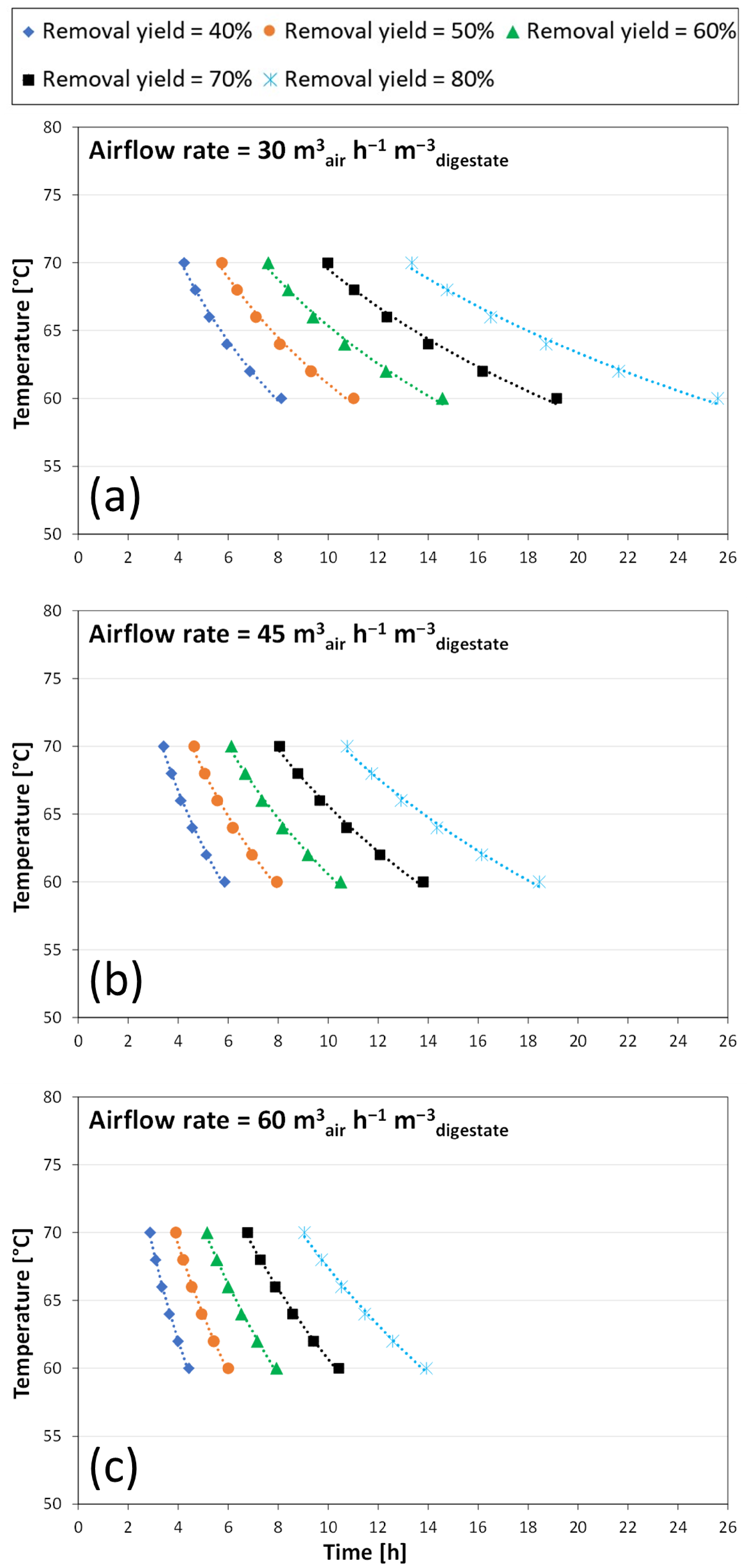
3.2. Modelling the Stripping Performance
- for airflow rates equal to 30 m3air h−1 m−3digestate
- for airflow rates equal to 45 m3air h−1 m−3digestate
- for airflow rates equal to 60 m3air h−1 m−3digestate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dadrasnia, A.; de Bona Muñoz, I.; Yáñez, E.H.; Lamkaddam, I.U.; Mora, M.; Ponsá, S.; Ahmed, M.; Argelaguet, L.L.; Williams, P.M.; Oatley-Radcliffe, D.L. Sustainable nutrient recovery from animal manure: A review of current best practice technology and the potential for freeze concentration. J. Clean. Prod. 2021, 315, 128106. [Google Scholar] [CrossRef]
- Rayne, N.; Aula, L. Livestock Manure and the Impacts on Soil Health: A Review. Soil Syst. 2020, 4, 64. [Google Scholar] [CrossRef]
- Loss, A.; Couto, R.D.R.; Brunetto, G.; Veiga, M.D.; Toselli, M.; Baldi, E. Animal manure as fertilizer: Changes in soil attributes, productivity and food composition. Int. J. Res. Granthaalayah 2019, 7, 307. [Google Scholar] [CrossRef]
- Cai, A.; Xu, M.; Wang, B.; Zhang, W.; Liang, G.; Hou, E.; Luo, Y. Manure acts as a better fertilizer for increasing crop yields than synthetic fertilizer does by improving soil fertility. Soil Tillage Res. 2019, 189, 168–175. [Google Scholar] [CrossRef]
- Stando, K.; Korzeniewska, E.; Felis, E.; Harnisz, M.; Bajkacz, S. Uptake of Pharmaceutical Pollutants and Their Metabolites from Soil Fertilized with Manure to Parsley Tissues. Molecules 2022, 27, 4378. [Google Scholar] [CrossRef] [PubMed]
- Malomo, G.A.; Madugu, A.S.; Bolu, S.A. Sustainable animal manure management strategies and practices. In Agricultural Waste and Residues; IntechOpen: London, UK, 2018; Volume 119. [Google Scholar]
- Rashmi, I.; Roy, T.; Kartika, K.S.; Pal, R.; Coumar, V.; Kala, S.; Shinoji, K.C. Organic and inorganic fertilizer contaminants in agriculture: Impact on soil and water resources. In Contaminants in Agriculture: Sources, Impacts and Management; Springer: Cham, Switzerland, 2020; pp. 3–41. [Google Scholar]
- Köninger, J.; Lugato, E.; Panagos, P.; Briones, M. Manure management and soil biodiversity: Towards more sustainable food systems in the EU. Agric. Syst. 2021, 194, 103251. [Google Scholar] [CrossRef]
- Huygens, D.; Orveillon, G.; Lugato, E.; Tavazzi, S.; Comero, S.; Jones, A.; Gawlik, B.; Saveyn, H. Technical Proposals for the Safe Use of Processed Manure above the Threshold Established for Nitrate Vulnerable Zones by the Nitrates Directive (91/676/EEC); Publications Office of the European Union: Luxembourg, 2020. [Google Scholar] [CrossRef]
- European Parliament and Council. Consolidated Text: Council Directive of 12 December 1991 Concerning the Protection of Waters against Pollution Caused by Nitrates from Agricultural Sources (91/676/EEC). 1991. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A01991L0676-20081211 (accessed on 6 November 2022).
- Decreto Ministeriale 19 Aprile 1999. Approvazione del Codice di Buona Pratica Agricola [Ministerial Decree 19 April 1999. Code of Good Agricultural Practice]. 1999. Available online: https://www.gazzettaufficiale.it/eli/id/1999/05/04/099A3435/sg (accessed on 19 May 2023).
- Decreto Legislativo 3 Aprile 2006, n. 152. Norme in Materia Ambientale [Legislative Decree 3 April 2006 n. 152. Environmental Regulation]. 2006. Available online: https://www.gazzettaufficiale.it/dettaglio/codici/materiaAmbientale (accessed on 19 May 2023).
- Decreto 25 Febbraio 2016 Criteri e Norme Tecniche Generali per la Disciplina Regionale Dell’utilizzazione Agronomica degli Effluenti di Allevamento e Delle Acque Reflue, Nonche’ Per la Produzione e L’utilizzazione Agronomica del Digestato [Decree of 25 February 2016. Criteria and General Technical Standards for the Regional Regulation of the Agronomic Use of Livestock Manure and Wastewater, and for the Production and Agronomic Use of Digestate]. 2016. Available online: https://www.gazzettaufficiale.it/eli/id/2016/04/18/16A02762/sg (accessed on 19 May 2023).
- Musacchio, A.; Re, V.; Mas-Pla, J.; Sacchi, E. EU Nitrates Directive, from theory to practice: Environmental effectiveness and influence of regional governance on its performance. Ambio 2020, 49, 504–516. [Google Scholar] [CrossRef] [Green Version]
- Pandey, B.; Chen, L. Technologies to recover nitrogen from livestock manure—A review. Sci. Total Environ. 2021, 784, 147098. [Google Scholar] [CrossRef]
- Yang, D.; Chen, Q.; Liu, R.; Song, L.; Zhang, Y.; Dai, X. Ammonia recovery from anaerobic digestate: State of the art, challenges and prospects. Bioresour. Technol. 2022, 363, 127957. [Google Scholar] [CrossRef]
- Baldi, M.; Collivignarelli, M.C.; Abbà, A.; Benigna, I. The Valorization of Ammonia in Manure Digestate by Means of Alternative Stripping Reactors. Sustainability 2018, 10, 3073. [Google Scholar] [CrossRef] [Green Version]
- Abbà, A.; Domini, M.; Baldi, M.; Pedrazzani, R.; Bertanza, G. Ammonia Recovery from Livestock Manure Digestate through an Air-Bubble Stripping Reactor: Evaluation of Performance and Energy Balance. Energies 2023, 16, 1643. [Google Scholar] [CrossRef]
- Limoli, A.; Langone, M.; Andreottola, G. Ammonia removal from raw manure digestate by means of a turbulent mixing stripping process. J. Environ. Manag. 2016, 176, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Collivignarelli, C.; Bertanza, G.; Baldi, M.; Avezzù, F. Ammonia stripping from MSW landfill leachate in bubble reactors: Process modeling and optimization. Waste Manag. Res. 1998, 16, 455–466. [Google Scholar] [CrossRef]
- Campos, J.C.; Moura, D.; Costa, A.P.; Yokoyama, L.; Araujo, F.V.D.F.; Cammarota, M.C.; Cardillo, L. Evaluation of pH, alkalinity and temperature during air stripping process for ammonia removal from landfill leachate. J. Environ. Sci. Health A 2013, 48, 1105–1113. [Google Scholar] [CrossRef]
- Avezzù, F.; Baldi, M.; Bertanza, G.; Collivignarelli, C. Ammonia stripping from high loaded wastewaters: Technical economical comparison of different plant configurations. In Advanced Wastewater Treatment, Recycling and Reuse; IWA Publishing: London, UK, 1998; pp. 943–946. [Google Scholar]
- Kim, E.J.; Kim, H.; Lee, E. Influence of ammonia stripping parameters on the efficiency and mass transfer rate of ammonia removal. Appl. Sci. 2021, 11, 441. [Google Scholar] [CrossRef]
- Kinidi, L.; Tan, I.A.W.; Abdul Wahab, N.B.; Tamrin, K.F.B.; Hipolito, C.N.; Salleh, S.F. Recent development in ammonia stripping process for industrial wastewater treatment. Int. J. Chem. Eng. 2018, 2018, 3181087. [Google Scholar] [CrossRef]
- Guštin, S.; Marinšek-Logar, R. Effect of pH, temperature and air flow rate on the continuous ammonia stripping of the anaerobic digestion effluent. Process Saf. Environ. Prot. 2011, 89, 61–66. [Google Scholar] [CrossRef]
- Değermenci, N.; Ata, O.N.; Yildız, E. Ammonia removal by air stripping in a semi-batch jet loop reactor. J. Ind. Eng. Chem. 2012, 18, 399–404. [Google Scholar] [CrossRef]
- Maghfiroh, M.; Park, N.; Chang, H.; Jung, J.; Ahn, K.; Lim, H.; Kim, W. Water spray reactor for ammonia removal via air stripping: An evaluation on mass transfer and process efficiency. J. Environ. Chem. Eng. 2022, 10, 108498. [Google Scholar] [CrossRef]
- Zhao, Q.B.; Ma, J.; Zeb, I.; Yu, L.; Chen, S.; Zheng, Y.M.; Frear, C. Ammonia recovery from anaerobic digester effluent through direct aeration. J. Chem. Eng. 2015, 279, 31–37. [Google Scholar] [CrossRef]
- Monfet, E.; Aubry, G.; Ramirez, A.A. Nutrient removal and recovery from digestate: A review of the technology. Biofuels 2018, 9, 247–262. [Google Scholar] [CrossRef]
| Test | T (°C) | Qair (m3air h−1 m−3digestate) |
|---|---|---|
| A | 60 | 30 |
| B | 65 | 30 |
| C | 70 | 30 |
| D | 60 | 45 |
| E | 65 | 45 |
| F | 70 | 45 |
| G | 60 | 60 |
| H | 65 | 60 |
| I | 70 | 60 |
| Parameter | Unit of Measurement | Value |
|---|---|---|
| pH | - | 8.2–9.1 |
| Ammoniacal nitrogen (N-NH4+) | (mg L−1) | 2090–2450 |
| Total Nitrogen (TN) | (mg L−1) | 2350–3050 |
| Total solids (TS) | (%) | 2.03–2.61 |
| Volatile solids (VS) | (%) | 1.17–1.68 |
| Total Phosphorus (TP) | (mg L−1) | 695–1095 |
| Test | Qair (m3air h−1 m−3digestate) | T (°C) | Parameter | Time (h) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 2 | 4 | 6 | 8 | 12 | ||||
| A | 30 | 60 | pH | 8.70 | 8.93 | 9.31 | 9.71 | 9.94 | 10.09 | 10.16 | 10.14 |
| N-NH4+ (mg L−1) | 1600 | 2350 | 2390 | 2270 | 2020 | 1840 | 1630 | 1220 | |||
| TN (mg L−1) | 2380 | 3050 | 3030 | 2960 | 2750 | 2430 | 2390 | 2050 | |||
| B | 65 | pH | 8.60 | 8.87 | 9.38 | 9.76 | 10.01 | 10.14 | 10.17 | 10.12 | |
| N-NH4+ (mg L−1) | 2070 | 2240 | 2160 | 2070 | 1690 | 1490 | 1130 | 540 | |||
| TN (mg L−1) | 3040 | 2640 | 2410 | 2380 | 2080 | 1900 | 1740 | 1330 | |||
| C | 70 | pH | 8.32 | 8.88 | 9.37 | 9.85 | 10.04 | 10.07 | 10.05 | 9.92 | |
| N-NH4+ (mg L−1) | 2200 | 2230 | 2150 | 1970 | 1530 | 1290 | 950 | 770 | |||
| TN (mg L−1) | 3020 | 2800 | 2830 | 2540 | 2210 | 2060 | 1580 | 1110 | |||
| D | 45 | 60 | pH | 8.20 | 8.82 | 9.30 | 9.78 | 9.80 | 9.98 | 9.91 | 9.85 |
| N-NH4+ (mg L−1) | 2100 | 2090 | 2320 | 1960 | 1620 | 1340 | 1190 | 770 | |||
| TN (mg L−1) | 2980 | 2830 | 2600 | 2120 | 2030 | 1850 | 1640 | 1080 | |||
| E | 65 | pH | 8.30 | 9.03 | 9.36 | 9.83 | 10.05 | 10.13 | 10.12 | 9.98 | |
| N-NH4+ (mg L−1) | 2440 | 2450 | 2420 | 2150 | 1690 | 1390 | 1120 | 850 | |||
| TN (mg L−1) | 3050 | 2980 | 2770 | 2490 | 2160 | 1870 | 1460 | 1160 | |||
| F | 70 | pH | 8.63 | 9.25 | 9.76 | 10.20 | 10.30 | 10.38 | 10.32 | 10.13 | |
| N-NH4+ (mg L−1) | 2580 | 2430 | 2410 | 2110 | 1490 | 1040 | 730 | 460 | |||
| TN (mg L−1) | 3190 | 3050 | 2830 | 2530 | 2080 | 1250 | 1100 | 970 | |||
| G | 60 | 60 | pH | 9.07 | 9.08 | 9.57 | 9.93 | 10.15 | 10.23 | 10.17 | n.a. |
| N-NH4+ (mg L−1) | 1120 | 2230 | 2350 | 2170 | 1750 | 1380 | 1090 | n.a. | |||
| TN (mg L−1) | 1760 | 2740 | 2820 | 2640 | 1920 | 1730 | 1360 | n.a. | |||
| H | 65 | pH | 9.12 | 8.82 | 9.15 | 9.59 | 9.93 | 10.11 | 10.01 | 9.85 | |
| N-NH4+ (mg L−1) | 1350 | 2290 | 2330 | 1970 | 1750 | 1270 | 940 | 640 | |||
| TN (mg L−1) | 1470 | 2350 | 2590 | 2250 | 1890 | 1520 | 1340 | 940 | |||
| I | 70 | pH | 8.38 | 9.40 | 9.82 | 10.04 | 10.11 | 9.88 | 9.50 | 8.63 | |
| N-NH4+ (mg L−1) | 2240 | 2250 | 2070 | 1740 | 1180 | 880 | 700 | 520 | |||
| TN (mg L−1) | 3130 | 2880 | 2600 | 2340 | 2030 | 1860 | 1750 | 1520 | |||
| Test | T (°C) | Qair (m3air h−1 m−3digestate) | Vlost (L) | N-NH4+ | TN | ||
|---|---|---|---|---|---|---|---|
| η (%) | η‘ (%) | η (%) | η‘ (%) | ||||
| A | 60 | 30 | 45 | 48.1 | 49.6 | 32.8 | 34.8 |
| B | 65 | 60 | 75.9 | 76.9 | 49.6 | 51.6 | |
| C | 70 | 76 | 65.5 | 67.2 | 60.4 | 62.4 | |
| D | 60 | 45 | 67 | 63.2 | 64.8 | 61.8 | 63.5 |
| E | 65 | 109 | 65.3 | 67.8 | 61.1 | 63.9 | |
| F | 70 | 210 | 81.1 | 83.7 | 68.2 | 72.6 | |
| G | 60 | 60 | 98 | 51.1 | 54.3 | 50.4 | 53.6 |
| H | 65 | 220 | 72.1 | 76.2 | 60.0 | 65.9 | |
| I | 70 | 260 | 76.9 | 80.9 | 47.2 | 56.4 | |
| Test | T (°C) | Qair (m3air h−1 m−3digestate) | pH0 | pH Average | (N-NH4+)0 (mg L−1) | k (h−1) |
|---|---|---|---|---|---|---|
| A | 60 | 30 | 8.70 | 10.00 | 2426 | 0.0602 |
| B | 65 | 8.60 | 10.04 | 2085 | 0.0971 | |
| C | 70 | 8.32 | 10.02 | 1975 | 0.1179 | |
| D | 60 | 45 | 8.20 | 9.88 | 1964 | 0.0916 |
| E | 65 | 8.30 | 9.99 | 2396 | 0.1096 | |
| F | 70 | 8.63 | 10.28 | 2005 | 0.1539 | |
| G | 60 | 60 | 9.07 | 10.09 | 2398 | 0.1111 |
| H | 65 | 9.12 | 10.04 | 1744 | 0.1554 | |
| I | 70 | 8.38 | 10.01 | 2054 | 0.1733 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbà, A.; Domini, M.; Baldi, M.; Collivignarelli, M.C.; Bertanza, G. Investigation of the Main Parameters Influencing the Kinetics of an Ammonia Stripping Plant Treating Swine Digestate. Sustainability 2023, 15, 10494. https://doi.org/10.3390/su151310494
Abbà A, Domini M, Baldi M, Collivignarelli MC, Bertanza G. Investigation of the Main Parameters Influencing the Kinetics of an Ammonia Stripping Plant Treating Swine Digestate. Sustainability. 2023; 15(13):10494. https://doi.org/10.3390/su151310494
Chicago/Turabian StyleAbbà, Alessandro, Marta Domini, Marco Baldi, Maria Cristina Collivignarelli, and Giorgio Bertanza. 2023. "Investigation of the Main Parameters Influencing the Kinetics of an Ammonia Stripping Plant Treating Swine Digestate" Sustainability 15, no. 13: 10494. https://doi.org/10.3390/su151310494






