Preparation, Optimization, and Characterization of Inclusion Complexes of Cinnamomum longepaniculatum Essential Oil in β-Cyclodextrin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Inclusion Complexes
2.3. Encapsulation Efficiency (EE) and Loading Efficiency (LE)
2.4. The Response Surface Methodology
2.4.1. Single-Factor Experiments
2.4.2. Optimization of Experimental Design
2.5. Fourier Transform Infrared (FTIR) Spectroscopy
2.6. X-ray Diffractogram (XRD) Analysis
2.7. Thermogravimetry/Derivative Thermogravimetry (TG/DTG) Analysis
2.8. Differential Scanning Calorimetry (DSC)
2.9. Morphological Examination
2.10. Statistical Analysis
3. Results and Discussion
3.1. Effects of Single Factors
3.2. Optimization of CLEO/β-CD-IC
3.3. FTIR Spectroscopy
3.4. XRD Analysis
3.5. TG/DTG and DSC
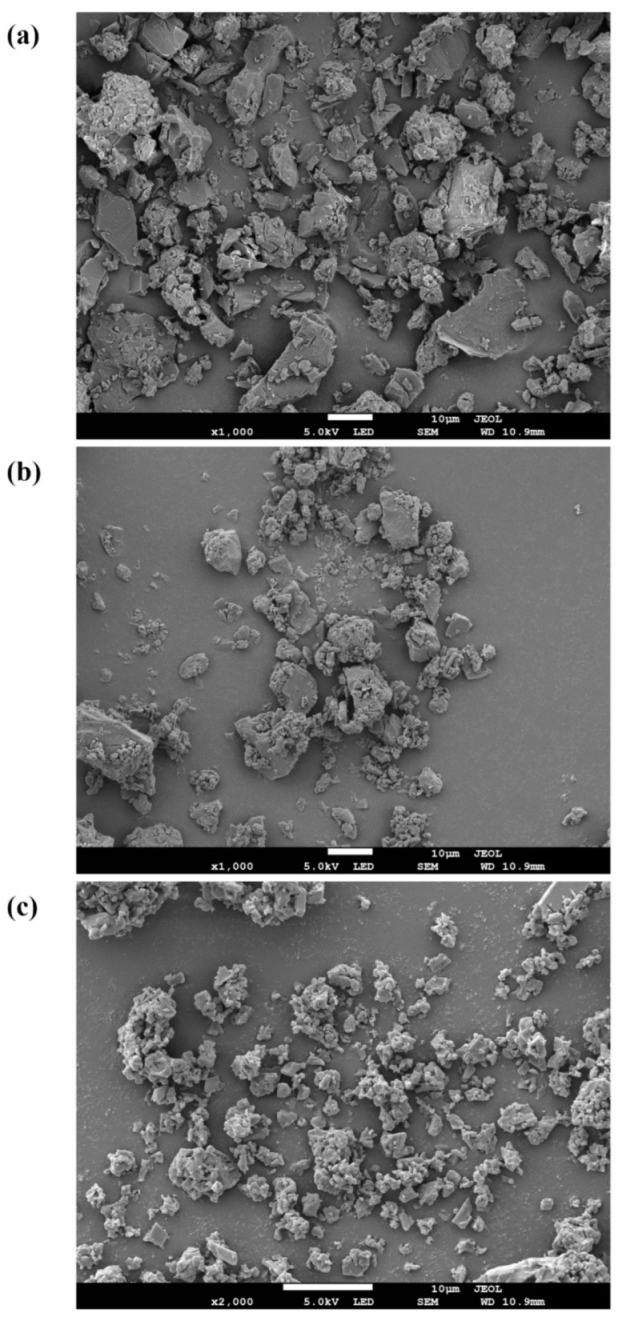
3.6. Morphological Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, X.; Yan, Y.; Zhou, W.H.; Feng, R.Z.; Shuai, Y.K.; Yang, L.; Liu, M.J.; He, X.Y.; Wei, Q. Transcriptome and metabolome reveal the accumulation of secondary metabolites in different varieties of Cinnamomum longepaniculatum. BMC Plant Biol. 2022, 22, 243. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, L.; Zou, Y.; Luo, S.; Wang, X.; Liang, Y.; Du, Y.; Feng, R.; Wei, Q. Antibacterial activity and mechanism of three isomeric terpineols of Cinnamomum longepaniculatum leaf oil. Folia Microbiol. 2021, 66, 59–67. [Google Scholar] [CrossRef]
- Yin, H.; Wang, C.; Yue, J.; Jiao, S.S.; Wang, X.H.; Jiang, L.L. Extraction of Cinnamomum longipaniculatum Essential Oil and Its Chemical Composition, Antioxidation and Antibacterial Properties. Stored Proced. 2020, 20, 183–191. [Google Scholar]
- Li, L.; Li, Z.W.; Yin, Z.Q.; Wei, Q.; Jia, R.Y.; Zhou, L.J.; Xu, J.; Song, X.; Zhou, Y.; Du, Y.H.; et al. Antibacterial activity of leaf essential oil and its constituents from Cinnamomum longepaniculatum. Int. J. Clin. Exp. Med. 2014, 7, 1721–1727. [Google Scholar]
- Du, Y.H.; Feng, R.Z.; Li, Q.; Wei, Q.; Yin, Z.Q.; Zhou, L.J.; Tao, C.; Jia, R.Y. Anti-inflammatory activity of leaf essential oil from Cinnamomum longepaniculatum (Gamble) N. Chao. Int. J. Clin. Exp. Med. 2014, 7, 5612–5620. [Google Scholar]
- Su, Z.; Qin, Y.; Zhang, K.; Bi, Y.; Kong, F. Inclusion Complex of Exocarpium Citri Grandis Essential Oil with β-Cyclodextrin: Characterization, Stability, and Antioxidant Activity. J. Food Sci. 2019, 84, 1592–1599. [Google Scholar] [CrossRef]
- Cao, C.; Wei, D.; Xu, L.; Hu, J.; Qi, J.; Zhou, Y. Characterization of tea tree essential oil and large-ring cyclodextrins (CD(9)-CD(22)) inclusion complex and evaluation of its thermal stability and volatility. J. Sci. Food Agric. 2021, 101, 2877–2883. [Google Scholar] [CrossRef]
- Mura, P. Advantages of the combined use of cyclodextrins and nanocarriers in drug delivery: A review. Int. J. Pharm. 2020, 579, 119181. [Google Scholar] [CrossRef] [PubMed]
- Hădărugă, N.G.; Szakal, R.N.; Chirilă, C.A.; Lukinich-Gruia, A.T.; Păunescu, V.; Muntean, C.; Rusu, G.; Bujancă, G.; Hădărugă, D.I. Complexation of Danube common nase (Chondrostoma nasus L.) oil by β-cyclodextrin and 2-hydroxypropyl-β-cyclodextrin. Food Chem. 2020, 303, 125419. [Google Scholar] [CrossRef]
- Marques, C.S.F.; Barreto, N.S.; Oliveira, S.S.C.; Santos, A.L.S.; Branquinha, M.H.; Sousa, D.P.; Castro, M.; Andrade, L.N.; Pereira, M.M.; Silva, C.F.D.; et al. β-Cyclodextrin/Isopentyl Caffeate Inclusion Complex: Synthesis, Characterization and Antileishmanial Activity. Molecules 2020, 25, 4181. [Google Scholar] [CrossRef]
- Kringel, D.H.; Antunes, M.D.; Klein, B.; Crizel, R.L.; Wagner, R.; de Oliveira, R.P.; Dias, A.R.G.; Zavareze, E.D.R. Production, Characterization, and Stability of Orange or Eucalyptus Essential Oil/β-Cyclodextrin Inclusion Complex. J. Food Sci. 2017, 82, 2598–2605. [Google Scholar] [CrossRef]
- Shrestha, M.; Ho, T.M.; Bhandari, B.R. Encapsulation of tea tree oil by amorphous beta-cyclodextrin powder. Food Chem. 2017, 221, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Cunha, F.V.M.; do Nascimento Caldas Trindade, G.; da Silva Azevedo, P.S.; Coêlho, A.G.; Braz, E.M.; Pereira de Sousa Neto, B.; de Rezende, D.C.; de Sousa, D.P.; de Assis Oliveira, F.; Nunes, L.C.C. Ethyl ferulate/β-cyclodextrin inclusion complex inhibits edema formation. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 115, 111057. [Google Scholar] [CrossRef] [PubMed]
- Mu, K.; Jiang, K.; Wang, Y.; Zhao, Z.; Cang, S.; Bi, K.; Li, Q.; Liu, R. The Biological Fate of Pharmaceutical Excipient β-Cyclodextrin: Pharmacokinetics, Tissue Distribution, Excretion, and Metabolism of β-Cyclodextrin in Rats. Molecules 2022, 27, 1138. [Google Scholar] [CrossRef]
- Lin, L.; Liao, X.; Li, C.; Abdel-Samie, M.A.; Siva, S.; Cui, H. Cold nitrogen plasma modified cuminaldehyde/β-cyclodextrin inclusion complex and its application in vegetable juices preservation. Food Res. Int. 2021, 141, 110132. [Google Scholar] [CrossRef]
- Fuentes Campo, A.; Sancho, M.I.; Melo, G.; Dávila, Y.A.; Gasull, E. In vitro and in vivo inhibition of Hass avocado polyphenol oxidase enzymatic browning by paeonol, β-cyclodextrin, and paeonol:β-cyclodextrin inclusion complex. J. Biosci. Bioeng. 2019, 127, 703–709. [Google Scholar] [CrossRef]
- Kringel, D.H.; Lang, G.H.; Dias, Á.R.G.; Gandra, E.A.; Valente Gandra, T.K.; da Rosa Zavareze, E. Impact of encapsulated orange essential oil with β-cyclodextrin on technological, digestibility, sensory properties of wheat cakes as well as Aspergillus flavus spoilage. J. Sci. Food Agric. 2021, 101, 5599–5607. [Google Scholar] [CrossRef]
- Hogenbom, J.; Istanbouli, M.; Faraone, N. Novel β-Cyclodextrin and Catnip Essential Oil Inclusion Complex and Its Tick Repellent Properties. Molecules 2021, 26, 7391. [Google Scholar] [CrossRef]
- Hogenbom, J.; Jones, A.; Wang, H.V.; Pickett, L.J.; Faraone, N. Synthesis and Characterization of β-Cyclodextrin-Essential Oil Inclusion Complexes for Tick Repellent Development. Polymers 2021, 13, 1892. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.S.; Carvalho, S.G.; Bertoli, L.D.; Villanova, J.C.O.; Pinheiro, P.F.; Dos Santos, D.C.M.; Yoshida, M.I.; de Freitas, J.C.C.; Cipriano, D.F.; Bernardes, P.C. β-Cyclodextrin inclusion complexes with essential oils: Obtention, characterization, antimicrobial activity and potential application for food preservative sachets. Food Res. Int. 2019, 119, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yu, J.; Jiao, C.; Tong, J.; Zhang, L.; Chang, Y.; Sun, W.; Jin, Q.; Cai, Y. Optimization of quercetin extraction method in Dendrobium officinale by response surface methodology. Heliyon 2019, 5, e02374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, C.H.; Drummond, L.; Sun, D.W.; Zhang, Z.-H. Evaluation of natural hog casings modified by surfactant solutions combined with lactic acid by response surface methodology. LWT-Food Sci. Technol. 2014, 58, 427–438. [Google Scholar] [CrossRef]
- Feng, C.H. Optimizing Procedures of Ultrasound-Assisted Extraction of Waste Orange Peels by Response Surface Methodology. Molecules 2022, 27, 2268. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.H.; Sun, D.W. Optimisation of immersion vacuum cooling operation and quality of Irish cooked sausages by using response surface methodology. Int. J. Food Sci. Technol. 2014, 49, 1850–1858. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, Z.Q.; Wei, Q.; Jia, R.Y.; Fan, J.; Zhou, L.J.; Du, Y.H. Study on preparation process and stability of beta-cyclodextrin inclusion compound in volatile oil of Cinnamomum longepaniculatum leaves. Zhongguo Zhong Yao Za Zhi 2013, 38, 2105–2108. [Google Scholar]
- Yang, Y.H.; Li, X.Z.; Zhang, S. Preparation methods and release kinetics of Litsea cubeba essential oil microcapsules. RSC Adv. 2018, 8, 29980–29987. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Zhang, J.; Yu, X.; Shu, Y.; Zhang, S.; Zhang, Y. Extraction optimization by using response surface methodology and purification of yellow pigment from Gardenia jasminoides var. radicans Makikno. Food Sci. Nutr. 2021, 9, 822–832. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, C.; Cheng, X.; Li, G.; Zhu, X. β-Cyclodextrin Inclusion Complex Containing Litsea cubeba Essential Oil: Preparation, Optimization, Physicochemical, and Antifungal Characterization. Coatings 2020, 10, 850. [Google Scholar] [CrossRef]
- Bai, C.; Xu, P.; Qi, W.; Kong, Q.; Xiong, G. Preparation and characterization of a sustained-release bio-preservative based on β-cyclodextrin encapsulated eugenol. Food Biosci. 2021, 42, 101192. [Google Scholar] [CrossRef]
- He, D.; Yan, L.; Hu, Y.; Wu, Q.; Wu, M.; Choi, J.I.; Tong, H. Optimization of Porphyran Extraction from Pyropia yezoensis by Response Surface Methodology and Its Lipid-Lowering Effects. Mar. Drugs 2021, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- Torres-Alvarez, C.; Castillo, S.; Sánchez-García, E.; Aguilera González, C.; Galindo-Rodríguez, S.A.; Gabaldón-Hernández, J.A.; Báez-González, J.G. Inclusion Complexes of Concentrated Orange Oils and β-Cyclodextrin: Physicochemical and Biological Characterizations. Molecules 2020, 25, 5109. [Google Scholar] [CrossRef] [PubMed]
- Petrović, G.M.; Stojanović, G.S.; Radulović, N.S. Encapsulation of cinnamon oil in β-cyclodextrin. J. Med. Plants Res. 2010, 4, 1382–1390. [Google Scholar]
- Ma, S.; Zhao, Z.; Liu, P. Optimization of preparation process of β-cyclodextrin inclusion compound of clove essential oil and evaluation of heat stability and antioxidant activities in vitro. J. Food Meas. Charact. 2018, 12, 2057–2067. [Google Scholar] [CrossRef]
- Li, H.; Chang, S.L.; Chang, T.R.; You, Y.; Zhao, B. Inclusion complexes of cannabidiol with β-cyclodextrin and its derivative: Physicochemical properties, water solubility, and antioxidant activity. J. Mol. Liq. 2021, 334, 116070. [Google Scholar] [CrossRef]
- Sampath, S.; Subramani, S.; Janardhanam, S.; Subramani, P.; Yuvaraj, A.; Chellan, R. Bioactive compound 1,8-Cineole selectively induces G2/M arrest in A431 cells through the upregulation of the p53 signaling pathway and molecular docking studies. Phytomedicine 2018, 46, 57–68. [Google Scholar] [CrossRef]
- Biernacka, M.; Ilyich, T.; Zavodnik, I.; Pałecz, B.; Stepniak, A. Studies of the Formation and Stability of Ezetimibe-Cyclodextrin Inclusion Complexes. Int. J. Mol. Sci. 2021, 23, 455. [Google Scholar] [CrossRef]
- Mashaqbeh, H.; Obaidat, R.; Al-Shar’i, N. Evaluation and Characterization of Curcumin-β-Cyclodextrin and Cyclodextrin-Based Nanosponge Inclusion Complexation. Polymers 2021, 13, 4073. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. Formation of Rutin-β-Cyclodextrin Inclusion Complexes by Supercritical Antisolvent Precipitation. Polymers 2021, 13, 246. [Google Scholar] [CrossRef]
- Gao, S.; Liu, Y.; Jiang, J.; Ji, Q.; Ye, F. Physicochemical properties and fungicidal activity of inclusion complexes of fungicide chlorothalonil with β-cyclodextrin and hydroxypropyl-β-cyclodextrin. J. Mol. Liq. 2019, 293, 111513. [Google Scholar] [CrossRef]
- Sbârcea, L.; Udrescu, L.; Ledeţi, I.; Szabadai, Z.; Fuliaş, A.; Sbârcea, C. β-Cyclodextrin inclusion complexes of lisinopril and zofenopril. J. Therm. Anal. Calorim. 2016, 123, 2377–2390. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Yu, T.; Yuan, L.; Rao, G.; Li, D.; Mu, C. Preparation, physicochemical characterization and release behavior of the inclusion complex of trans-anethole and β-cyclodextrin. Food Res. Int. 2015, 74, 55–62. [Google Scholar] [CrossRef]
- Tao, F.; Hill, L.E.; Peng, Y.; Gomes, C.L. Synthesis and characterization of β-cyclodextrin inclusion complexes of thymol and thyme oil for antimicrobial delivery applications. LWT-Food Sci. Technol. 2014, 59, 247–255. [Google Scholar] [CrossRef]
- Fauziah, C.I.; Zaibunnisa, A.H.; Osman, H.; Aida, W. Thermal Analysis and Surface Morphology Study of Cholesterol: Β-Cyclodextrin Inclusion Complex. Adv. Mater. Res. 2013, 812, 221–225. [Google Scholar] [CrossRef]
- Oliveira Brito Pereira Bezerra Martins, A.; Wanderley, A.G.; Alcântara, I.S.; Rodrigues, L.B.; Cesário, F.; Correia de Oliveira, M.R.; Castro, F.F.E.; Albuquerque, T.R.; da Silva, M.S.A.; Ribeiro-Filho, J.; et al. Anti-Inflammatory and Physicochemical Characterization of the Croton Rhamnifolioides Essential Oil Inclusion Complex in β-Cyclodextrin. Biology 2020, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Dos Passos Menezes, P.; de Oliveira Araujo, F.; Andrade, T.A.; Trindade, I.A.S.; de Araujo-Filho, H.G.; de Souza Siqueira Quintans, J.; Quintans-Junior, L.J.; Menezes, L.R.A.; de Almeida, R.N.; Braga, R.M.; et al. Physicochemical Characterization and Antinociceptive Effect of β-cyclodextrin/Lippia pedunculosa Essential Oil in Mice. Curr. Top. Med. Chem. 2018, 18, 797–807. [Google Scholar] [CrossRef]

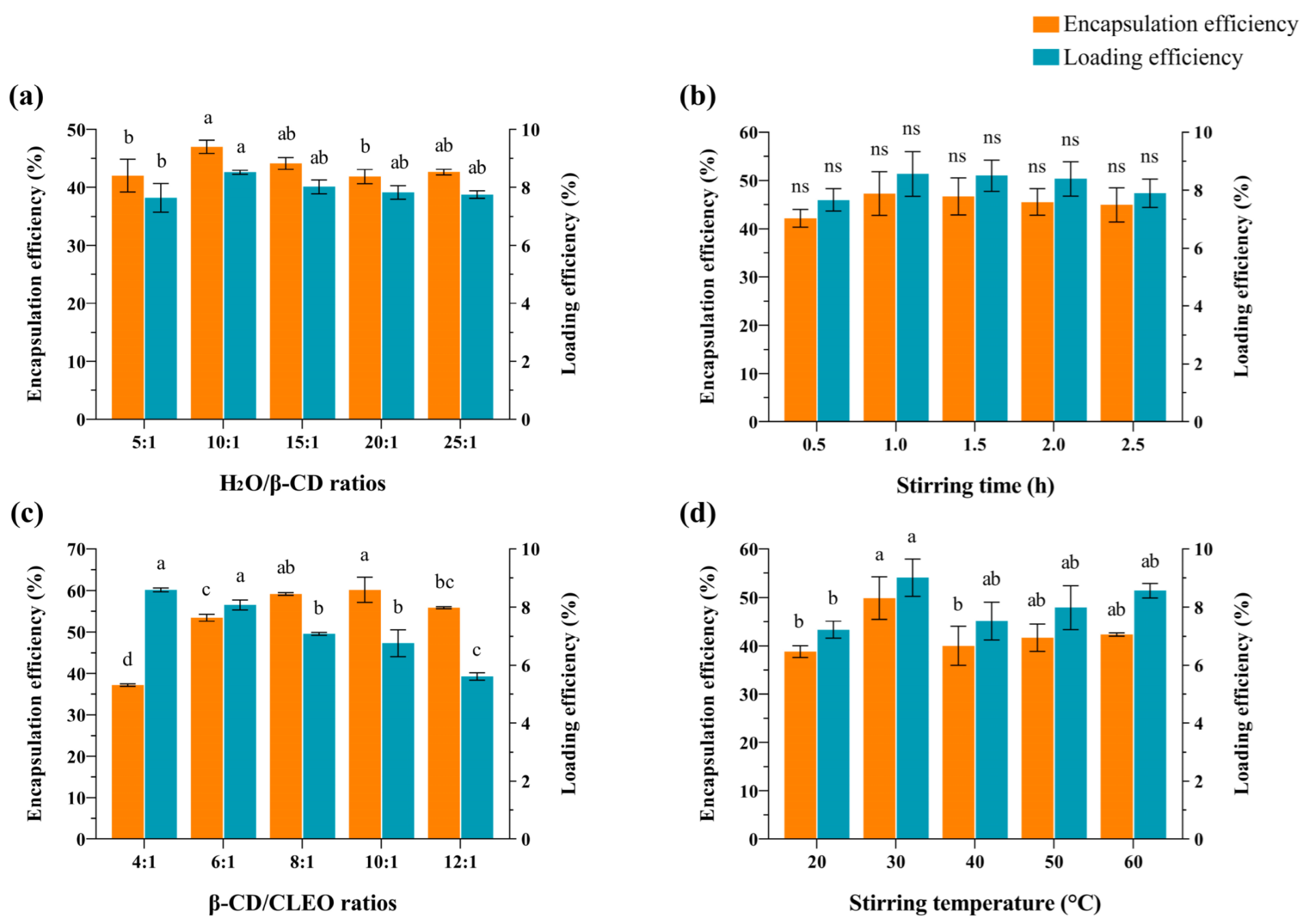

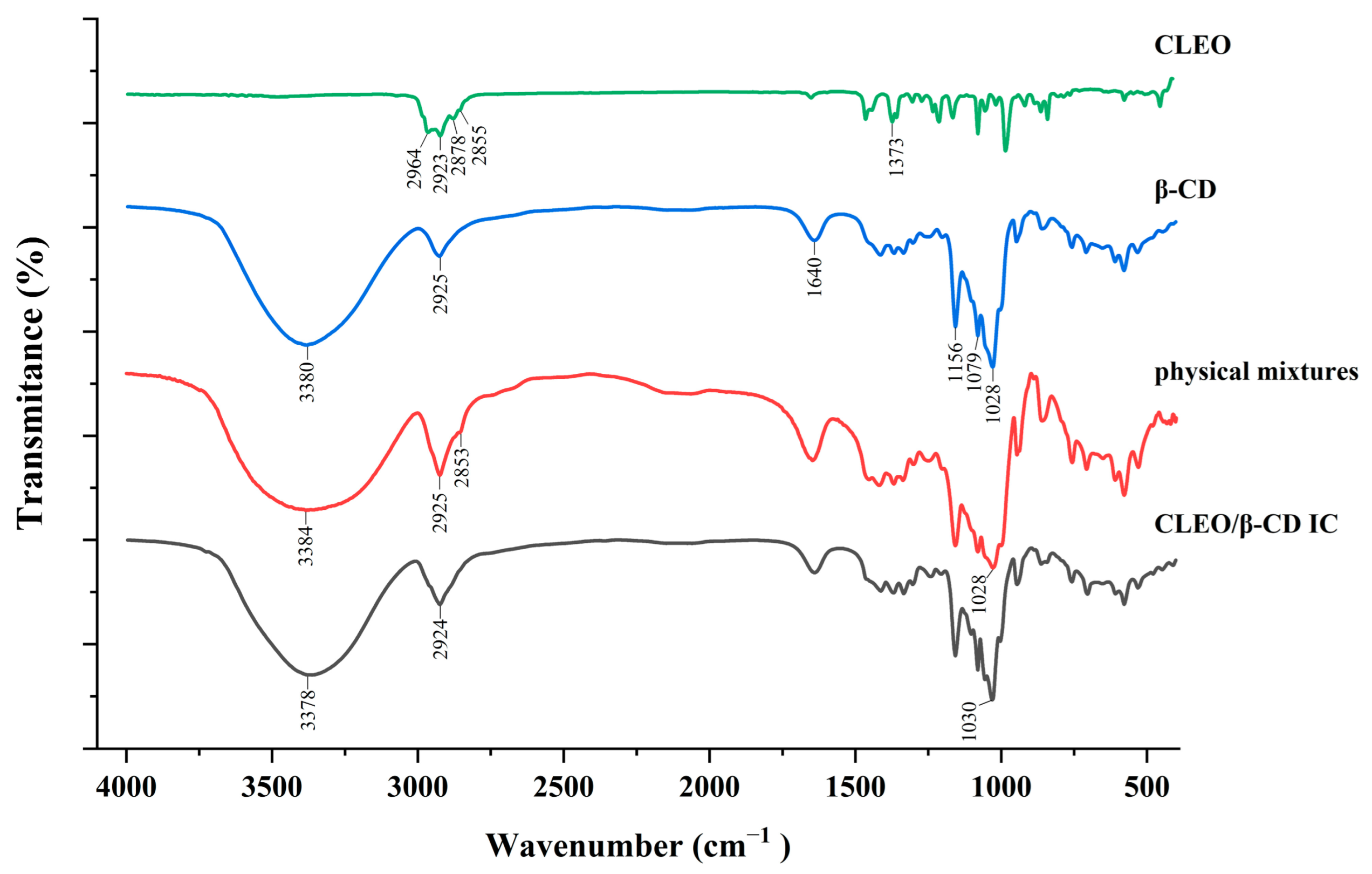
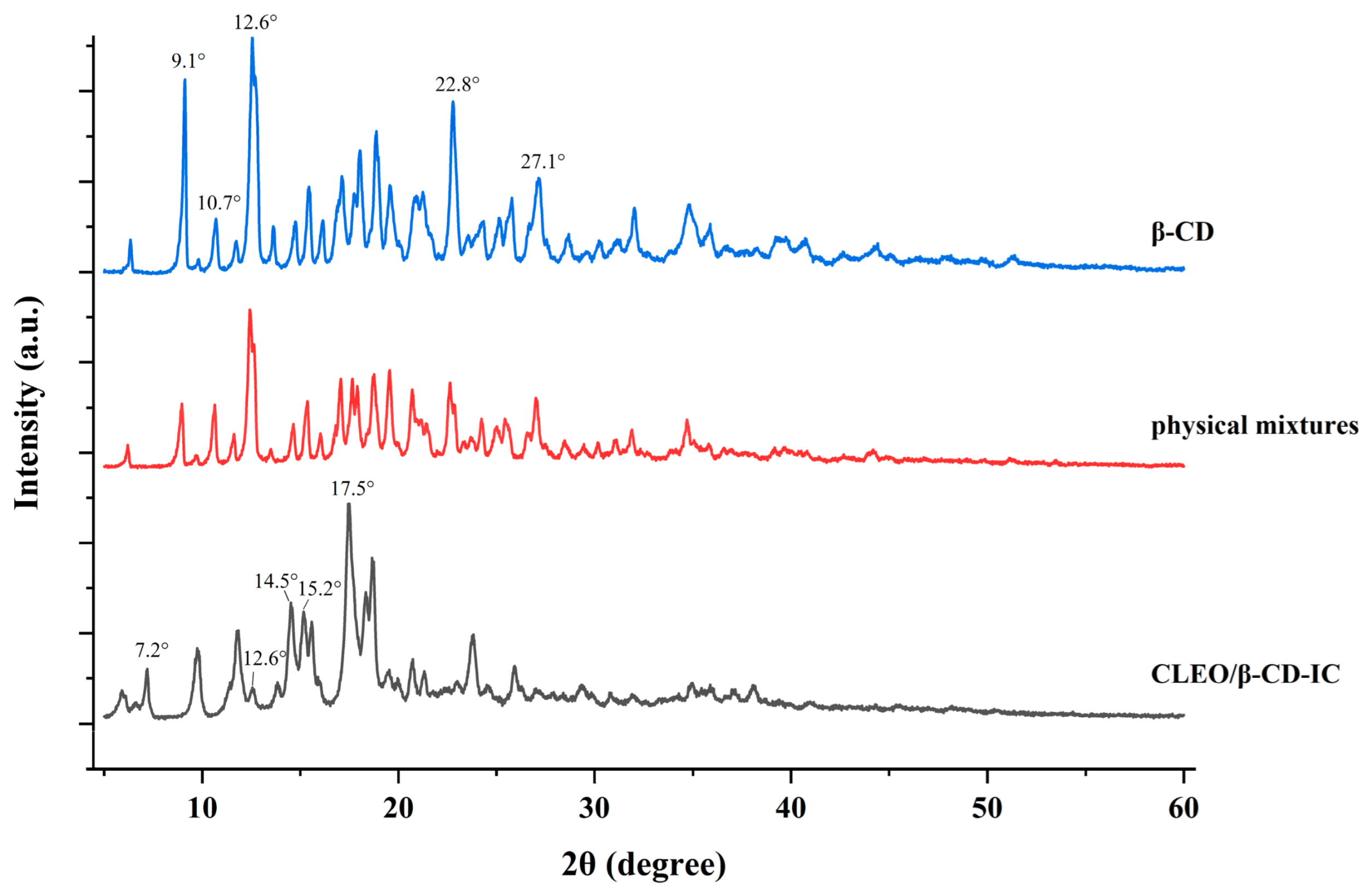
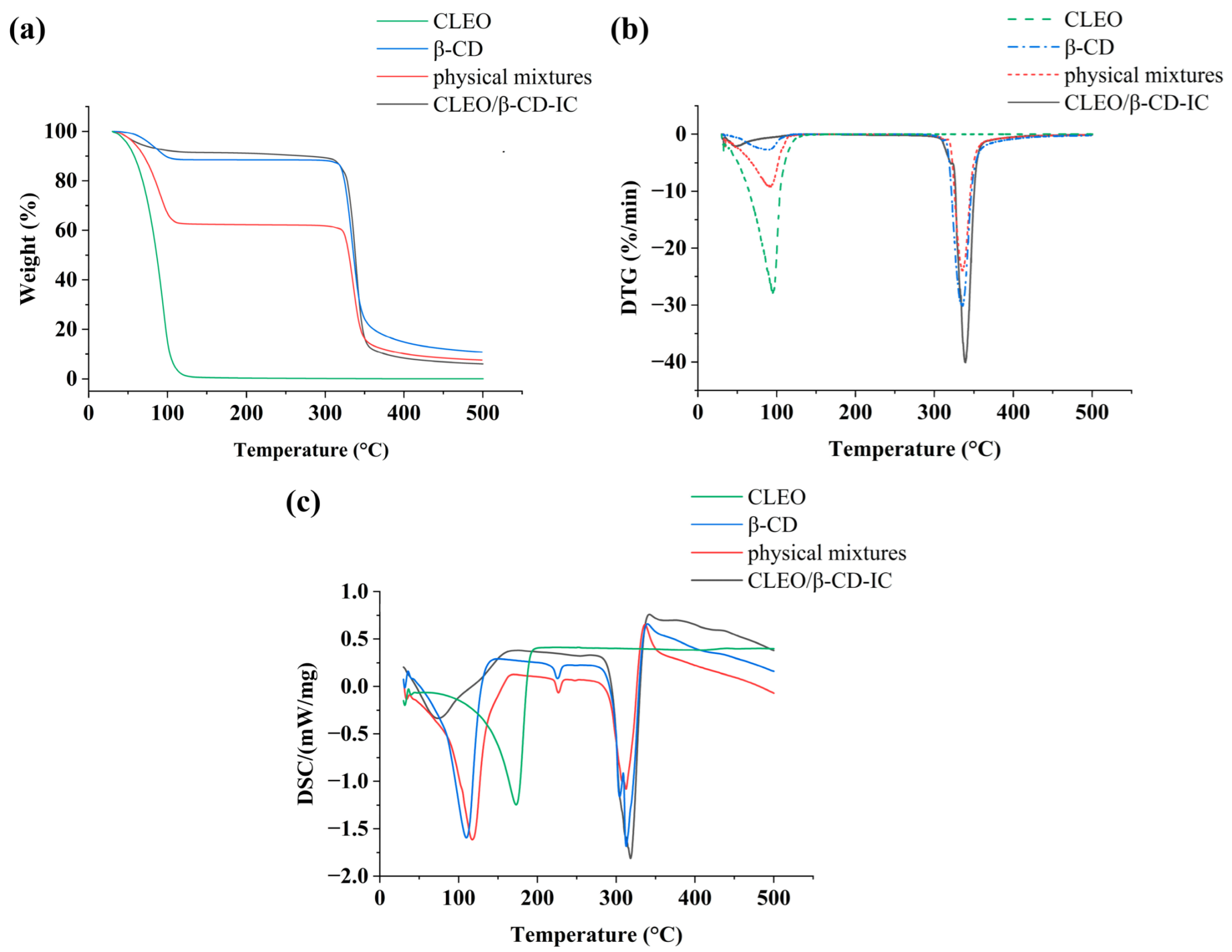
| Variable | Symbol | Level of Variation | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| H2O/β-CD ratio (g/mL) | A | 5:1 | 10:1 | 15:1 |
| β-CD/CLEO ratio (g/mL) | B | 6:1 | 8:1 | 10:1 |
| Stirring temperature (°C) | C | 20 | 30 | 40 |
| Runs | A | B | C | Y |
|---|---|---|---|---|
| H2O/β-CD Ratio (mL/g) | β-CD/CLEO Ratio (g/mL) | Stirring Temperature (°C) | Encapsulation Efficiency (%) | |
| 1 | 5 | 4 | 30 | 36.10 |
| 2 | 15 | 6 | 20 | 52.65 |
| 3 | 15 | 8 | 30 | 61.27 |
| 4 | 10 | 8 | 20 | 67.98 |
| 5 | 10 | 8 | 40 | 63.59 |
| 6 | 10 | 6 | 30 | 54.02 |
| 7 | 10 | 6 | 30 | 52.84 |
| 8 | 5 | 8 | 30 | 63.09 |
| 9 | 15 | 4 | 30 | 36.07 |
| 10 | 10 | 6 | 30 | 54.58 |
| 11 | 10 | 6 | 30 | 54.17 |
| 12 | 5 | 6 | 40 | 48.30 |
| 13 | 10 | 4 | 20 | 34.56 |
| 14 | 10 | 4 | 40 | 37.19 |
| 15 | 5 | 6 | 20 | 52.92 |
| 16 | 15 | 6 | 40 | 45.42 |
| 17 | 10 | 6 | 30 | 52.16 |
| Variance Origin | Sum of Squares | df | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 1661.61 | 9 | 184.62 | 53.15 | <0.0001 | significant |
| A | 3.13 | 1 | 3.13 | 0.8996 | 0.3745 | - |
| B | 1568.28 | 1 | 1568.28 | 451.46 | <0.0001 | significant |
| C | 23.15 | 1 | 23.15 | 6.67 | 0.0364 | significant |
| A2 | 31.03 | 1 | 31.03 | 8.93 | 0.0203 | significant |
| B2 | 12.27 | 1 | 12.27 | 3.53 | 0.1023 | - |
| C2 | 4.35 | 1 | 4.35 | 1.25 | 0.2998 | - |
| AB | 0.8010 | 1 | 0.8010 | 0.2306 | 0.6457 | - |
| AC | 1.70 | 1 | 1.70 | 0.4902 | 0.5064 | - |
| BC | 12.32 | 1 | 12.32 | 3.55 | 0.1017 | - |
| Residual | 24.32 | 7 | 3.47 | - | - | - |
| Lack of Fit | 20.21 | 3 | 6.74 | 6.57 | 0.0503 | not significant |
| Pure Error | 4.10 | 4 | 1.03 | - | - | - |
| Cor Total | 1685.93 | 16 | - | - | - | - |
| R2 | 0.9856 | - | - | - | - | - |
| Adjusted R2 | 0.9670 | - | - | - | - | significant |
| Model | 1661.61 | 9 | 184.62 | 53.15 | <0.0001 | significant |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Zhao, X.; Wang, C.; Fang, Q.; Zhong, L.; Wei, Q. Preparation, Optimization, and Characterization of Inclusion Complexes of Cinnamomum longepaniculatum Essential Oil in β-Cyclodextrin. Sustainability 2022, 14, 9513. https://doi.org/10.3390/su14159513
Yan Y, Zhao X, Wang C, Fang Q, Zhong L, Wei Q. Preparation, Optimization, and Characterization of Inclusion Complexes of Cinnamomum longepaniculatum Essential Oil in β-Cyclodextrin. Sustainability. 2022; 14(15):9513. https://doi.org/10.3390/su14159513
Chicago/Turabian StyleYan, Yue, Xin Zhao, Chao Wang, Qiong Fang, Lu Zhong, and Qin Wei. 2022. "Preparation, Optimization, and Characterization of Inclusion Complexes of Cinnamomum longepaniculatum Essential Oil in β-Cyclodextrin" Sustainability 14, no. 15: 9513. https://doi.org/10.3390/su14159513





