Application of Synthesized Biomass Bamboo Charcoal–Iron Oxide “BC/Fe” Nanocomposite Adsorbents in the Removal of Cationic Methylene Blue Dye Contaminants from Wastewater by Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Adsorbent Magnetic “BC/Fe” Bamboo Charcoa–Iron Oxide Nanocomposite Materials Synthesis and Characterization
2.3. Batch Kinetics and Equilibrium Adsorption Experimental Procedure
2.4. Adsorption Kinetics and Equilibrium Adsorption Isotherm Data Analysis
3. Results and Discussion
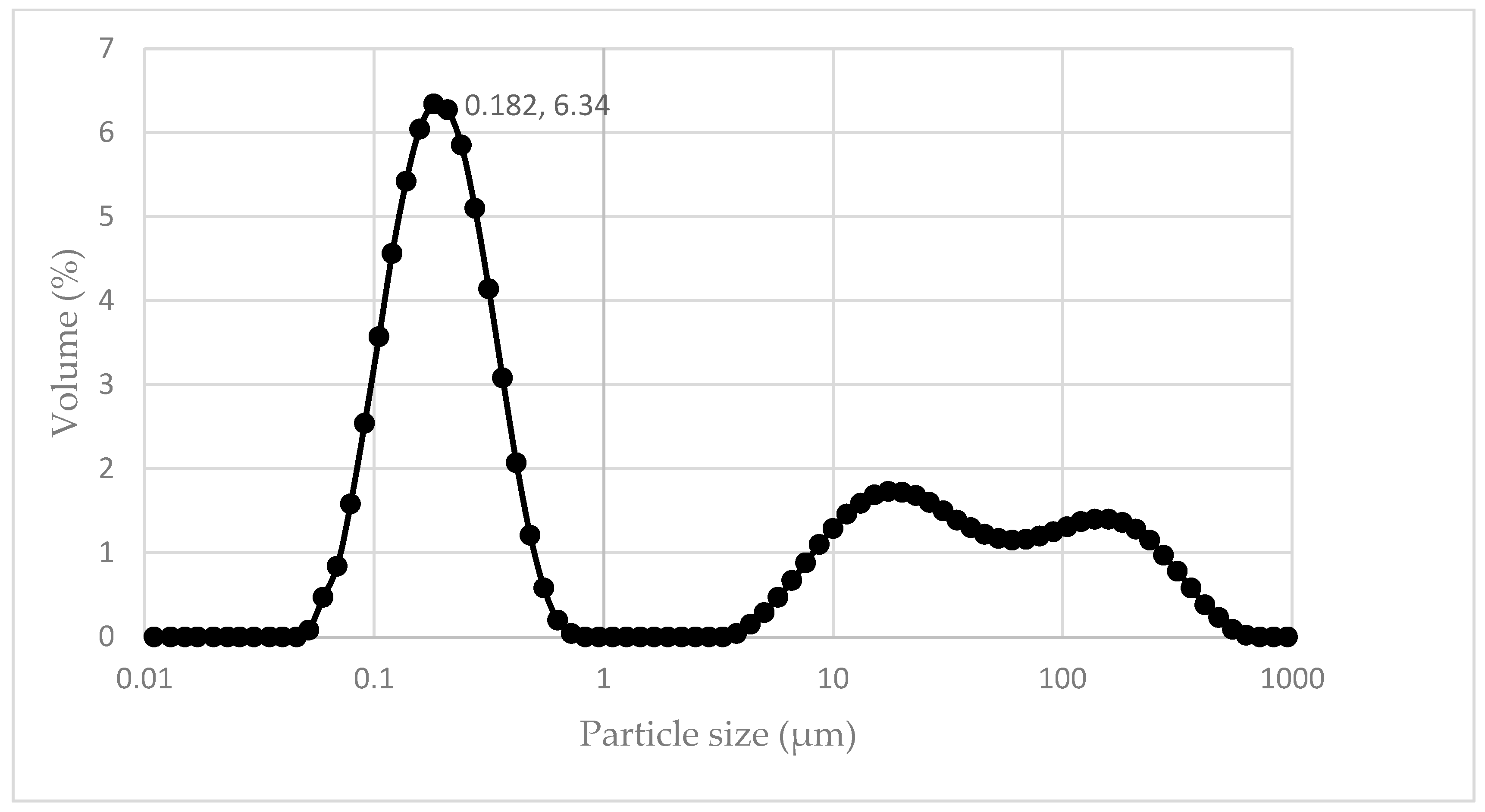
3.1. Characteristics of Synthesized Adsorbent Magnetic “BC/Fe” Bamboo Charcoa–Iron Oxide Nanocomposite Materials
3.2. Studies on MB Dye Adsorption Kinetics by Magnetic “BC/Fe” Bamboo Charcoa–Iron Oxide (BC/Fe) under Different Process Conditions
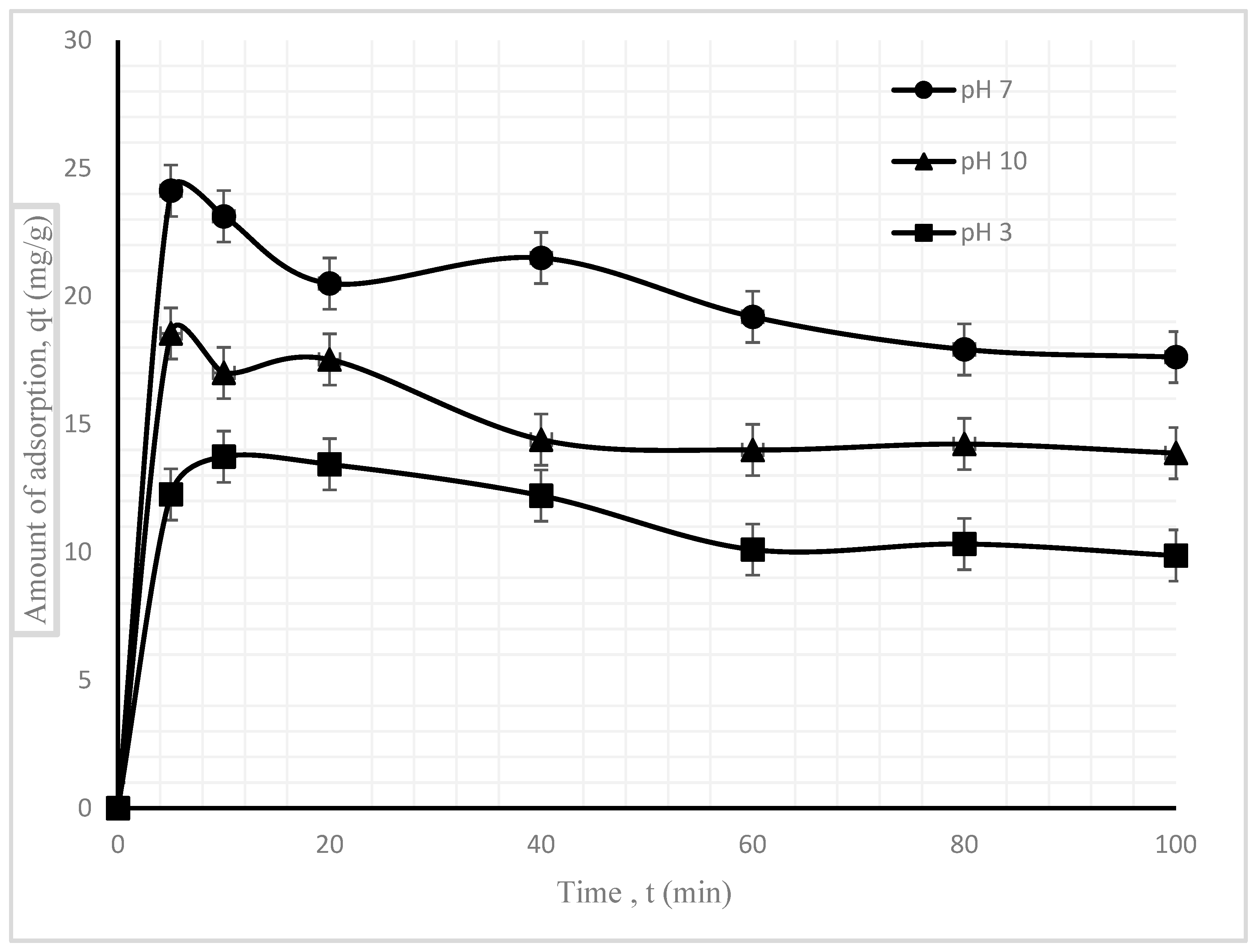
3.2.1. Effect of Solution pH
3.2.2. Effect of Contact Time and Adsorbate MB Dye Concentration and Adsorption Kinetics
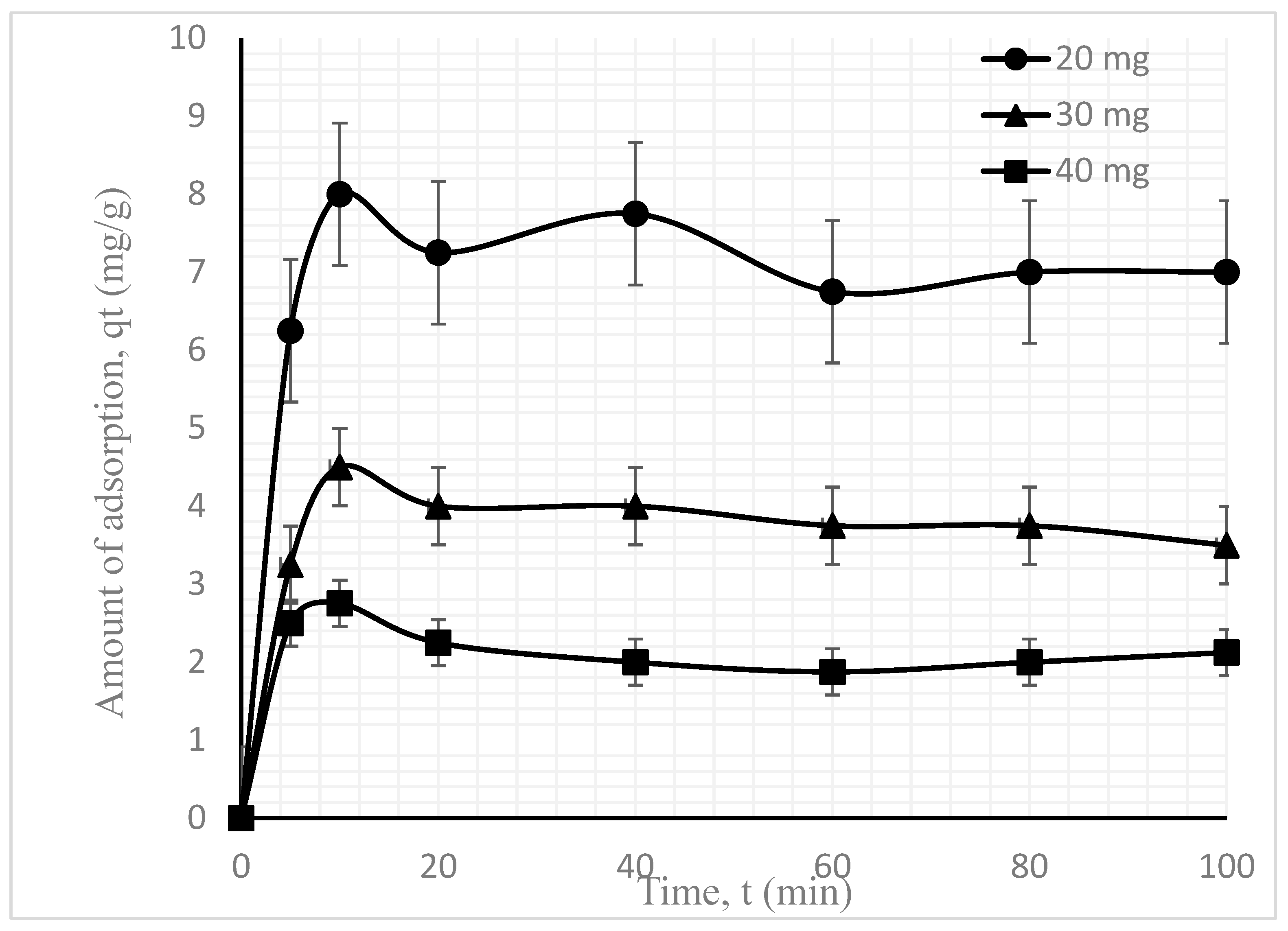
3.2.3. MB Dye Adsorption Kinetics under Varying Adsorbent Doses
3.2.4. Effect of Solution Temperature
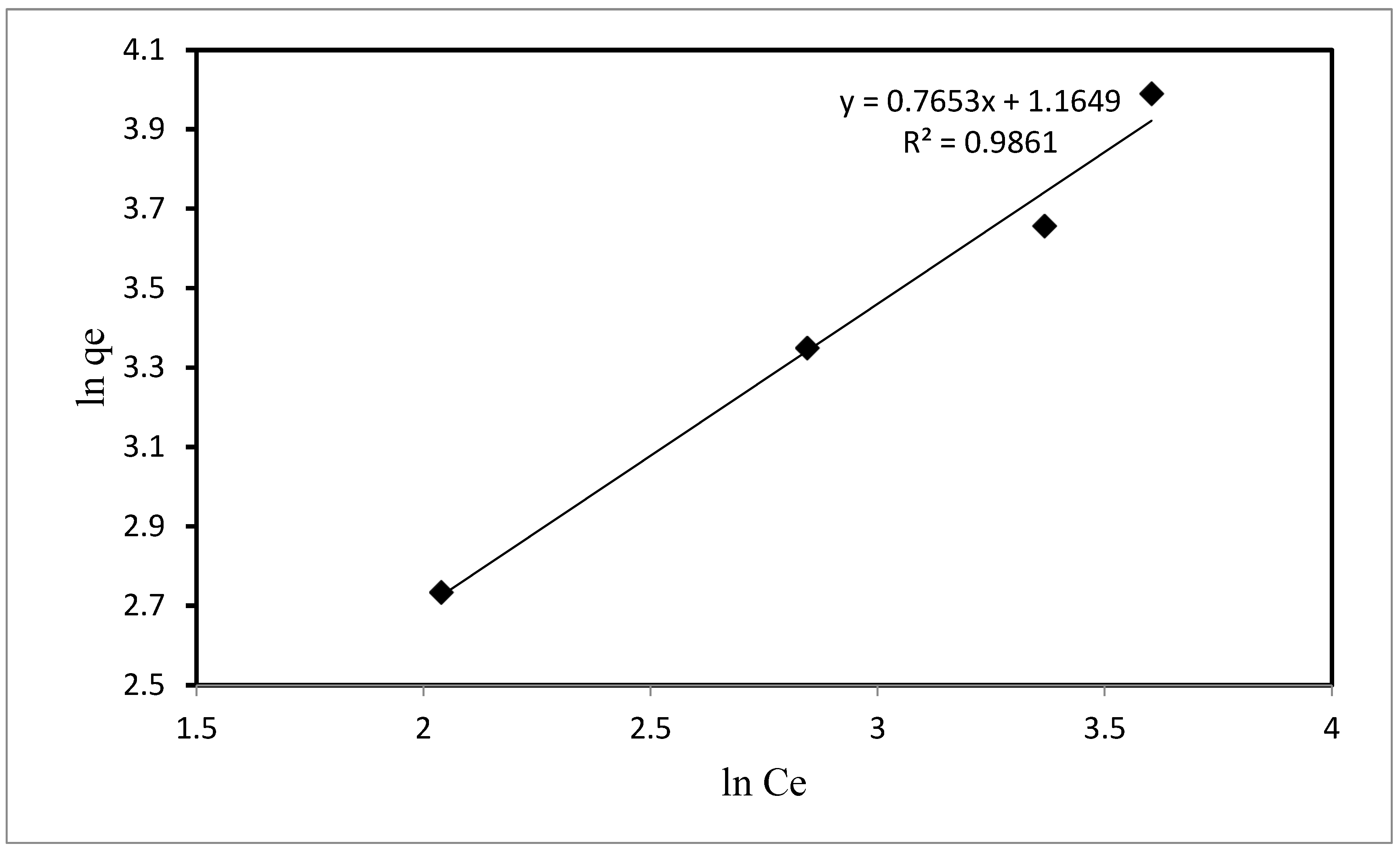
3.3. Mechanism of Adsorption and Isotherm Studies
4. Recovery, Regeneration by Desorption, Reusability, and Limitations of This Present Work
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karaer, H.; Kaya, I. Synthesis, characterization of magnetic chitosan/active charcoal composite and using at the adsorption of methylene blue and reactive blue4. Microporous Mesoporous Mater. 2016, 232, 26–38. [Google Scholar] [CrossRef]
- Buthelezi, P.; Olaniran, A.; Pillay, B. Textile dye removal from wastewater effluents using bioflocculants produced by indigenous bacterial isolates. Molecules 2012, 17, 14260–14274. [Google Scholar] [CrossRef] [PubMed]
- Yagub, M.T.; Sen, T.K.; Afroze, A.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Sivashankar, R.; Sathya, A.B.; Vasantharaj, K.; Sivasubramanian, V. Magnetic composite an environmental super adsorbent for dye sequestration-A review. Environ. Nanotechnol. Monit. Manag. 2014, 1–2, 36–49. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Ang, H.M. Equilibrium, kinetics, and thermodynamics of methylene blue adsorption by pine tree leaves. Water Air Soil Pollut. 2012, 223, 5267–5282. [Google Scholar] [CrossRef]
- Bo, Q.; Lee, C.; Yoo, J.S.; Piao, Y. Facile scalable synthesis of highly monodisperse small silica nanoparticles using alkaline buffer solution and their application for efficient sentinel lymph node mapping. J. Mater. Chem. B 2017, 5, 586. [Google Scholar]
- Afroze, S.; Sen, T.K. A review on heavy metal ions and dye adsorption from water by agriculturalsSolid waste adsorbents. Water Air Soil Pollut. 2018, 229, 225. [Google Scholar] [CrossRef]
- Amar, I.A.; Sharif, A.; Alkhayali, M.M.; Jabji, M.A.; Altohami, F.; Abdul Qadir, M.A.; Ahwid, M.A. Adsorptive removal of methylene blue dye from aqueous solutions using CoFe1.9Mo0.1O4 magnetic nanoparticles. Iran. J. Energy Environ. 2018, 9, 247–254. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and application of granular activated carbon from biomass waste materials for water treatment: A review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Obey, G.; Adelaide, M.; Ramaraj, R. Biochar derived from non-customized matamba fruit shell as an adsorbent for wastewater treatment. J. Bioresour. Bioprod. 2022, 7, 109–115. [Google Scholar] [CrossRef]
- Gan, C.; Liu, Y.G.; Tan, X.F.; Wang, S.F.; Zeng, G.M.; Zheng, B.H.; Liu, W. Effect porous zinc-biochar nanocomposite on Cr (vi) adsorption from aqueous solution. RSC Adv. 2015, 5, 35107–35115. [Google Scholar] [CrossRef]
- Liu, S.J.; Liu, Y.G.; Tan, X.F.; Liu, S.B.; Li, M.F.; Liu, N.; Yin, Z.H.; Tian, S.R.; Zhou, Y.H. Facile synthesis of MnOx-loaded biochar for the removal of doxycycline hydrochloride: Effect of the ambient conditions and co-exixting heavy metal. J. Chem. Technol. Biotechnol. 2019, 94, 2187–2197. [Google Scholar]
- Zhou, L.; Liu, Y.G.; Liu, S.B.; Yin, Y.C.; Zeng, G.M.; Tan, X.F. Investigation of the adsorption reduction mechanism by hexavalent chromium by ramie biochar. Bioresour. Technol. 2016, 218, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, S.; Lai, C.W.; Gan, S.; Zamiri, G.; Pivehzhani, O.A.; Johan, M.R. Application of efficient magnetic practice and activated carbon for dye removal from wastewater. ACS Omega 2020, 5, 20684–20697. Available online: http://pubs.acs.org/journal/acsodf (accessed on 9 March 2023). [CrossRef]
- Sharma, A.; Mangla, D.; Chaudhry, S.A. Recent advances in magnetic composites as adsorbents for wastewater remediation. J. Environ. Manag. 2022, 306, 114483. [Google Scholar] [CrossRef]
- Zhao, Q.; Xu, T.; Song, X.; Nie, S.; Choi, S.-E. Preparation and application in water treatment of magnetic biochar. Front. Bioeng. Biotechnol. 2021, 9, 769667. [Google Scholar] [CrossRef]
- Hameed, B.; Din, A.; Ahmad, A. Adsorption of methylene blue onto bamboo-based activated carbon: Kinetics and equilibrium studies. J. Hazard. Mater. 2006, 141, 819–825. [Google Scholar] [CrossRef]
- Isa, S.S.M.; Ramli, M.M.; Che, H.D.S.C.; Anhar, N.A.M. Different carbonization process of bamboo charcoal using gigantochloa albociliata. AIP Conf. Proc. 2017, 1885, 020226. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, S.; Chen, M.; Gu, H.; Rapole, S.P.; Pallavkar, S.; Ho, T.C.; Hopper, J.; Guo, Z. Magnetic nanocomposites for environmental remediation. Adv. Powder Technol. 2013, 24, 459–467. [Google Scholar] [CrossRef]
- Nishioka, H.; Sen, T.K. Solvothermal synthesis and characterization of magnetic bamboo charcoal (BC) nanocomposites. J. Inst. Eng. (India) Ser. E 2019, 100, 155–165. [Google Scholar] [CrossRef]
- Mohapatra, M.; Anand, S. Synthesis, and applications of nano-structured iron oxides/hydroxides—A review. Int. J. Eng. Sci. Technol. 2010, 2, 127–146. [Google Scholar] [CrossRef]
- Hoa, L.T.M.; Dung, T.T.; Danh, T.M.; Duc, N.H.; Chien, D.M. Preparation, and characterization of magnetic nanoparticles coated with polyethylene glycol. J. Phys. Conf. Ser. 2009, 187, 012048. [Google Scholar] [CrossRef]
- Krolow, M.Z.C.; Hartwig, C.A.; Link, G.C.; Raubach, C.W.; Pereira, J.S.F.; Picoloto, R.S.; Gonçalves, M.R.F.; Carreño, N.L.V.; Mesko, M.F. Synthesis and characterization of carbon nanocomposites. In Carbon Nanostructures; Springer: Berlin/Heidelberg, Germany, 2011; pp. 33–47. [Google Scholar] [CrossRef]
- Altingtig, E.; Altundag, H.; Tuzen, M.; Sari, A. Effective removal of methylene blue from aqueous solutions using magnetic loaded activated carbon as novel adsorbent. Chem. Eng. Res. Desig. 2017, 122, 151–163. [Google Scholar] [CrossRef]
- Yadav, S.; Asthana, A.; Chakraborty, R.; Jain, B.; Singh, A.K.; Sónia, A.; Carabineiro, A.; Hasan Susan, M.A.B. Cationic dye removal using novel magnetic/activated charcoal/cyclodextrin/alginate polymer nanocomposite. Nanomaterials 2020, 10, 170. [Google Scholar] [CrossRef]
- Wahyuni, E.T.; Rendo, D.; Suherman, S. Removal of methylene blue dye in water by using recoverable natural zeolite/Fe3O4 adsorbent. Glob. NEST J. 2021, 23, 119–126. [Google Scholar]
- Torrellas, S.S.; Boutahala, M.; Boukhalfa, N.; Munoz, M. Article Effective adsorption of methylene blue dye onto magnetic nanocomposites. modelling and reuse studies. Appl. Sci. 2019, 9, 4563. [Google Scholar] [CrossRef]
- Hashem, F.S. Adsorption of methylene blue from aqueous solutions using Fe3O4/bentonite nanocomposite. Hydrol. Curr. Res. 2012, 3, 143. [Google Scholar]
- Hamidzadeh, S.; Torabbeigi, M.; Jamaleddin Shahta, S. Removal of crystal violet from water by magnetically modified activated carbon and nanomagnetic iron oxide. J. Environ. Health Sci. Eng. 2015, 13, 8. [Google Scholar] [CrossRef]
- Juang, R.S.; Yei, Y.C.; Liao, C.S.; Lin, K.S.; Lu, H.C.; Wang, S.F.; Sun, A.C. Synthesis of magnetic Fe3O4/activated carbon nanocomposites with high surface area as recoverable adsorbents. J. Taiwan Inst. Chem. Eng. 2018, 90, 51–60. [Google Scholar] [CrossRef]
- Rai, B.N.; Sing, R.S. Bioremediation of congo red dye in immobilized batch and continuous packed bed bioreactor by brevibacillus parabrevis using coconut shell biochar. Bioresour. Technol. 2018, 252, 37–43. [Google Scholar]
- Vikrant, K.; Giri, B.S.; Raza, N.; Roy, K.; Kim, K.H.; Rai, B.N.; Singh, R.S. Recent advancement in bioremediation of dye: Current status and challenges. Bioresour. Technol. 2018, 253, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Abuzerr, S.; Darwish, M.; Mahvi, A.H. Simultaneous removal of cationic methylene blue and anionic reactive red 198 dyes using magnetic activated carbon nanoparticles: Equilibrium and kinetics analysis. Water Sci. Technol. 2017, 2, 534–545. [Google Scholar] [CrossRef]
- Ahmed, M.J.K.; Ahmaruzzaman, M. Activated charcoal-magnetic nanocomposites for remediation of simulated dye polluted wastewater. Water Sci. Technol. 2015, 71, 1361–1366. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K. Removal of anionic dye Congo red from aqueous solution by raw pine and acid-treated pine cone powder as adsorbent: Equilibrium, thermodynamic, kinetics, mechanism and process design. Water Res. 2012, 46, 1933–1946. [Google Scholar] [CrossRef]
- Freundlich, H. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica, platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Kaur, S.; Rani, S.; Mahajan, R.K. Adsorption kinetics for the removal of hazardous dye congo red by biowaste materials as adsorbents. J. Chem. 2013, 2013, 628582. [Google Scholar] [CrossRef]
- Beydaghdari, M.; Hooriabad Saboor, F.; Babapoor, A.; Karve, V.V.; Asgari, M. Recent advances in MOF-based adsorbents for dye removal from the aquatic environment. Energies 2022, 15, 2023. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K.; Phan, C. Synthesis and characterization of slow pyrolysis pine cone bio-char in the removal of organic and inorganic pollutants from aqueous solution by adsorption: Kinetic, equilibrium, mechanism and thermodynamic. Bioresour. Technol. 2017, 246, 76–81. [Google Scholar] [CrossRef]
- Diez, E.; Redondo, C.; Gomez, J.M.; Miranda, R.; Rodringuez, A. Zeolite adsorbents for selective removal of Co(II) and Li (II) from aqueous solution. Water 2023, 15, 270. [Google Scholar] [CrossRef]
- Afroze, S.; Sen, T.K.; Ang, H.M. Adsorption removal of zinc (II) from aqueous phase by raw and base modified Eucalyptus sheathiana bark: Kinetics, mechanism and equilibrium study. Process Saf. Environ. Protect. 2016, 102, 336–352. [Google Scholar] [CrossRef]
- Malik, P.K. Dye removal from wastewater using activated carbon developed from sawdust: Adsorption equilibrium and kinetics. J. Hazard. Mater. 2004, 113, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Zuhara, S.; Pradhan, S.P.; Zakaria, Y.; Shetty, A.R.; McKay, G. Removal of methylene blue from water using magnetic GTL-derived biosolids: Study of adsorption isotherms and kinetic models. Molecules 2023, 28, 1511. [Google Scholar] [CrossRef]
- Auta, M.; Hameed, B.H. Chitosaneclay composite as highly effective and lowcost adsorbent for batch and fixed-bed adsorption of methylene blue. Chem. Eng. J. 2014, 237, 352–361. [Google Scholar] [CrossRef]
- Bulut, Y.; Karaer, H. Adsorption of methylene blue from aqueous solution by crosslinked chitosan/bentonite composite. J. Dispers. Sci. Technol. 2015, 36, 61–67. [Google Scholar] [CrossRef]
- Mahdavinia, G.R.; Massoudi, A.; Baghban, A.; Shokri, E. Study of adsorption of cationic dye on magnetic kappa-carrageenan/PVA nanocomposite hydrogels. J. Environ. Chem. Eng. 2014, 2, 1578–1587. [Google Scholar] [CrossRef]
- Fan, L.; Luo, C.; Li, X.; Lu, F.; Qiu, H.; Sun, M. Fabrication of novel magnetic chitosan grafted with graphene oxide to enhance adsorption properties for methyl blue. J. Hazard. Mater. 2012, 215–216, 272–279. [Google Scholar] [CrossRef]






| Initial Dye Concentration in ppm | Pseudo-Second-Order Rate Constant, K2 (g/mg-min) | Calculated Amount of Dye Adsorption at Equilibrium, qe (mg/g) | Experimental Amount of Dye Adsorption at Equilibrium, qe (mg/g) | Linear Regression Coefficient, R2 |
|---|---|---|---|---|
| 10 | 0.1945 | 9.53 | 9.50 | 0.995 |
| 20 | 0.0799 | 13.36 | 14.25 | 0.994 |
| 30 | 0.0835 | 13.48 | 15.12 | 0.9965 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sen, T.K. Application of Synthesized Biomass Bamboo Charcoal–Iron Oxide “BC/Fe” Nanocomposite Adsorbents in the Removal of Cationic Methylene Blue Dye Contaminants from Wastewater by Adsorption. Sustainability 2023, 15, 8841. https://doi.org/10.3390/su15118841
Sen TK. Application of Synthesized Biomass Bamboo Charcoal–Iron Oxide “BC/Fe” Nanocomposite Adsorbents in the Removal of Cationic Methylene Blue Dye Contaminants from Wastewater by Adsorption. Sustainability. 2023; 15(11):8841. https://doi.org/10.3390/su15118841
Chicago/Turabian StyleSen, Tushar Kanti. 2023. "Application of Synthesized Biomass Bamboo Charcoal–Iron Oxide “BC/Fe” Nanocomposite Adsorbents in the Removal of Cationic Methylene Blue Dye Contaminants from Wastewater by Adsorption" Sustainability 15, no. 11: 8841. https://doi.org/10.3390/su15118841





