Microalgae Biomass and Lipids as Feedstock for Biofuels: Sustainable Biotechnology Strategies
Abstract
:1. Introduction
1.1. Lipids in Microalgal Biomass
1.2. Lipid Biosynthetic Pathways in Microalgal Biomass
1.3. Polyunsaturated Fatty Acids Biosynthesis
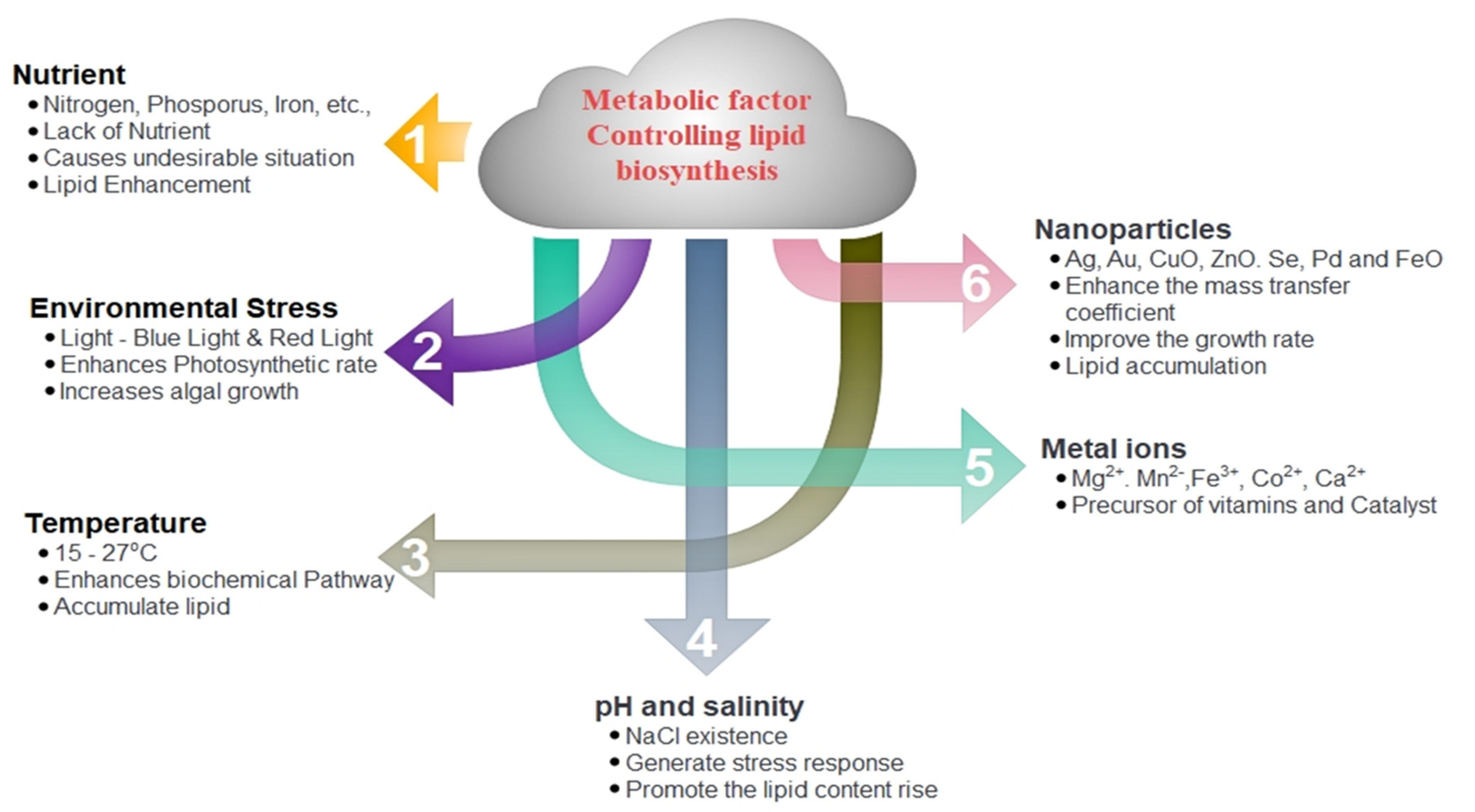
2. Enhancement of Biomass and Lipid Production from Microalgae
2.1. Conventional Methods
2.1.1. Nutrient Limitation
2.1.2. Environmental Stress
2.1.3. Temperature
2.1.4. pH and Salinity
2.1.5. Metal Ions
2.1.6. Nanoparticles
2.2. Challenges and Limitations of Convention Methods
3. Biotechnological Approaches for the Improvement of Biomass and Lipid Production
3.1. Selection Screening and Improvement of Potent Microalgal Strains (Bioprospecting)
3.2. Molecular and Metabolic Engineering Approaches
3.2.1. Lipids and Fatty Acids Biosynthesis Pathway Engineering
3.2.2. Engineering Photosynthetic Capability
Genetic Modification of NADPH Generation
3.2.3. Genetic Transformation Approaches
3.2.4. In Silico Metabolic Engineering Tools
3.3. Transcriptional Regulations
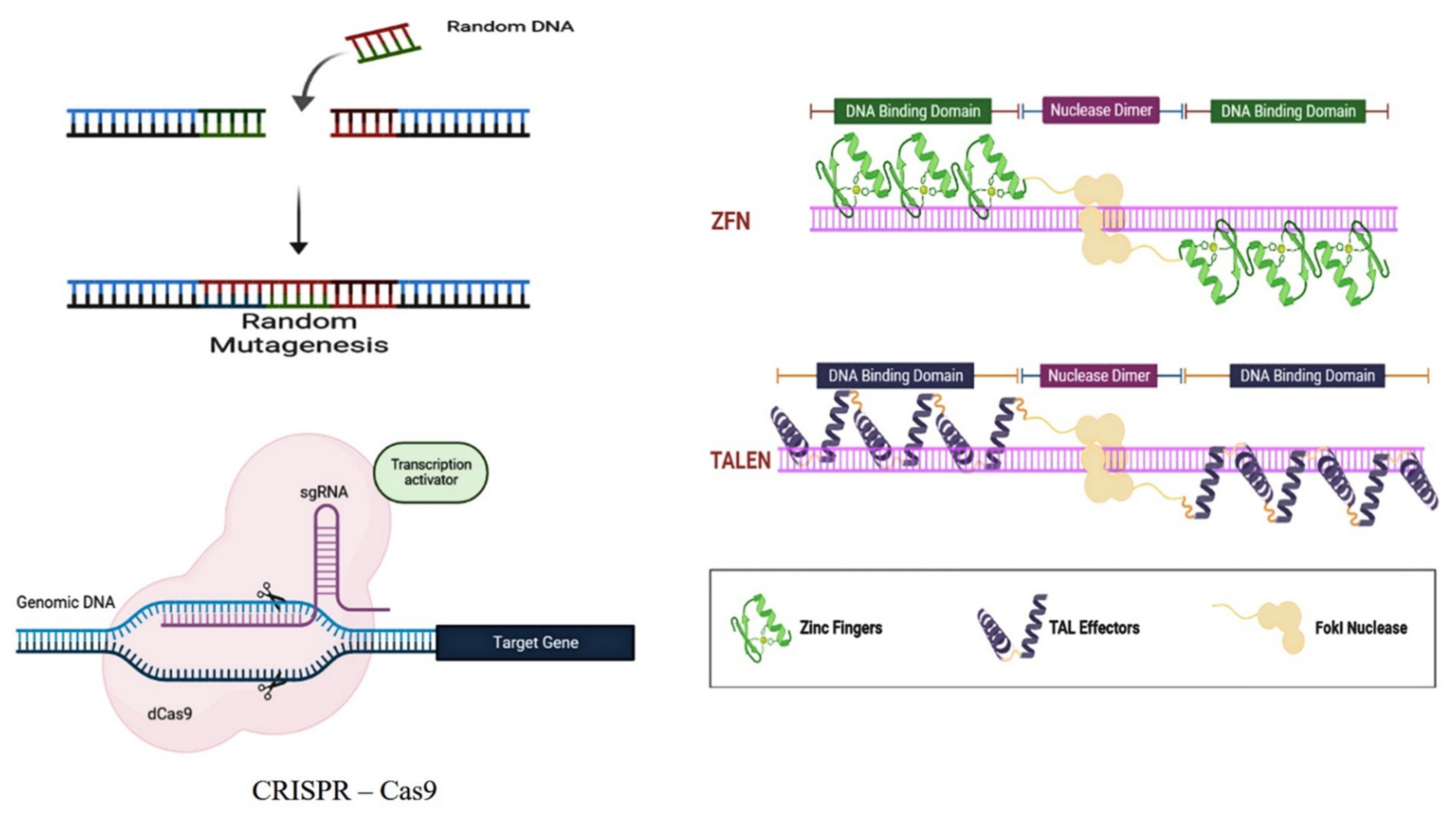
3.4. Gene Editing Tools for the Development of Biofuel from Microalgae
3.4.1. CRIPSR Associated Lipid
3.4.2. CRISPRi Technology
3.4.3. ZFN-Mediated Lipid Enhancement
3.5. Co-Culturing Techniques
3.5.1. Microalgae and Microalgae
3.5.2. Microalgae and Bacteria
3.5.3. Microalgae and Fungi
3.5.4. Microalgae and Yeast
4. Biotechnologically Enhanced Lipids as the Substrate for Biodiesel Production
5. Integrated Approaches (Microalgae Lipid and Pigment Production)
5.1. Lipids in Microalgae
5.2. Pigments in Microalgae
6. Economic and Commercialization Feasibility
7. Future Aspects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, L.D.; Li, Z.H.; Hiltunen, E. Strategies for Lipid Production Improvement in Microalgae as a Biodiesel Feedstock. Biomed. Res. Int. 2016, 2016, 8792548. [Google Scholar] [CrossRef] [Green Version]
- Alishah Aratboni, H.; Rafiei, N.; Garcia-Granados, R.; Alemzadeh, A.; Morones-Ramírez, J.R. Biomass and Lipid Induction Strategies in Microalgae for Biofuel Production and Other Applications. Microb. Cell Fact. 2019, 18, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal Triacylglycerols as Feedstocks for Biofuel Production: Perspectives and Advances. Plant. J. 2008, 54, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Brindhadevi, K.; Mathimani, T.; Ganesan, R.; Sekar, M.; Shanmugam, S.; Phuong, T.N.; Alharbi, S.A.; Chinnathambi, A.; Chanasut, U.; Whangchai, K. Central Composite Design for the Optimization of CaO and Fe2(SO4)3 Facilitated Transesterification of Scenedesmus Sp. Oil for Fatty Acid Methyl Ester Production. Fuel 2022, 321, 124096. [Google Scholar] [CrossRef]
- Chu, W.-L. Strategies to Enhance Production of Microalgal Biomass and Lipids for Biofuel Feedstock. Eur. J. Phycol. 2017, 52, 419–437. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Q. Regulatory Mechanisms of Lipid Biosynthesis in Microalgae. Biol. Rev. Camb. Philos. Soc. 2021, 96, 2373–2391. [Google Scholar] [CrossRef] [PubMed]
- Leu, S.; Boussiba, S. Advances in the Production of High-Value Products by Microalgae. Ind. Biotechnol. 2014, 10, 169–183. [Google Scholar] [CrossRef]
- Yu, W.-L.; Ansari, W.; Schoepp, N.G.; Hannon, M.J.; Mayfield, S.P.; Burkart, M.D. Modifications of the Metabolic Pathways of Lipid and Triacylglycerol Production in Microalgae. Microb. Cell Fact. 2011, 10, 91. [Google Scholar] [CrossRef] [Green Version]
- Blatti, J.L.; Michaud, J.; Burkart, M.D. Engineering Fatty Acid Biosynthesis in Microalgae for Sustainable Biodiesel. Curr. Opin. Chem. Biol. 2013, 17, 496–505. [Google Scholar] [CrossRef]
- Kumar, G.; Shekh, A.; Jakhu, S.; Sharma, Y.; Kapoor, R.; Sharma, T.R. Bioengineering of Microalgae: Recent Advances, Perspectives, and Regulatory Challenges for Industrial Application. Front. Bioeng. Biotechnol. 2020, 8, 914. [Google Scholar] [CrossRef]
- Li, H.-Y.; Lu, Y.; Zheng, J.-W.; Yang, W.-D.; Liu, J.-S. Biochemical and Genetic Engineering of Diatoms for Polyunsaturated Fatty Acid Biosynthesis. Mar. Drugs 2014, 12, 153–166. [Google Scholar] [CrossRef]
- Sharma, K.K.; Schuhmann, H.; Schenk, P.M. High Lipid Induction in Microalgae for Biodiesel Production. Energies 2012, 5, 1532–1553. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.-M.; Ren, L.-J.; Zhao, Q.-Y.; Ji, X.-J.; Huang, H. Enhancement of Lipid Accumulation in Microalgae by Metabolic Engineering. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 552–566. [Google Scholar] [CrossRef]
- Guarnieri, M.T.; Nag, A.; Smolinski, S.L.; Darzins, A.; Seibert, M.; Pienkos, P.T. Examination of Triacylglycerol Biosynthetic Pathways via de Novo Transcriptomic and Proteomic Analyses in an Unsequenced Microalga. PLoS ONE 2011, 6, e25851. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The Promising Future of Microalgae: Current Status, Challenges, and Optimization of a Sustainable and Renewable Industry for Biofuels, Feed, and Other Products. Microb. Cell Fact. 2018, 17, 1–21. [Google Scholar] [CrossRef]
- Shokravi, Z.; Shokravi, H.; Chyuan, O.H.; Lau, W.J.; Koloor, S.S.R.; Petrů, M.; Ismail, A.F. Improving ‘Lipid Productivity’ in Microalgae by Bilateral Enhancement of Biomass and Lipid Contents: A Review. Sustainability 2020, 12, 9083. [Google Scholar] [CrossRef]
- Banerjee, C.; Dubey, K.K.; Shukla, P. Metabolic Engineering of Microalgal Based Biofuel Production: Prospects and Challenges. Front. Microbiol. 2016, 7, 432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Zhao, W.; Mao, X.; Li, Y.; Wu, T.; Chen, F. High-Value Biomass from Microalgae Production Platforms: Strategies and Progress Based on Carbon Metabolism and Energy Conversion. Biotechnol. Biofuels 2018, 11, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, J.; Balamurugan, S.; Li, T.; Cai, J.-X.; Chen, T.-T.; Wang, X.; Yang, W.-D.; Li, H.-Y. Biotechnological Approaches to Enhance Biofuel Producing Potential of Microalgae. Fuel 2021, 302, 121169. [Google Scholar] [CrossRef]
- Ahorsu, R.; Medina, F.; Constantí, M. Significance and Challenges of Biomass as a Suitable Feedstock for Bioenergy and Biochemical Production: A Review. Energies 2018, 11, 3366. [Google Scholar] [CrossRef]
- Thuy Lan Chi, N.; Mathimani, T.; Manigandan, S.; Shanmugam, S.; Thi Ha, N.; Cam Nhung, T.; Ali Alharbi, S.; Chinnathambi, A.; Brindhadevi, K.; Chanasut, U.; et al. Small Scale Photobioreactor, Outdoor Open Pond Cultivation of Chlorella Sp. and Harvesting at Log and Stationary Growth Phase towards Lipids and Methyl Ester Production. Fuel 2022, 319, 123813. [Google Scholar] [CrossRef]
- Marrone, B.L.; Lacey, R.E.; Anderson, D.B.; Bonner, J.; Coons, J.; Dale, T.; Downes, C.M.; Fernando, S.; Fuller, C.; Goodall, B.; et al. Review of the Harvesting and Extraction Program within the National Alliance for Advanced Biofuels and Bioproducts. Algal Res. 2018, 33, 470–485. [Google Scholar] [CrossRef]
- Mutanda, T.; Ramesh, D.; Karthikeyan, S.; Kumari, S.; Anandraj, A.; Bux, F. Bioprospecting for Hyper-Lipid Producing Microalgal Strains for Sustainable Biofuel Production. Bioresour. Technol. 2011, 102, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.; Barreira, L.; Mozes, A.; Florindo, C.; Polo, C.; Duarte, C.V.; Custódio, L.; Varela, J. Microplate-Based High Throughput Screening Procedure for the Isolation of Lipid-Rich Marine Microalgae. Biotechnol. Biofuels 2011, 4, 61. [Google Scholar] [CrossRef] [Green Version]
- Lam, G.P.; Vermuë, M.H.; Eppink, M.H.M.; Wijffels, R.H.; van den Berg, C. Multi-Product Microalgae Biorefineries: From Concept towards Reality. Trends Biotechnol. 2017, 36, 216–227. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.-Y.; Raman, A.A.A.; Ibrahim, S. Microalgae Lipid and Biomass for Biofuel Production: A Comprehensive Review on Lipid Enhancement Strategies and Their Effects on Fatty Acid Composition. Renew. Sustain. Energy Rev. 2018, 97, 200–232. [Google Scholar] [CrossRef]
- Adesanya, V.O.; Cadena, E.; Scott, S.A.; Smith, A.G. Life Cycle Assessment on Microalgal Biodiesel Production Using a Hybrid Cultivation System. Bioresour. Technol. 2014, 163, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Pellegrino, J. Life-Cycle Assessment of Five Microalgae-to-Biofuels Processes of Varying Complexity. J. Renew. Sustain. Energy 2015, 7, 043136. [Google Scholar] [CrossRef]
- Ahmad, A.; Banat, F.; Alsafar, H.; Hasan, S.W. Recent Breakthroughs in Integrated Biomolecular and Biotechnological Approaches for Enhanced Lipid and Carotenoid Production from Microalgae. Phytochem. Rev. 2022, 1–21. [Google Scholar] [CrossRef]
- D’Alessandro, E.B.; Antoniosi Filho, N.R. Concepts and Studies on Lipid and Pigments of Microalgae: A Review. Renew. Sustain. Energy Rev. 2016, 58, 832–841. [Google Scholar] [CrossRef]
- Anwar, M.; Lou, S.; Chen, L.; Li, H.; Hu, Z. Recent Advancement and Strategy on Bio-Hydrogen Production from Photosynthetic Microalgae. Bioresour. Technol. 2019, 292, 121972. [Google Scholar] [CrossRef]
- Atabani, A.E.; Tyagi, V.K.; Fongaro, G.; Treichel, H.; Pugazhendhi, A.; Hoang, A.T. Integrated Biorefineries, Circular Bio-Economy, and Valorization of Organic Waste Streams with Respect to Bio-Products. Biomass Convers. Biorefin. 2022, 12, 565. [Google Scholar] [CrossRef]
- Balamurugan, S.; Wang, X.; Wang, H.-L.; An, C.-J.; Li, H.; Li, D.-W.; Yang, W.-D.; Liu, J.-S.; Li, H.-Y. Occurrence of Plastidial Triacylglycerol Synthesis and the Potential Regulatory Role of AGPAT in the Model Diatom Phaeodactylum tricornutum. Biotechnol. Biofuels 2017, 10, 97. [Google Scholar] [CrossRef] [Green Version]
- Bamary, Z.; Einali, A. Changes in Carbon Partitioning and Pattern of Antioxidant Enzyme Activity Induced by Arginine Treatment in the Green Microalga Dunaliella salina under Long-Term Salinity. Microb. Ecol. 2022, 84, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Banerjee, S.; Ghosh, A.K.; Das, D. Maneuvering the Genetic and Metabolic Pathway for Improving Biofuel Production in Algae: Present Status and Future Prospective. Renew. Sustain. Energy Rev. 2020, 133, 110155. [Google Scholar] [CrossRef]
- Barrangou, R.; Birmingham, A.; Wiemann, S.; Beijersbergen, R.L.; Hornung, V.; Smith, A.v.B. Advances in CRISPR-Cas9 Genome Engineering: Lessons Learned from RNA Interference. Nucleic Acids Res. 2015, 43, 3407–3419. [Google Scholar] [CrossRef] [Green Version]
- Beal, C.M.; Hebner, R.E.; Webber, M.E.; Ruoff, R.S.; Seibert, A.F.; King, C.W. Comprehensive Evaluation of Algal Biofuel Production: Experimental and Target Results. Energies 2012, 5, 1943–1981. [Google Scholar] [CrossRef]
- Beckett, R.P.; Zavarzina, A.G.; Liers, C. Oxidoreductases and Cellulases in Lichens: Possible Roles in Lichen Biology and Soil Organic Matter Turnover. Fungal Biol. 2013, 117, 431–438. [Google Scholar] [CrossRef]
- Benedetti, M.; Vecchi, V.; Barera, S.; Dall’Osto, L. Biomass from Microalgae: The Potential of Domestication towards Sustainable Biofactories. Microb. Cell Fact. 2018, 17, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benemann, J.R.; Tillett, D.M.; Weissman, J.C. Microalgae Biotechnology. Trends Biotechnol. 1987, 5, 47–53. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Agrawal, K.; Verma, P. Algal Biofuels: An Economic and Effective Alternative of Fossil Fuels. In Clean Energy Production Technologies; Springer: Singapore, 2020; pp. 207–227. ISBN 9789811571893. [Google Scholar]
- Bogen, C.; Klassen, V.; Wichmann, J.; La Russa, M.; Doebbe, A.; Grundmann, M.; Uronen, P.; Kruse, O.; Mussgnug, J.H. Identification of Monoraphidium contortum as a Promising Species for Liquid Biofuel Production. Bioresour. Technol. 2013, 133, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.; Godfrey, V.; Wahlen, B.; Seefeldt, L.; Bugbee, B. Understanding Precision Nitrogen Stress to Optimize the Growth and Lipid Content Tradeoff in Oleaginous Green Microalgae. Bioresour. Technol. 2013, 131, 188–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udayan, A.; Pandey, A.K.; Sirohi, R.; Sreekumar, N.; Sang, B.-I.; Sim, S.J.; Kim, S.H.; Pandey, A. Production of Microalgae with High Lipid Content and Their Potential as Sources of Nutraceuticals. Phytochem. Rev. 2022, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A. High-Value Products from Microalgae—Their Development and Commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Boyle, N.R.; Page, M.D.; Liu, B.; Blaby, I.K.; Casero, D.; Kropat, J.; Cokus, S.J.; Hong-Hermesdorf, A.; Shaw, J.; Karpowicz, S.J.; et al. Three Acyltransferases and Nitrogen-Responsive Regulator Are Implicated in Nitrogen Starvation-Induced Triacylglycerol Accumulation in Chlamydomonas. J. Biol. Chem. 2012, 287, 15811–15825. [Google Scholar] [CrossRef] [Green Version]
- Brar, A.; Kumar, M.; Soni, T.; Vivekanand, V.; Pareek, N. Insights into the Genetic and Metabolic Engineering Approaches to Enhance the Competence of Microalgae as Biofuel Resource: A Review. Bioresour. Technol. 2021, 339, 125597. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.K.; Beer, T.; Batten, D. Life Cycle Assessment of Biodiesel Production from Microalgae in Ponds. Bioresour. Technol. 2011, 102, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, K.H.M.; Guaratini, T.; Barros, M.P.; Falcão, V.R.; Tonon, A.P.; Lopes, N.P.; Campos, S.; Torres, M.A.; Souza, A.O.; Colepicolo, P.; et al. Metabolites from Algae with Economical Impact. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 146, 60–78. [Google Scholar] [CrossRef]
- Chen, M.; Schliep, M.; Willows, R.D.; Cai, Z.-L.; Neilan, B.A.; Scheer, H. A Red-Shifted Chlorophyll. Science 2010, 329, 1318–1319. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae Biorefinery: High Value Products Perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef]
- Chia, S.R.; Ong, H.C.; Chew, K.W.; Show, P.L.; Phang, S.-M.; Ling, T.C.; Nagarajan, D.; Lee, D.-J.; Chang, J.-S. Sustainable Approaches for Algae Utilisation in Bioenergy Production. Renew. Energy 2018, 129, 838–852. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from Microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.U.; Park, J.M. Biodiesel Production by Various Oleaginous Microorganisms from Organic Wastes. Bioresour. Technol. 2018, 256, 502–508. [Google Scholar] [CrossRef]
- Chowdhury, H.; Loganathan, B. Third-Generation Biofuels from Microalgae: A Review. Curr. Opin. Green Sustain. Chem. 2019, 20, 39–44. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Freitas, B.C.B.; Rosa, G.M.; Moraes, L.; Morais, M.G.; Mitchell, B.G. Operational and Economic Aspects of Spirulina-Based Biorefinery. Bioresour. Technol. 2019, 292, 121946. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, N.M.D.; Parisien, A.; Wang, B.; Lan, C.Q. Enhancement of Lipid Production Using Biochemical, Genetic and Transcription Factor Engineering Approaches. J. Biotechnol. 2009, 141, 31–41. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.P.; Aguilar-Hernandez, I.; Cardenas-Chavez, D.L.; Ornelas-Soto, N.; Romero-Ogawa, M.A.; Parra-Saldivar, R. Extraction and Purification of High-Value Metabolites from Microalgae: Essential Lipids, Astaxanthin and Phycobiliproteins: High-Value Metabolites Form Algae. Microb. Biotechnol. 2015, 8, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Daroch, M.; Geng, S.; Wang, G. Recent Advances in Liquid Biofuel Production from Algal Feedstocks. Appl. Energy 2013, 102, 1371–1381. [Google Scholar] [CrossRef]
- Das, P.K.; Rani, J.; Rawat, S.; Kumar, S. Microalgal Co-Cultivation for Biofuel Production and Bioremediation: Current Status and Benefits. Bioenergy Res. 2022, 15, 1–26. [Google Scholar] [CrossRef]
- Doudna, J.A.; Charpentier, E. Genome Editing. The New Frontier of Genome Engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef]
- Fan, J.; Yan, C.; Andre, C.; Shanklin, J.; Schwender, J.; Xu, C. Oil Accumulation Is Controlled by Carbon Precursor Supply for Fatty Acid Synthesis in Chlamydomonas reinhardtii. Plant Cell Physiol. 2012, 53, 1380–1390. [Google Scholar] [CrossRef] [Green Version]
- Gan, S.Y.; Maggs, C.A. Random Mutagenesis and Precise Gene Editing Technologies: Applications in Algal Crop Improvement and Functional Genomics. Eur. J. Phycol. 2017, 52, 466–481. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Y.; Shen, Y.; Yan, D.; He, X.; Dai, J.; Wu, Q. Oil Accumulation Mechanisms of the Oleaginous Microalga Chlorella protothecoides Revealed through Its Genome, Transcriptomes, and Proteomes. BMC Genom. 2014, 15, 582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, C.; Zhai, Y.; Ding, Y.; Wu, Q. Application of Sweet Sorghum for Biodiesel Production by Heterotrophic Microalga Chlorella protothecoides. Appl. Energy 2010, 87, 756–761. [Google Scholar] [CrossRef]
- Gautam, K.; Tripathi, J.K.; Pareek, A.; Sharma, D.K. Growth and Secretome Analysis of Possible Synergistic Interaction between Green Algae and Cyanobacteria. J. Biosci. Bioeng. 2019, 127, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Gimpel, J.A.; Specht, E.A.; Georgianna, D.R.; Mayfield, S.P. Advances in Microalgae Engineering and Synthetic Biology Applications for Biofuel Production. Curr. Opin. Chem. Biol. 2013, 17, 489–495. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Pires, J.C.M.; Simões, M. Biotechnological Potential of Synechocystis salina Co-Cultures with Selected Microalgae and Cyanobacteria: Nutrients Removal, Biomass and Lipid Production. Bioresour. Technol. 2016, 200, 279–286. [Google Scholar] [CrossRef]
- Gong, Y.; Guo, X.; Wan, X.; Liang, Z.; Jiang, M. Characterization of a Novel Thioesterase (PtTE) from Phaeodactylum tricornutum. J. Basic Microbiol. 2011, 51, 666–672. [Google Scholar] [CrossRef]
- Gong, Y.; Jiang, M. Biodiesel Production with Microalgae as Feedstock: From Strains to Biodiesel. Biotechnol. Lett. 2011, 33, 1269–1284. [Google Scholar] [CrossRef]
- Goswami, R.K.; Mehariya, S.; Verma, P.; Lavecchia, R.; Zuorro, A. Microalgae-Based Biorefineries for Sustainable Resource Recovery from Wastewater. J. Water Proc. Eng. 2021, 40, 101747. [Google Scholar] [CrossRef]
- Griffiths, M.J.; van Hille, R.P.; Harrison, S.T.L. Lipid Productivity, Settling Potential and Fatty Acid Profile of 11 Microalgal Species Grown under Nitrogen Replete and Limited Conditions. J. Appl. Phycol. 2012, 24, 989–1001. [Google Scholar] [CrossRef]
- Guschina, I.A.; Harwood, J.L. Lipids and Lipid Metabolism in Eukaryotic Algae. Prog. Lipid Res. 2006, 45, 160–186. [Google Scholar] [CrossRef] [PubMed]
- Botero, C.D.; Restrepo, D.L.; Cardona, C.A. A Comprehensive Review on the Implementation of the Biorefinery Concept in Biodiesel Production Plants. Biofuel Res. J. 2017, 4, 691–703. [Google Scholar] [CrossRef]
- Halim, R.; Danquah, M.K.; Webley, P.A. Extraction of Oil from Microalgae for Biodiesel Production: A Review. Biotechnol. Adv. 2012, 30, 709–732. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.-J.; Su, C.-H.; Chien, L.-J. Accumulation of Lipid Production in Chlorella minutissima by Triacylglycerol Biosynthesis-Related Genes Cloned from Saccharomyces cerevisiae and Yarrowia lipolytica. J. Microbiol. 2012, 50, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Mimouni, V.; Lukomska, E.; Morant-Manceau, A.; Bougaran, G. Carbon Partitioning and Lipid Remodeling during Phosphorus and Nitrogen Starvation in the Marine Microalga Diacronema lutheri (Haptophyta). J. Phycol. 2020, 56, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Chen, F.; Wei, D.; Zhang, X.; Chen, G. Biodiesel Production by Microalgal Biotechnology. Appl. Energy 2010, 87, 38–46. [Google Scholar] [CrossRef]
- Jagadevan, S.; Banerjee, A.; Banerjee, C.; Guria, C.; Tiwari, R.; Baweja, M.; Shukla, P. Recent Developments in Synthetic Biology and Metabolic Engineering in Microalgae towards Biofuel Production. Biotechnol. Biofuels 2018, 11, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Javed, F.; Aslam, M.; Rashid, N.; Shamair, Z.; Khan, A.L.; Yasin, M.; Fazal, T.; Hafeez, A.; Rehman, F.; Rehman, M.S.U.; et al. Microalgae-Based Biofuels, Resource Recovery and Wastewater Treatment: A Pathway towards Sustainable Biorefinery. Fuel 2019, 255, 115826. [Google Scholar] [CrossRef]
- Jayappriyan, K.R.; Rajkumar, R.; Venkatakrishnan, V.; Nagaraj, S.; Rengasamy, R. In Vitro Anticancer Activity of Natural β-Carotene from Dunaliella salina EU5891199 in PC-3 Cells. Biomed. Prev. Nutr. 2013, 3, 99–105. [Google Scholar] [CrossRef]
- Jeon, S.; Lim, J.-M.; Lee, H.-G.; Shin, S.-E.; Kang, N.K.; Park, Y.-I.; Oh, H.-M.; Jeong, W.-J.; Jeong, B.-R.; Chang, Y.K. Current Status and Perspectives of Genome Editing Technology for Microalgae. Biotechnol. Biofuels 2017, 10, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, M.-K.; Kim, H.-C.; Sapireddy, V.R.; Yun, H.-S.; Abou-Shanab, R.A.I.; Choi, J.; Lee, W.; Timmes, T.C.; Inamuddin; Jeon, B.-H. Simultaneous Nutrient Removal and Lipid Production from Pretreated Piggery Wastewater by Chlorella vulgaris YSW-04. Appl. Microbiol. Biotechnol. 2013, 97, 2701–2710. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Yuan, Q. Removal of Nitrogen from Wastewater Using Microalgae and Microalgae–Bacteria Consortia. Cogent Environ. Sci. 2016, 2, 1275089. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA-Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Jinkerson, R.E.; Jonikas, M.C. Molecular Techniques to Interrogate and Edit the Chlamydomonas Nuclear Genome. Plant. J. 2015, 82, 393–412. [Google Scholar] [CrossRef]
- Kao, P.-H.; Ng, I.-S. CRISPRi Mediated Phosphoenolpyruvate Carboxylase Regulation to Enhance the Production of Lipid in Chlamydomonas reinhardtii. Bioresour. Technol. 2017, 245, 1527–1537. [Google Scholar] [CrossRef]
- Kasai, Y.; Oshima, K.; Ikeda, F.; Abe, J.; Yoshimitsu, Y.; Harayama, S. Construction of a Self-Cloning System in the Unicellular Green Alga Pseudochoricystis ellipsoidea. Biotechnol. Biofuels 2015, 8, 94. [Google Scholar] [CrossRef] [Green Version]
- Khatodia, S.; Bhatotia, K.; Passricha, N.; Khurana, S.M.P.; Tuteja, N. The CRISPR/Cas Genome-Editing Tool: Application in Improvement of Crops. Front. Plant Sci. 2016, 7, 506. [Google Scholar] [CrossRef] [Green Version]
- De Clerck, O.; Guiry, M.D.; Leliaert, F.; Samyn, Y.; Verbruggen, H. Algal Taxonomy: A Road to Nowhere? J. Phycol. 2013, 49, 215–225. [Google Scholar] [CrossRef]
- Fon-Sing, S.; Borowitzka, M.A. Isolation and Screening of Euryhaline Tetraselmis Spp. Suitable for Large-Scale Outdoor Culture in Hypersaline Media for Biofuels. J. Appl. Phycol. 2016, 28, 1–14. [Google Scholar] [CrossRef]
- Jain, P.; Arora, N.; Mehtani, J.; Pruthi, V.; Majumder, C.B. Pretreated Algal Bloom as a Substantial Nutrient Source for Microalgae Cultivation for Biodiesel Production. Bioresour. Technol. 2017, 242, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Přibyl, P.; Cepák, V.; Zachleder, V. Production of Lipids in 10 Strains of Chlorella and Parachlorella, and Enhanced Lipid Productivity in Chlorella vulgaris. Appl. Microbiol. Biotechnol. 2012, 94, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yang, T.; Zhu, W.; Zhao, Y. Wastewater Treatment and Biofuel Production through Attached Culture of Chlorella vulgaris in a Porous Substratum Biofilm Reactor. J. Appl. Phycol. 2017, 29, 833–841. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, W.; Shao, H.; Liu, T. A Comparative Analysis of Biomass and Lipid Content in Five Tribonema Sp. Strains at Autotrophic, Heterotrophic and Mixotrophic Cultivation. Algal. Res. 2017, 24, 284–289. [Google Scholar] [CrossRef]
- Kitcha, S.; Cheirsilp, B. Enhanced Lipid Production by Co-Cultivation and Co-Encapsulation of Oleaginous Yeast Trichosporonoides Spathulata with Microalgae in Alginate Gel Beads. Appl. Biochem. Biotechnol. 2014, 173, 522–534. [Google Scholar] [CrossRef] [PubMed]
- La, A.; Perré, P.; Taidi, B. Process for Symbiotic Culture of Saccharomyces cerevisiae and Chlorella vulgaris for in Situ CO2 Mitigation. Appl. Microbiol. Biotechnol. 2019, 103, 731–745. [Google Scholar] [CrossRef]
- Lamers, P.P.; Janssen, M.; De Vos, R.C.H.; Bino, R.J.; Wijffels, R.H. Carotenoid and Fatty Acid Metabolism in Nitrogen-Starved Dunaliella salina, a Unicellular Green Microalga. J. Biotechnol. 2012, 162, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Larkum, A.W.D.; Ross, I.L.; Kruse, O.; Hankamer, B. Selection, Breeding and Engineering of Microalgae for Bioenergy and Biofuel Production. Trends Biotechnol. 2012, 30, 198–205. [Google Scholar] [CrossRef]
- Laurens, L.M.L.; Markham, J.; Templeton, D.W.; Christensen, E.D.; Van Wychen, S.; Vadelius, E.W.; Chen-Glasser, M.; Dong, T.; Davis, R.; Pienkos, P.T. Development of Algae Biorefinery Concepts for Biofuels and Bioproducts; a Perspective on Process-Compatible Products and Their Impact on Cost-Reduction. Energy Environ. Sci. 2017, 10, 1716–1738. [Google Scholar] [CrossRef] [Green Version]
- Li, D.-W.; Cen, S.-Y.; Liu, Y.-H.; Balamurugan, S.; Zheng, X.-Y.; Alimujiang, A.; Yang, W.-D.; Liu, J.-S.; Li, H.-Y. A Type 2 Diacylglycerol Acyltransferase Accelerates the Triacylglycerol Biosynthesis in Heterokont Oleaginous Microalga Nannochloropsis oceanica. J. Biotechnol. 2016, 229, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, J.; Lim, P.-E.; Wei, D. Enhanced Single Cell Oil Production by Mixed Culture of Chlorella pyrenoidosa and Rhodotorula glutinis Using Cassava Bagasse Hydrolysate as Carbon Source. Bioresour. Technol. 2018, 255, 140–148. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, H.; Deng, Q.; Cao, L. Characterization of Fibrolytic and Lipid Accumulating Fungi Isolated from Fresh Cattle Feces. Environ. Sci. Pollut. Res. Int. 2014, 21, 9228–9233. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, S.M.; Laamanen, C.A.; Basiliko, N.; Scott, J.A. Utilization of Lipid-Extracted Biomass (LEB) to Improve the Economic Feasibility of Biodiesel Production from Green Microalgae. Environ. Rev. 2020, 28, 325–338. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for Biodiesel Production and Other Applications: A Review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef] [Green Version]
- Mehrabadi, A.; Craggs, R.; Farid, M.M. Biodiesel Production Potential of Wastewater Treatment High Rate Algal Pond Biomass. Bioresour. Technol. 2016, 221, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Yang, J.; Xu, X.; Zhang, L.; Nie, Q.; Xian, M. Biodiesel Production from Oleaginous Microorganisms. Renew. Energy 2009, 34, 1–5. [Google Scholar] [CrossRef]
- Mishra, S.; Roy, M.; Mohanty, K. Microalgal Bioenergy Production under Zero-Waste Biorefinery Approach: Recent Advances and Future Perspectives. Bioresour. Technol. 2019, 292, 122008. [Google Scholar] [CrossRef]
- Msanne, J.; Xu, D.; Konda, A.R.; Casas-Mollano, J.A.; Awada, T.; Cahoon, E.B.; Cerutti, H. Metabolic and Gene Expression Changes Triggered by Nitrogen Deprivation in the Photoautotrophically Grown Microalgae Chlamydomonas reinhardtii and Coccomyxa Sp. C-169. Phytochemistry 2012, 75, 50–59. [Google Scholar] [CrossRef] [Green Version]
- Mussgnug, J.H. Genetic Tools and Techniques for Chlamydomonas reinhardtii. Appl. Microbiol. Biotechnol. 2015, 99, 5407–5418. [Google Scholar] [CrossRef]
- Mussgnug, J.H.; Klassen, V.; Schlüter, A.; Kruse, O. Microalgae as Substrates for Fermentative Biogas Production in a Combined Biorefinery Concept. J. Biotechnol. 2010, 150, 51–56. [Google Scholar] [CrossRef]
- Naghshbandi, M.P.; Tabatabaei, M.; Aghbashlo, M.; Aftab, M.N.; Iqbal, I. Metabolic Engineering of Microalgae for Biofuel Production. Methods Mol. Biol. 2020, 1980, 153–172. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M.T.; Freitag, J.F.; Cavanhi, V.A.F.; Colla, L.M. Microalgae Harvesting by Fungal-Assisted Bioflocculation. Rev. Environ. Sci. Biotechnol. 2020, 19, 369–388. [Google Scholar] [CrossRef]
- Ng, I.-S.; Tan, S.-I.; Kao, P.-H.; Chang, Y.-K.; Chang, J.-S. Recent Developments on Genetic Engineering of Microalgae for Biofuels and Bio-based Chemicals. Biotechnol. J. 2017, 12, 1600644. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.-F.; Wang, X.; Hu, D.-X.; Balamurugan, S.; Li, D.-W.; Yang, W.-D.; Liu, J.-S.; Li, H.-Y. Molecular Characterization of a Glycerol-3-Phosphate Acyltransferase Reveals Key Features Essential for Triacylglycerol Production in Phaeodactylum tricornutum. Biotechnol. Biofuels 2016, 9, 60. [Google Scholar] [CrossRef] [Green Version]
- Nizami, A.-S.; Rehan, M. Towards Nanotechnology-Based Biofuel Industry. Biofuel Res. J. 2018, 5, 798–799. [Google Scholar] [CrossRef] [Green Version]
- Otondo, A.; Kokabian, B.; Stuart-Dahl, S.; Gude, V.G. Energetic Evaluation of Wastewater Treatment Using Microalgae, Chlorella vulgaris. J. Environ. Chem. Eng. 2018, 6, 3213–3222. [Google Scholar] [CrossRef]
- Peng, L.; Fu, D.; Chu, H.; Wang, Z.; Qi, H. Biofuel Production from Microalgae: A Review. Environ. Chem. Lett. 2020, 18, 285–297. [Google Scholar] [CrossRef]
- Qin, L.; Wei, D.; Wang, Z.; Alam, M.A. Advantage Assessment of Mixed Culture of Chlorella vulgaris and Yarrowia lipolytica for Treatment of Liquid Digestate of Yeast Industry and Cogeneration of Biofuel Feedstock. Appl. Biochem. Biotechnol. 2019, 187, 856–869. [Google Scholar] [CrossRef]
- Qu, Z.; Duan, P.; Cao, X.; Liu, M.; Lin, L.; Li, M. Comparison of Monoculture and Mixed Culture (Scenedesmus obliquus and Wild algae) for C, N, and P Removal and Lipid Production. Environ. Sci. Pollut. Res. Int. 2019, 26, 20961–20968. [Google Scholar] [CrossRef]
- Radakovits, R.; Eduafo, P.M.; Posewitz, M.C. Genetic Engineering of Fatty Acid Chain Length in Phaeodactylum tricornutum. Metab. Eng. 2011, 13, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Radakovits, R.; Jinkerson, R.E.; Darzins, A.; Posewitz, M.C. Genetic Engineering of Algae for Enhanced Biofuel Production. Eukaryot. Cell 2010, 9, 486–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahpeyma, S.S.; Raheb, J. Microalgae Biodiesel as a Valuable Alternative to Fossil Fuels. Bioenergy Res. 2019, 12, 958–965. [Google Scholar] [CrossRef]
- Rai, V.; Muthuraj, M.; Gandhi, M.N.; Das, D.; Srivastava, S. Real-Time ITRAQ-Based Proteome Profiling Revealed the Central Metabolism Involved in Nitrogen Starvation Induced Lipid Accumulation in Microalgae. Sci. Rep. 2017, 7, 45732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banu, J.R.; Preethi, S.; Kavitha, S.; Gunasekaran, M.; Kumar, G. Microalgae Based Biorefinery Promoting Circular Bioeconomy-Techno Economic and Life-Cycle Analysis. Bioresour. Technol. 2020, 302, 122822. [Google Scholar] [CrossRef]
- Ramanan, R.; Kim, B.-H.; Cho, D.-H.; Oh, H.-M.; Kim, H.-S. Algae-Bacteria Interactions: Evolution, Ecology and Emerging Applications. Biotechnol. Adv. 2016, 34, 14–29. [Google Scholar] [CrossRef] [Green Version]
- Rashid, N.; Ryu, A.J.; Jeong, K.J.; Lee, B.; Chang, Y.-K. Co-Cultivation of Two Freshwater Microalgae Species to Improve Biomass Productivity and Biodiesel Production. Energy Convers. Manag. 2019, 196, 640–648. [Google Scholar] [CrossRef]
- Ray, A.; Banerjee, S.; Das, D. Microalgal Bio-Flocculation: Present Scenario and Prospects for Commercialization. Environ. Sci. Pollut. Res. Int. 2021, 28, 26294–26312. [Google Scholar] [CrossRef]
- Ray, A.; Nayak, M.; Ghosh, A. A Review on Co-Culturing of Microalgae: A Greener Strategy towards Sustainable Biofuels Production. Sci. Total Environ. 2022, 802, 149765. [Google Scholar] [CrossRef] [PubMed]
- Romero-García, J.M.; Gutiérrez, C.D.B.; Toro, J.C.S.; Alzate, C.A.C.; Castro, E. Environmental Assessment of Biorefineries. In Biosynthetic Technology and Environmental Challenges; Springer: Singapore, 2018; pp. 377–401. ISBN 9789811074332. [Google Scholar]
- Santos, C.A.; Ferreira, M.E.; da Silva, T.L.; Gouveia, L.; Novais, J.M.; Reis, A. A Symbiotic Gas Exchange between Bioreactors Enhances Microalgal Biomass and Lipid Productivities: Taking Advantage of Complementary Nutritional Modes. J. Ind. Microbiol. Biotechnol. 2011, 38, 909–917. [Google Scholar] [CrossRef]
- Mussgnug, J.H.; Thomas-Hall, S.; Rupprecht, J.; Foo, A.; Klassen, V.; McDowall, A.; Schenk, P.M.; Kruse, O.; Hankamer, B. Engineering Photosynthetic Light Capture: Impacts on Improved Solar Energy to Biomass Conversion. Plant Biotechnol. J. 2007, 5, 802–814. [Google Scholar] [CrossRef]
- Schroda, M. RNA Silencing in Chlamydomonas: Mechanisms and Tools. Curr. Genet. 2006, 49, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Schuhmann, H.; Lim, D.K.Y.; Schenk, P.M. Perspectives on Metabolic Engineering for Increased Lipid Contents in Microalgae. Biofuels 2012, 3, 71–86. [Google Scholar] [CrossRef]
- Shahid, A.; Malik, S.; Zhu, H.; Xu, J.; Nawaz, M.Z.; Nawaz, S.; Asraful Alam, M.; Mehmood, M.A. Cultivating Microalgae in Wastewater for Biomass Production, Pollutant Removal, and Atmospheric Carbon Mitigation; a Review. Sci. Total Environ. 2020, 704, 135303. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S.; Ngo, H.-H.; Wu, Y.-R. Advanced CRISPR/Cas-Based Genome Editing Tools for Microbial Biofuels Production: A Review. Renew. Energy 2020, 149, 1107–1119. [Google Scholar] [CrossRef]
- Shi, S.; Valle-Rodríguez, J.O.; Siewers, V.; Nielsen, J. Prospects for Microbial Biodiesel Production. Biotechnol. J. 2011, 6, 277–285. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.-E.; Lim, J.-M.; Koh, H.G.; Kim, E.K.; Kang, N.K.; Jeon, S.; Kwon, S.; Shin, W.-S.; Lee, B.; Hwangbo, K.; et al. CRISPR/Cas9-Induced Knockout and Knock-in Mutations in Chlamydomonas reinhardtii. Sci. Rep. 2016, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.S.; Jeong, J.; Nguyen, T.H.T.; Kim, J.Y.H.; Jin, E.; Sim, S.J. Targeted Knockout of Phospholipase A2 to Increase Lipid Productivity in Chlamydomonas reinhardtii for Biodiesel Production. Bioresour. Technol. 2019, 271, 368–374. [Google Scholar] [CrossRef]
- Singh, A.; Nigam, P.S.; Murphy, J.D. Mechanism and Challenges in Commercialisation of Algal Biofuels. Bioresour. Technol. 2011, 102, 26–34. [Google Scholar] [CrossRef]
- Kaushik, G. Applied Environmental Biotechnology: Present Scenario and Future Trends; Kaushik, G., Ed.; Springer: New Delhi, India, 2015; ISBN 9788132221227. [Google Scholar]
- Singh, U.B.; Ahluwalia, A.S. Microalgae: A Promising Tool for Carbon Sequestration. Mitig. Adapt. Strateg. Glob. Chang. 2013, 18, 73–95. [Google Scholar] [CrossRef]
- Sizova, I.; Greiner, A.; Awasthi, M.; Kateriya, S.; Hegemann, P. Nuclear Gene Targeting in Chlamydomonas Using Engineered Zinc-Finger Nucleases. Plant J. 2013, 73, 873–882. [Google Scholar] [CrossRef]
- Skjånes, K.; Rebours, C.; Lindblad, P. Potential for Green Microalgae to Produce Hydrogen, Pharmaceuticals and Other High Value Products in a Combined Process. Crit. Rev. Biotechnol. 2013, 33, 172–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spicer, A.; Molnar, A. Gene Editing of Microalgae: Scientific Progress and Regulatory Challenges in Europe. Biology 2018, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial Applications of Microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Srinuanpan, S.; Cheirsilp, B.; Prasertsan, P.; Kato, Y.; Asano, Y. Photoautotrophic Cultivation of Oleaginous Microalgae and Co-Pelletization with Filamentous Fungi for Cost-Effective Harvesting Process and Improved Lipid Yield. Aquac. Int. 2018, 26, 1493–1509. [Google Scholar] [CrossRef]
- Suali, E.; Sarbatly, R. Conversion of Microalgae to Biofuel. Renew. Sustain. Energy Rev. 2012, 16, 4316–4342. [Google Scholar] [CrossRef]
- Sun, X.-M.; Ren, L.-J.; Zhao, Q.-Y.; Ji, X.-J.; Huang, H. Microalgae for the Production of Lipid and Carotenoids: A Review with Focus on Stress Regulation and Adaptation. Biotechnol. Biofuels 2018, 11, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trentacoste, E.M.; Shrestha, R.P.; Smith, S.R.; Glé, C.; Hartmann, A.C.; Hildebrand, M.; Gerwick, W.H. Metabolic Engineering of Lipid Catabolism Increases Microalgal Lipid Accumulation without Compromising Growth. Proc. Natl. Acad. Sci. USA 2013, 110, 19748–19753. [Google Scholar] [CrossRef] [Green Version]
- Tribelli, P.M.; López, N.I. Poly(3-Hydroxybutyrate) Influences Biofilm Formation and Motility in the Novel Antarctic Species Pseudomonas extremaustralis under Cold Conditions. Extremophiles 2011, 15, 541–547. [Google Scholar] [CrossRef]
- Vanthoor-Koopmans, M.; Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H.M. Biorefinery of Microalgae for Food and Fuel. Bioresour. Technol. 2013, 135, 142–149. [Google Scholar] [CrossRef]
- Vo Hoang Nhat, P.; Ngo, H.H.; Guo, W.S.; Chang, S.W.; Nguyen, D.D.; Nguyen, P.D.; Bui, X.T.; Zhang, X.B.; Guo, J.B. Can Algae-Based Technologies Be an Affordable Green Process for Biofuel Production and Wastewater Remediation? Bioresour. Technol. 2018, 256, 491–501. [Google Scholar] [CrossRef]
- Ort, D.R.; Melis, A. Optimizing Antenna Size to Maximize Photosynthetic Efficiency. Plant Physiol. 2011, 155, 79–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Chen, X.; Li, H.; Wang, J.; Hu, Z. Artificial MiRNA Inhibition of Phosphoenolpyruvate Carboxylase Increases Fatty Acid Production in a Green Microalga Chlamydomonas reinhardtii. Biotechnol. Biofuels 2017, 10, 91. [Google Scholar] [CrossRef] [Green Version]
- Wayama, M.; Ota, S.; Matsuura, H.; Nango, N.; Hirata, A.; Kawano, S. Three-Dimensional Ultrastructural Study of Oil and Astaxanthin Accumulation during Encystment in the Green Alga Haematococcus pluvialis. PLoS ONE 2013, 8, e53618. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.W.M.; Lee, Y.K. The Dilemma for Lipid Productivity in Green Microalgae: Importance of Substrate Provision in Improving Oil Yield without Sacrificing Growth. Biotechnol. Biofuels 2016, 9, 255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wrede, D.; Taha, M.; Miranda, A.F.; Kadali, K.; Stevenson, T.; Ball, A.S.; Mouradov, A. Co-Cultivation of Fungal and Microalgal Cells as an Efficient System for Harvesting Microalgal Cells, Lipid Production and Wastewater Treatment. PLoS ONE 2014, 9, e113497. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Li, X.; Yu, J.; Wang, Q. Increased Hydrogen Production in Co-Culture of Chlamydomonas reinhardtii and Bradyrhizobium japonicum. Bioresour. Technol. 2012, 123, 184–188. [Google Scholar] [CrossRef]
- Xue, F.; Miao, J.; Zhang, X.; Tan, T. A New Strategy for Lipid Production by Mix Cultivation of Spirulina platensis and Rhodotorula glutinis. Appl. Biochem. Biotechnol. 2010, 160, 498–503. [Google Scholar] [CrossRef]
- Xue, J.; Balamurugan, S.; Li, D.-W.; Liu, Y.-H.; Zeng, H.; Wang, L.; Yang, W.-D.; Liu, J.-S.; Li, H.-Y. Glucose-6-Phosphate Dehydrogenase as a Target for Highly Efficient Fatty Acid Biosynthesis in Microalgae by Enhancing NADPH Supply. Metab. Eng. 2017, 41, 212–221. [Google Scholar] [CrossRef]
- Xue, J.; Li, T.; Chen, T.-T.; Balamurugan, S.; Yang, W.-D.; Li, H.-Y. Regulation of Malate-Pyruvate Pathway Unifies the Adequate Provision of Metabolic Carbon Precursors and NADPH in Tetradesmus obliquus. Algal Res. 2021, 57, 102340. [Google Scholar] [CrossRef]
- Xue, J.; Niu, Y.-F.; Huang, T.; Yang, W.-D.; Liu, J.-S.; Li, H.-Y. Genetic Improvement of the Microalga Phaeodactylum tricornutum for Boosting Neutral Lipid Accumulation. Metab. Eng. 2015, 27, 1–9. [Google Scholar] [CrossRef]
- Yan, J.; Cheng, R.; Lin, X.; You, S.; Li, K.; Rong, H.; Ma, Y. Overexpression of Acetyl-CoA Synthetase Increased the Biomass and Fatty Acid Proportion in Microalga Schizochytrium. Appl. Microbiol. Biotechnol. 2013, 97, 1933–1939. [Google Scholar] [CrossRef]
- Yang, B.; Liu, J.; Jiang, Y.; Chen, F. Chlorella Species as Hosts for Genetic Engineering and Expression of Heterologous Proteins: Progress, Challenge and Perspective. Biotechnol. J. 2016, 11, 1244–1261. [Google Scholar] [CrossRef]
- Yang, B.; Liu, J.; Ma, X.; Guo, B.; Liu, B.; Wu, T.; Jiang, Y.; Chen, F. Genetic Engineering of the Calvin Cycle toward Enhanced Photosynthetic CO2 Fixation in Microalgae. Biotechnol. Biofuels 2017, 10, 229. [Google Scholar] [CrossRef]
- Yao, L.; Cengic, I.; Anfelt, J.; Hudson, E.P. Multiple Gene Repression in Cyanobacteria Using CRISPRi. ACS Synth. Biol. 2016, 5, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-W.; Hu, I.-C.; Chen, C.-Y.; Ho, S.-H.; Lee, D.-J.; Chang, J.-S. Microalgae-Based Biorefinery—From Biofuels to Natural Products. Bioresour. Technol. 2013, 135, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Zanchett, G.; Oliveira-Filho, E.C. Cyanobacteria and Cyanotoxins: From Impacts on Aquatic Ecosystems and Human Health to Anticarcinogenic Effects. Toxins 2013, 5, 1896–1917. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, J.; Zhai, X.; Che, J.; Xiu, Z.; Chi, Z. Carbonate Assisted Lipid Extraction and Biodiesel Production from Wet Microalgal Biomass and Recycling Waste Carbonate for CO2 Supply in Microalgae Cultivation. Sci. Total Environ. 2021, 779, 146445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ji, H.; Gong, G.; Zhang, X.; Tan, T. Synergistic Effects of Oleaginous Yeast Rhodotorula glutinis and Microalga Chlorella vulgaris for Enhancement of Biomass and Lipid Yields. Bioresour. Technol. 2014, 164, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Yu, X.; Li, J.; Tang, X.; Huang, Z. Enhancing Lipid Productivity by Co-Cultivation of Chlorella Sp. U4341 and Monoraphidium Sp. FXY-10. J. Biosci. Bioeng. 2014, 118, 72–77. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, G.; Sun, S.; Hu, C.; Liu, J. Co-Pelletization of Microalgae and Fungi for Efficient Nutrient Purification and Biogas Upgrading. Bioresour. Technol. 2019, 289, 121656. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Huo, S.; Qin, L. A Microalgae-Based Biodiesel Refinery: Sustainability Concerns and Challenges. Int. J. Green Energy 2015, 12, 595–602. [Google Scholar] [CrossRef]
- Zhu, L.; Li, S.; Hu, T.; Nugroho, Y.K.; Yin, Z.; Hu, D.; Chu, R.; Mo, F.; Liu, C.; Hiltunen, E. Effects of Nitrogen Source Heterogeneity on Nutrient Removal and Biodiesel Production of Mono- and Mix-Cultured Microalgae. Energy Convers. Manag. 2019, 201, 112144. [Google Scholar] [CrossRef]
- Zoller, S.; Lutzoni, F. Slow Algae, Fast Fungi: Exceptionally High Nucleotide Substitution Rate Differences between Lichenized Fungi Omphalina and Their Symbiotic Green Algae Coccomyxa. Mol. Phylogenet. Evol. 2003, 29, 629–640. [Google Scholar] [CrossRef]
- Sharmila, V.G.; Kumar, M.D.; Pugazhendi, A.; Bajhaiya, A.K.; Gugulothu, P.; Banu, J.R. Biofuel Production from Macroalgae: Present Scenario and Future Scope. Bioengineered 2021, 12, 9216–9238. [Google Scholar] [CrossRef]


| S.No | Microalgae | Lipid Productivity (mg L−1 d−1) | Production Process | Operational Parameters | Genetic/Metabolic Approach | Targeted Genes | Value-Added Product | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | Chlorella minutissima | 1.37 | Continuous | Temperature—25 °C–30 °C, pH—5 to 8 Mixotrophic | Molecular | Co-expression of five acyltransferases | Biodiesel and also antioxidants | [16] |
| 2 | Chlorella sorokiniana | 0.85 | Batch | Temperature—25 °C pH—6 heterotrophic | Metabolic | Ribulose-bisphosphate carboxylase and acetyl–CoA carboxylase | Biodiesel | [16] |
| 3 | Neochloris oleoabundans | 1.13 | Batch | Temperature—25 °C pH—8 Mixotrophic | Molecular | Glycerol-3-phospahte acyltransferase | Triacylglycerols Biodiesel | [43] |
| 4 | Chlorella vulgaris | 0.91 | Batch | Temperature—25 °C pH—10 Autotrophic | Molecular | Carbonic anhydrase | Biodiesel | [21] |
| 5 | Chlorella pyrenoidosa | 1.45 | Fed-Batch | Temperature—25 °C–30 °C pH—7 to 10 Mixotrophic | Molecular | NAH (H) kinase | Biodiesel and PUFA | [43] |
| 6 | Phaeodactylum Tricornutum | 1.11 | Batch | Temp—25 °C pH—8 to 10 Mixotrophic | Molecular | Pyruvate dehydrogenase | Biodiesel | [16] |
| 7 | Chlamydomonas reinhardtii | 109 | Semi-continuous | Temp—25 °C pH—5 to 10 | Molecular | acetyl–CoA-synthetase | Biodiesel | [44] |
| S.No | Microalgae | Culture System | Biomass Productivity | Lipid Productivity | Lipid Content | Type of Inducer | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Chlorella vulgaris | Algal bloom hydrolysate | 436 mg L−1d−1 | 188 mg L−1 d−1 | 53% | Nitrogen starvation | [92] |
| 2 | Monoraphidium dybowskii LB50 | BG-11 medium | 80.56 mg L−1d−1 | 31.12 mg L−1d−1 | 44.4% | Nitrogen starvation | [147] |
| 3 | Chlorella sorokiniana | Defined medium | 300 mg L−1d−1 | 502 mg L−1d−1 | ~25% AFDW lipids | IAA cytokinin kinetin (K) | [90] |
| 4 | Tetraselmis sp. | Charcoal -filtered seawater with nutrient enrichment | 201 mg L−1d−1 | 85.5 mg L−1d−1 | 45% | Salinity + Nitrogen | [91] |
| 5 | Para chlorella | ½ SS nutrient medium | 409-1291mg L−1d−1 | 161–604 mg L−1d−1 | 66% | Nutrient sulphur deprivation | [93] |
| 6 | Chlorococcum sp. | BG-11 | 175 mg L−1d−1 | 2.0–19.3 mg L−1d−1 | 56% | Nitrogen starvation | [94] |
| 7 | Nannochloroposis sp. | BG-11 medium(+ glucose or acetate) | 90–145 mg L−1d−1 | 324 mg L−1d−1 | 18.16–25.49% | Salinity | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babu, S.S.; Gondi, R.; Vincent, G.S.; JohnSamuel, G.C.; Jeyakumar, R.B. Microalgae Biomass and Lipids as Feedstock for Biofuels: Sustainable Biotechnology Strategies. Sustainability 2022, 14, 15070. https://doi.org/10.3390/su142215070
Babu SS, Gondi R, Vincent GS, JohnSamuel GC, Jeyakumar RB. Microalgae Biomass and Lipids as Feedstock for Biofuels: Sustainable Biotechnology Strategies. Sustainability. 2022; 14(22):15070. https://doi.org/10.3390/su142215070
Chicago/Turabian StyleBabu, Swathi Somaiyan, Rashmi Gondi, Godvin Sharmila Vincent, Godwin Christopher JohnSamuel, and Rajesh Banu Jeyakumar. 2022. "Microalgae Biomass and Lipids as Feedstock for Biofuels: Sustainable Biotechnology Strategies" Sustainability 14, no. 22: 15070. https://doi.org/10.3390/su142215070







